Changes in EEG Activity and Cognition Related to Physical Activity in Older Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Data Extraction and Assessment of Risk of Bias
3. Results
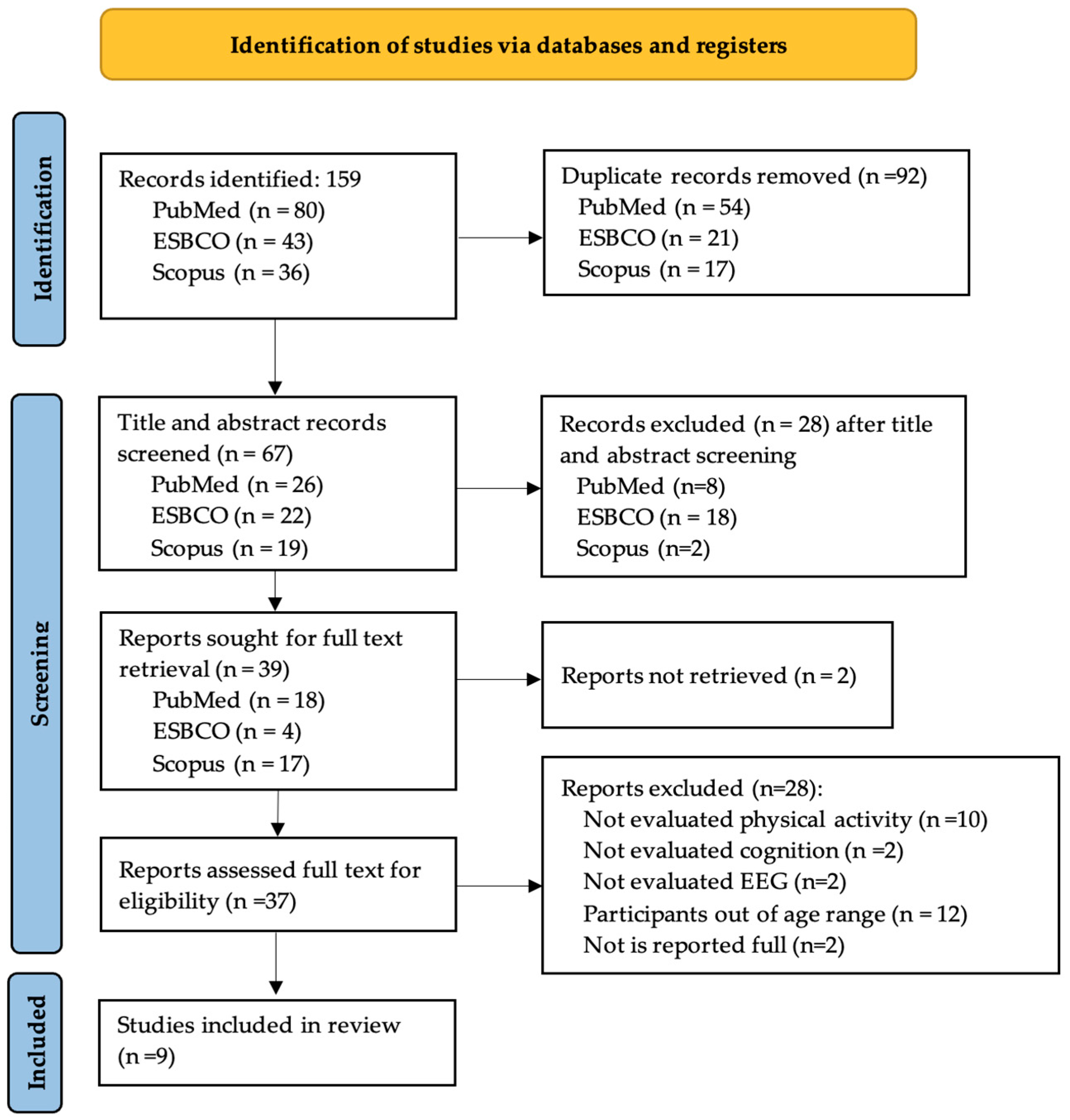
3.1. Search Results
3.2. Risk of Bias Assessment
- Q1: Was the research question or objective in this paper clearly stated and appropriate?
