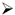Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.1.1. Inclusion Criteria
- ❖
- Age ≥ 18;
- ❖
- Definitive diagnosis of Psoriasis;
- ❖
- Not under treatment for psoriasis (for at least 1 year);
- ❖
- ≥15 teeth.
2.1.2. Exclusion Criteria
- ❖
- Pregnant and lactating women;
- ❖
- Current or previous neoplasm, chemotherapy, radiotherapy, medication-related osteonecrosis of the jaws;
- ❖
- Smoking habit;
- ❖
- dental and periodontal infections requiring dental treatment;
- ❖
- Removable dentures.
2.2. Data Collection
2.2.1. Sample Characteristics
- ✓
- Age;
- ✓
- Gender;
- ✓
- Comorbidities: a positive medical history for type 2 diabetes, hypertension, dyslipidemia, cardiovascular events, or immune-mediated inflammatory diseases (IMIDs), as well as habitual drug use.
2.2.2. Psoriasis Descriptive Variables
- ▪
- Psoriasis subtypes: diverse clinical forms encompass chronic plaque psoriasis, identifiable by well-demarcated, erythematous plaques covered with coarse scales; guttate psoriasis, characterized by the sudden onset of numerous small inflammatory plaques; pustular psoriasis, presenting as an acute, subacute, or chronic pustular eruption, and erythrodermic psoriasis, featuring widespread cutaneous erythema and scaling affecting a substantial portion or the entirety of the body surface area [11].
- ▪
- Body Surface Area (BSA): delineated as the percentage of total body surface involvement, with 1% representing an approximate area equivalent to the patient’s handprint.
- ▪
- Psoriasis Area and Severity Index (PASI): serves as a widely employed tool in psoriasis trials, offering an assessment and grading of the severity of psoriasis lesions along with the patient’s response to treatment. It generates a numeric score within the range of 0 to 72. Typically, a PASI score falling between 5 and 10 indicates moderate disease, while a score exceeding 10 is considered severe. The benchmark for efficacy in most clinical trials and the criterion endorsed by the Food and Drug Administration for evaluating new psoriasis treatments is a 75% reduction in the PASI score, commonly referred to as PASI 75 [7].
- ▪
- Dermatology Life Quality Index (DLQI): is a validated patient-reported instrument designed to assess the impact of skin diseases on health-related quality of life and daily activities. Comprising 10 questions, each response is evaluated on a scale from 0 to 3. The DLQI score is calculated by summing the scores for each question, yielding a maximum score of 30 and a minimum score of 0. Higher scores on the DLQI indicate a greater compromise in the quality of life, with a score exceeding 10 suggesting a severe impact on the patient’s life due to their skin condition [7]. In detail, DLQI score categorized the impact of the disease on the quality of life into none (0–1 score), small (2–5 score), moderate (6–10 score), very large (11–20 score), and extremely large (21–30 score) [3]. Developed by Finlay AY and Khan GK in 1994, the DLQI has been widely utilized in global clinical trials and research endeavors exploring the quality of life and disease burden associated with various dermatological conditions. Accessible in multiple languages representative of the participating countries, the DLQI can be accessed online (http://www.dermatology.org.uk/quality/quality-dlqi.html, accessed on 20 November 2023). The questionnaire was completed as part of a standardized interview to avoid different interpretations among patients and to mitigate its subjective nature [26] and was electronically attached to the patients’ medical records.
- ▪
- Psoriasis severity: mild psoriasis was characterized by the criteria of body surface area (BSA) ≤ 10, psoriasis area and severity index (PASI) ≤ 10, and dermatology life quality index (DLQI) ≤ 10; conversely, moderate to severe psoriasis was defined as (BSA > 10 or PASI > 10) and DLQI > 10.
- ▪
- Psoriasis lesions distribution and prevailing involvement (skin, mucosal, nail involvement, and diffuse distribution of lesions);
- ▪
- Psoriasis arthritis;
- ▪
- Years since psoriasis diagnosis.
2.2.3. Outcome Variables
- Oral lesions: psoriasis-specific lesions and nonspecific lesions of the oral mucosa reported on dental charts, along with mucosal, dental, and periodontal infections and treatment needs, were obtained from dental charts granted by the Complex Operating Unit of Odontostomatology of the same Hospital.
- Oral Health Impact Profile-14 (OHIP-14): scores have a potential range from 0 to 56, with the calculation involving the summation of the ordinal values assigned to each of the 14 items. Additionally, the domain scores within this instrument can span from 0 to 8. A higher OHIP-14 score is indicative of a more compromised Oral Health-Related Quality of Life (OHRQoL), while lower scores suggest a better OHRQoL [28]. The Italian-validated version of the questionnaire (provided as Supplementary File S1) [29], obtained from dental charts, was completed as part of a standardized interview to avoid different interpretations among patients and to mitigate its subjective nature [26]. To make the results more readable, an OHIP score of 0–14 was associated with excellent OHRQoL, an OHIP score of 15–28 with good OHRQoL, an OHIP score of 27–42 with medium OHRQoL and an OHIP score of 43–56 with low OHRQoL.
2.3. Data Analysis
3. Results
3.1. Qualitative Synthesis of the Collected Data
3.1.1. Sample Characteristics
3.1.2. Psoriasis Descriptive Variables