- Q2: Was the study population clearly specified and defined?
- Q3: Did the authors include a sample size justification?
- Q4: Were controls selected or recruited from the same or similar population that gave rise to the cases (including the same timeframe)?
- Q5: Were the definitions, inclusion and exclusion criteria, algorithms or processes used to identify or select cases and controls valid, reliable, and implemented consistently across all study participants?
- Q6: Were the cases clearly defined and differentiated from controls?
- Q7: If less than 100 percent of eligible cases and/or controls were selected for the study, were the cases and/or controls randomly selected from those eligible?
- Q8: Was there use of concurrent controls?
- Q9: Were the investigators able to confirm that the exposure/risk occurred prior to the development of the condition or event that defined a participant as a case?
- Q10: Were the measures of exposure/risk clearly defined, valid, reliable, and implemented consistently (including the same time period) across all study participants?
- Q11: Were the assessors of exposure/risk blinded to the case or control status of participants?
- Q12: Were key potential confounding variables measured and adjusted statistically in the analyses? If matching was used, did the investigators account for matching during study analysis?
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oken, 2006 [35] |  |  |  |  |  |  |  |  |  |  |  |  |
| Wang, 2014 [23] |  |  |  |  |  |  |  |  |  |  |  |  |
| Chuang, 2015 [36] |  |  |  |  |  |  |  |  |  |  |  |  |
| Gajewski, 2015 [37] |  |  |  |  |  |  |  |  |  |  |  |  |
| Schättin, 2016 [38] |  |  |  |  |  |  |  |  |  |  |  |  |
| Schättin, 2018 [39] |  |  |  |  |  |  |  |  |  |  |  |  |
| Gajewski, 2018 [40] |  |  |  |  |  |  |  |  |  |  |  |  |
| Schättin, 2019 [41] |  |  |  |  |  |  |  |  |  |  |  |  |
| Adcock, 2020 [42] |  |  |  |  |  |  |  |  |  |  |  |  |
 ); high risk of bias (
); high risk of bias ( ); unclear risk of bias (
); unclear risk of bias ( ).
).3.3. Physical Activity and Cognition and EEG in Aging
3.3.1. Effects of the Physical Activity on Cognition and EEG: Multiscale Entropy, Power Frequency
3.3.2. Effects of the Physical Activity on Cognition and EEG: Event-Related Potentials
| Study | Participants Characteristics | EEG | Cognitive Process Evaluated and Task | Physical Activity Program | Results |
|---|---|---|---|---|---|
| Oken, 2006 (RCT) [31] | Healthy men and women aged 65–85 years. Yoga group n = 44 Exercise group: n = 47 Control: n = 44 | Quantitative-EEG posterior median power frequency | Attention: Stroop Color Alertness: Word Test | Yoga classes (90 min) for 6-month Aerobic intervention: walking for 1 h (1 class per week) | = Yoga and aerobic intervention did not produce improvements in cognitive function = EEG auditory median power frequency |
| Wang, 2014 (OBS) [23] | Healthy men and women aged 66–70 years. Physically active: n = 24 Physically inactive n = 24 | EEG Multiscale entropy analysis | Visuo-spatial attention and working memory | Estimation of physical activity levels with questionnaire; participants to recall physical activity in the past 7 days | ↑ visuo-spatial attention and working memory in physically active group ↑ EEG multiscale entropy in physically active group |
| Chuang, 2015 (INT) [36] | Sedentary females aged 65–75 years. Dance Dance Revolution (DDR): n = 7 Brisk walking (BW): n = 11 Control group: n = 8 | EEG-ERP: N2, P3 | Selective attention: flanker task | Intervention: three times per week for 3 months, exergame DDR or BW | ↑ response speed in congruent and incongruent conditions in the flanker task with DDR and BW intervention ↓ N2 and P3 latencies in DDR and BW groups |
| Gajewski, 2015 (OBS) [37] | Healthy men aged 65–70 years. Low active: n = 20 Active: n = 20 | EEG-ERP: CNV, P2, N2, N450 | Attention: D2, Stroop Color, Digit-Symbol-Test Memory: Digit-Span-Test, Speed of processing: Trail Making Test | Estimation of physical activity levels with questionnaire: Lüdenscheid Activity Questionnaire | = speed of processing, attention, and memory in physically active group. ↑ Stroop task performance in physically active group = CNV amplitude in physically active group ↑ EEG-ERP: N2 and N450 amplitude in physically active group |
| Schättin, 2016 (RCS) [38] | Healthy men and women mean age 79.2 ± 7.3 years. Exergame: n = 13 Balance: n = 14 | EEG spectral power | Executive Functions: Test for Attentional Performance | Exergame or balance Training: 24 sessions for 8 to 10 weeks | ↑ Executive Functions in exergame group ↓ Theta relative power exergame group |
| Schättin, 2018 (OBS) [39] | Healthy men and women mean age 73.3 ± 5.9 years. Single group: n = 36 | EEG spectral power | Attention: Test of Attentional Performance | Estimation of physical activity levels with questionnaire: Physical Activity Questionnaire 50+ | = attention in physically active group ↑ EEG power in the alpha frequency in physically active group |
| Gajewski, 2018 (RCS) [40] | Healthy men and women aged 65–88 years. Physical training: n = 37 Control: n = 40 | EEG-ERP: P3a, P3b | Memory: Verbal Learning and Memory Test, Word Fluency Test, Rey-Osterrieth Complex Figure Working memory: Digit-Span Test | Physical training: cardiovascular, aerobic, and strength exercises. For 4 months, two times per week and 90 min per session | = memory in physically active group = EEG-ERP: P3a, P3b amplitude in physically active group |
| Schättin, 2019 (RCS) [41] | Healthy men and women mean age 69.4 ± 4.6 years. Single group: n = 17 | EEG: response-locked potentials | Attention: Test of Attentional Performance | Exergame Training: 30 sessions for 10 weeks | = Attention in physically active group = EEG: response-locked potentials in physically active group |
| Adcock, 2020 (INT) [42] | Healthy female 71.4 ± 5.8 years. Single group: n = 19 | EEG: power spectral density | Attention: Test of Attentional Performance | Exergame Training: 21 sessions for 7 weeks | ↑Attention in physically active group = EEG: alpha power spectral density in physically active group |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. World Social Report 2023: Leaving No One Behind in an Ageing World; DESA Publications: New York, NY, USA, 2023. [Google Scholar]
- Jiang, R.; Scheinost, D.; Zuo, N.; Wu, J.; Qi, S.; Liang, Q.; Zhi, D.; Luo, N.; Chung, Y.; Liu, S.; et al. A Neuroimaging Signature of Cognitive Aging from Whole-Brain Functional Connectivity. Adv. Sci. 2022, 9, 2201621. [Google Scholar] [CrossRef] [PubMed]
- Hernández Cortés, K.S.; Montoya Pedrón, A.; Hernández Cortés, N.M.; Bolaños Vaillant, S.; Romero García, L.; Hernández Cortés, K.S.; Montoya Pedrón, A.; Hernández Cortés, N.M.; Bolaños Vaillant, S.; Romero García, L. La Atrofia Cerebral Como Hallazgo o Factor Predictor Del Deterioro Cognitivo En El Envejecimiento Normal. Rev. Cuba. Med. Gen. Integral 2023, 39, e2239. [Google Scholar]
- Molina, D.S.M.; Camino, S.A.C. La atención al adulto mayor. Necesidad y posibilidad. Rev. Científica Arbitr. Multidiscip. PENTACIENCIAS 2024, 6, 260–272. [Google Scholar] [CrossRef]
- Martínez, N.; Santaella, E.; Rodríguez-García, A.-M. Benefits of Physical Activity for the Promotion of Active Aging in Elderly. Bibliographic Review. Retos 2021, 2021, 829–834. [Google Scholar]
- World Health Organization. Physical Activity and Sedentary Behaviour: A Brief to Support Older People. Available online: https://iris.who.int/handle/10665/365170 (accessed on 15 December 2023).
- Li, W.; Kim, K.-W.R.; Zhang, D.; Liu, B.; Dengler-Crish, C.M.; Wen, M.; Shi, L.; Pan, X.; Gu, Y.; Li, Y. Cost-Effectiveness of Physical Activity Interventions for Prevention and Management of Cognitive Decline and Dementia-a Systematic Review. Alzheimer’s Res. Ther. 2023, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Vázquez, J.; Cruz-Ortiz, M.; del Carmen Pérez-Rodríguez, M.; Cuevas-Guerrero, C.E. Aging: A Look at the Demographic Transition and Its Implications for Health Care. Rev. Enfermería Inst. Mex. Seguro Soc. 2018, 26, 273–280. [Google Scholar]
- Romero, N.; Romero-Ramos, Ó.; González, A.J. Physical Activity and Cognitive Functions in Older People: A Systematic Review of the Last 5 Years. Retos 2021, 39, 1017–1023. [Google Scholar]
- Tebar, W.R.; Ritti-Dias, R.M.; Saraiva, B.T.C.; Suetake, V.Y.B.; Delfino, L.D.; Christofaro, D.G.D. Physical Activity Levels Are Associated with Regional Bone Mineral Density in Boys. Phys. Sportsmed. 2019, 47, 336–340. [Google Scholar] [CrossRef]
- Diaz, V.; Bossio, M.; Justel, N. Towards a Healthy Aging: A Systematic Review about Music and Physical Exercise as Modulating Factors. Actual. Psicol. 2019, 33, 113–141. [Google Scholar]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise among Older People. BioMed Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef]
- Aldas-Vargas, C.; Chara-Plua, N.; Guerrero-Pluas, P.; Flores-Peña, R. Physical Activity in the Elderly. Dominio Cienc. 2020, 7, 64–77. [Google Scholar]
- Reyes-Rincón, H.; Campos-Uscanga, Y.; Reyes-Rincón, H.; Campos-Uscanga, Y. Beneficios de La Actividad Física En Espacios Naturales Sobre La Salud Del Adulto Mayor. Ene 2020, 14, 14207. [Google Scholar] [CrossRef]
- Sánchez, M.W. Evaluación de La Reserva Cognitiva Global Como Predictor Del Declive/Deterioro En Población de Mayores Autónomos e Institucionalizados. Ph.D. Thesis, Universidad Pontificia de Salamanca, Salamanca, Spain, 2018. [Google Scholar]
- Cabaco, A.S.; Wobbeking Sánchez, M.; Mejía-Ramírez, M.; Urchaga-Litago, J.D.; Castillo-Riedel, E.; Bonete-López, B. Mediation Effects of Cognitive, Physical, and Motivational Reserves on Cognitive Performance in Older People. Front. Psychol. 2022, 13, 1112308. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Kañevsky, A.; Leis, A.; Iturry, M.; Roncoroni, M.; Serrano, C.; Cristalli, D.; Ure, J.; Zuin, D. Papel de la actividad física en la prevención de deterioro cognitivo y demencia en adultos mayores: Una revisión sistemática. Neurol. Argent. 2020, 12, 124–137. [Google Scholar] [CrossRef]
- Wöbbeking-Sánchez, M.; Sánchez Cabaco, A.; Bonete-López, B.; Urchaga Litago, J.D.; Loureiro, M.J.; Mejía, M. Physical Activity and Life Satisfaction: An Empirical Study in a Population of Senior Citizens. Front. Psychol. 2021, 12, 636914. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Spies, C. Neuromonitoring in the Elderly. Curr. Opin. Anaesthesiol. 2019, 32, 101–107. [Google Scholar] [CrossRef]
- Omejc, N.; Peskar, M.; Miladinović, A.; Kavcic, V.; Džeroski, S.; Marusic, U. On the Influence of Aging on Classification Performance in the Visual EEG Oddball Paradigm Using Statistical and Temporal Features. Life 2023, 13, 391. [Google Scholar] [CrossRef]
- Yen, C.; Lin, C.-L.; Chiang, M.-C. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef]
- Bogéa Ribeiro, L.; da Silva Filho, M. Systematic Review on EEG Analysis to Diagnose and Treat Autism by Evaluating Functional Connectivity and Spectral Power. Neuropsychiatr. Dis. Treat. 2023, 19, 415–424. [Google Scholar] [CrossRef]
- Wang, C.-H.; Tsai, C.-L.; Tseng, P.; Yang, A.C.; Lo, M.-T.; Peng, C.-K.; Wang, H.-Y.; Muggleton, N.G.; Juan, C.-H.; Liang, W.-K. The Association of Physical Activity to Neural Adaptability during Visuo-Spatial Processing in Healthy Elderly Adults: A Multiscale Entropy Analysis. Brain Cogn. 2014, 92, 73–83. [Google Scholar] [CrossRef]
- Olichney, J.; Xia, J.; Church, K.J.; Moebius, H.J. Predictive Power of Cognitive Biomarkers in Neurodegenerative Disease Drug Development: Utility of the P300 Event-Related Potential. Neural Plast. 2022, 2022, 2104880. [Google Scholar] [CrossRef] [PubMed]
- Serafini, L.; Pesciarelli, F. Neural Timing of the Other-Race Effect across the Lifespan: A Review. Psychophysiology 2023, 60, e14203. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, A.; Assecondi, S.; Tagliabue, C.F.; Mazza, V. Power Shift and Connectivity Changes in Healthy Aging during Resting-State EEG. Neuroimage 2022, 256, 119247. [Google Scholar] [CrossRef] [PubMed]
- Grandi, F.; Tirapu Ustárroz, J. Neurociencia cognitiva del envejecimiento: Modelos explicativos. Rev. Esp. Geriatr. Gerontol. 2017, 52, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Courtney, S.M.; Hinault, T. When the Time Is Right: Temporal Dynamics of Brain Activity in Healthy Aging and Dementia. Prog. Neurobiol. 2021, 203, 102076. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Canuet, L.; Aoki, Y.; Hata, M.; Iwase, M.; Ikeda, S.; Nishida, K.; Ikeda, M. Healthy and Pathological Brain Aging: From the Perspective of Oscillations, Functional Connectivity, and Signal Complexity. Neuropsychobiology 2017, 75, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Rossi, S.; Babiloni, C.; Polich, J. Clinical Neurophysiology of Aging Brain: From Normal Aging to Neurodegeneration. Prog. Neurobiol. 2007, 83, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma Oscillations Weaken with Age in Healthy Elderly in Human EEG. Neuroimage 2020, 215, 116826. [Google Scholar] [CrossRef]
- Smailovic, U.; Koenig, T.; Laukka, E.J.; Kalpouzos, G.; Andersson, T.; Winblad, B.; Jelic, V. EEG Time Signature in Alzheimer’s Disease: Functional Brain Networks Falling Apart. Neuroimage Clin. 2019, 24, 102046. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Urrútia, G.; Bonfill, X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Oken, B.S.; Zajdel, D.; Kishiyama, S.; Flegal, K.; Dehen, C.; Haas, M.; Kraemer, D.F.; Lawrence, J.; Leyva, J. Randomized, Controlled, Six-Month Trial of Yoga in Healthy Seniors: Effects on Cognition and Quality of Life. Altern. Ther. Health Med. 2006, 12, 40–47. [Google Scholar] [PubMed]
- Chuang, L.-Y.; Hung, H.-Y.; Huang, C.-J.; Chang, Y.-K.; Hung, T.-M. A 3-Month Intervention of Dance Dance Revolution Improves Interference Control in Elderly Females: A Preliminary Investigation. Exp. Brain Res. 2015, 233, 1181–1188. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Long-Term Habitual Physical Activity Is Associated with Lower Distractibility in a Stroop Interference Task in Aging: Behavioral and ERP Evidence. Brain Cogn. 2015, 98, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Schättin, A.; Arner, R.; Gennaro, F.; de Bruin, E.D. Adaptations of Prefrontal Brain Activity, Executive Functions, and Gait in Healthy Elderly Following Exergame and Balance Training: A Randomized-Controlled Study. Front. Aging Neurosci. 2016, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Schättin, A.; Gennaro, F.; Egloff, M.; Vogt, S.; de Bruin, E.D. Physical Activity, Nutrition, Cognition, Neurophysiology, and Short-Time Synaptic Plasticity in Healthy Older Adults: A Cross-Sectional Study. Front. Aging Neurosci. 2018, 10, 242. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. ERP and Behavioral Effects of Physical and Cognitive Training on Working Memory in Aging: A Randomized Controlled Study. Neural Plast. 2018, 2018, 3454835. [Google Scholar] [CrossRef]
- Schättin, A.; Baier, C.; Mai, D.; Klamroth-Marganska, V.; Herter-Aeberli, I.; de Bruin, E.D. Effects of Exergame Training Combined with Omega-3 Fatty Acids on the Elderly Brain: A Randomized Double-Blind Placebo-Controlled Trial. BMC Geriatr. 2019, 19, 81. [Google Scholar] [CrossRef]
- Adcock, M.; Sonder, F.; Schättin, A.; Gennaro, F.; de Bruin, E.D. A Usability Study of a Multicomponent Video Game-Based Training for Older Adults. Eur. Rev. Aging Phys. Act. 2020, 17, 3. [Google Scholar] [CrossRef]
- Nakamura, M.; Chen, Q.; Sugi, T.; Ikeda, A.; Shibasaki, H. Technical Quality Evaluation of EEG Recording Based on Electroencephalographers’ Knowledge. Med. Eng. Phys. 2005, 27, 93–100. [Google Scholar] [CrossRef]
- Kappenman, E.S.; Luck, S.J. The Effects of Electrode Impedance on Data Quality and Statistical Significance in ERP Recordings. Psychophysiology 2010, 47, 888–904. [Google Scholar] [CrossRef] [PubMed]
- Radüntz, T. Signal Quality Evaluation of Emerging EEG Devices. Front. Physiol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Ghahfarrokhi, M.M.; Shirvani, H.; Rahimi, M.; Bazgir, B.; Shamsadini, A.; Sobhani, V. Feasibility and Preliminary Efficacy of Different Intensities of Functional Training in Elderly Type 2 Diabetes Patients with Cognitive Impairment: A Pilot Randomised Controlled Trial. BMC Geriatr. 2024, 24, 71. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.J.; Hortobágyi, T.; Karssemeijer, E.G.A.; Van der Zee, E.A.; Scherder, E.J.A.; van Heuvelen, M.J.G. Effects of Low- and High-Intensity Physical Exercise on Physical and Cognitive Function in Older Persons with Dementia: A Randomized Controlled Trial. Alzheimer’s Res. Ther. 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Chen, F.-T.; Li, R.-H.; Hillman, C.H.; Cline, T.L.; Chu, C.-H.; Hung, T.-M.; Chang, Y.-K. Effects of Acute Resistance Exercise on Executive Function: A Systematic Review of the Moderating Role of Intensity and Executive Function Domain. Sports Med. Open 2022, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Duque-Fernández, L.M.; Ornelas-Contreras, M.; Benavides-Pando, E.V. Actividad física y su relación con el envejecimiento y la capacidad funcional: Una revisión de la literatura de investigación. Psicol. Salud 2020, 30, 45–57. [Google Scholar] [CrossRef]
- Erickson, K.I.; Donofry, S.D.; Sewell, K.R.; Brown, B.M.; Stillman, C.M. Cognitive Aging and the Promise of Physical Activity. Annu. Rev. Clin. Psychol. 2022, 18, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical Activity as a Protective Factor for Dementia and Alzheimer’s Disease: Systematic Review, Meta-Analysis and Quality Assessment of Cohort and Case-Control Studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef]
- Makino, K.; Raina, P.; Griffith, L.E.; Lee, S.; Harada, K.; Katayama, O.; Tomida, K.; Morikawa, M.; Yamaguchi, R.; Nishijima, C.; et al. Lifetime Physical Activity and Late-Life Mild Cognitive Impairment in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2024, 25, 488–493.e3. [Google Scholar] [CrossRef]
- Zotcheva, E.; Håberg, A.K.; Wisløff, U.; Salvesen, Ø.; Selbæk, G.; Stensvold, D.; Ernstsen, L. Effects of 5 Years Aerobic Exercise on Cognition in Older Adults: The Generation 100 Study: A Randomized Controlled Trial. Sports Med. 2022, 52, 1689–1699. [Google Scholar] [CrossRef]
- Domingos, C.; Alves, C.P.; Sousa, E.; Rosa, A.; Pereira, J.G. Does Neurofeedback Training Improve Performance in Athletes? NeuroRegulation 2020, 7, 8. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Vetrovsky, T.; Balbim, G.M.; Sorte Silva, N.C.B.; Manca, A.; Deriu, F.; Kolmos, M.; Kruuse, C.; Liu-Ambrose, T.; Radák, Z.; et al. The Impact of Aerobic and Resistance Training Intensity on Markers of Neuroplasticity in Health and Disease. Ageing Res. Rev. 2022, 80, 101698. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical Activity and Brain Plasticity in Late Adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Vatinno, A.A.; Simpson, A.; Ramakrishnan, V.; Bonilha, H.S.; Bonilha, L.; Seo, N.J. The Prognostic Utility of Electroencephalography in Stroke Recovery: A Systematic Review and Meta-Analysis. Neurorehabil. Neural Repair. 2022, 36, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-G.; Thapa, N.; Park, H.-J.; Bae, S.; Park, K.W.; Park, J.-H.; Park, H. Virtual Reality and Exercise Training Enhance Brain, Cognitive, and Physical Health in Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2022, 19, 13300. [Google Scholar] [CrossRef]
- Dimitrova, J.; Hogan, M.; Khader, P.; O’Hora, D.; Kilmartin, L.; Walsh, J.C.; Roche, R.; Anderson-Hanley, C. Comparing the Effects of an Acute Bout of Physical Exercise with an Acute Bout of Interactive Mental and Physical Exercise on Electrophysiology and Executive Functioning in Younger and Older Adults. Aging Clin. Exp. Res. 2017, 29, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Hübner, L.; Godde, B.; Voelcker-Rehage, C. Acute Exercise as an Intervention to Trigger Motor Performance and EEG Beta Activity in Older Adults. Neural Plast. 2018, 2018, 4756785. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Hsieh, S.-S.; Chueh, T.-Y.; Huang, C.-J.; Hung, T.-M. The Effects of Barbell Resistance Exercise on Information Processing Speed and Conflict-Related ERP in Older Adults: A Crossover Randomized Controlled Trial. Sci. Rep. 2021, 11, 9137. [Google Scholar] [CrossRef]
- Gusatovic, J.; Gramkow, M.H.; Hasselbalch, S.G.; Frederiksen, K.S. Effects of Aerobic Exercise on Event-Related Potentials Related to Cognitive Performance: A Systematic Review. PeerJ 2022, 10, e13604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Serrano, L.M.; Wöbbeking-Sánchez, M.; De La Torre, L.; Pérez-Elvira, R.; Chávez-Hernández, M.E. Changes in EEG Activity and Cognition Related to Physical Activity in Older Adults: A Systematic Review. Life 2024, 14, 440. https://doi.org/10.3390/life14040440
Rodríguez-Serrano LM, Wöbbeking-Sánchez M, De La Torre L, Pérez-Elvira R, Chávez-Hernández ME. Changes in EEG Activity and Cognition Related to Physical Activity in Older Adults: A Systematic Review. Life. 2024; 14(4):440. https://doi.org/10.3390/life14040440
Chicago/Turabian StyleRodríguez-Serrano, Luis Miguel, Marina Wöbbeking-Sánchez, Lizbeth De La Torre, Ruben Pérez-Elvira, and María Elena Chávez-Hernández. 2024. "Changes in EEG Activity and Cognition Related to Physical Activity in Older Adults: A Systematic Review" Life 14, no. 4: 440. https://doi.org/10.3390/life14040440
APA StyleRodríguez-Serrano, L. M., Wöbbeking-Sánchez, M., De La Torre, L., Pérez-Elvira, R., & Chávez-Hernández, M. E. (2024). Changes in EEG Activity and Cognition Related to Physical Activity in Older Adults: A Systematic Review. Life, 14(4), 440. https://doi.org/10.3390/life14040440






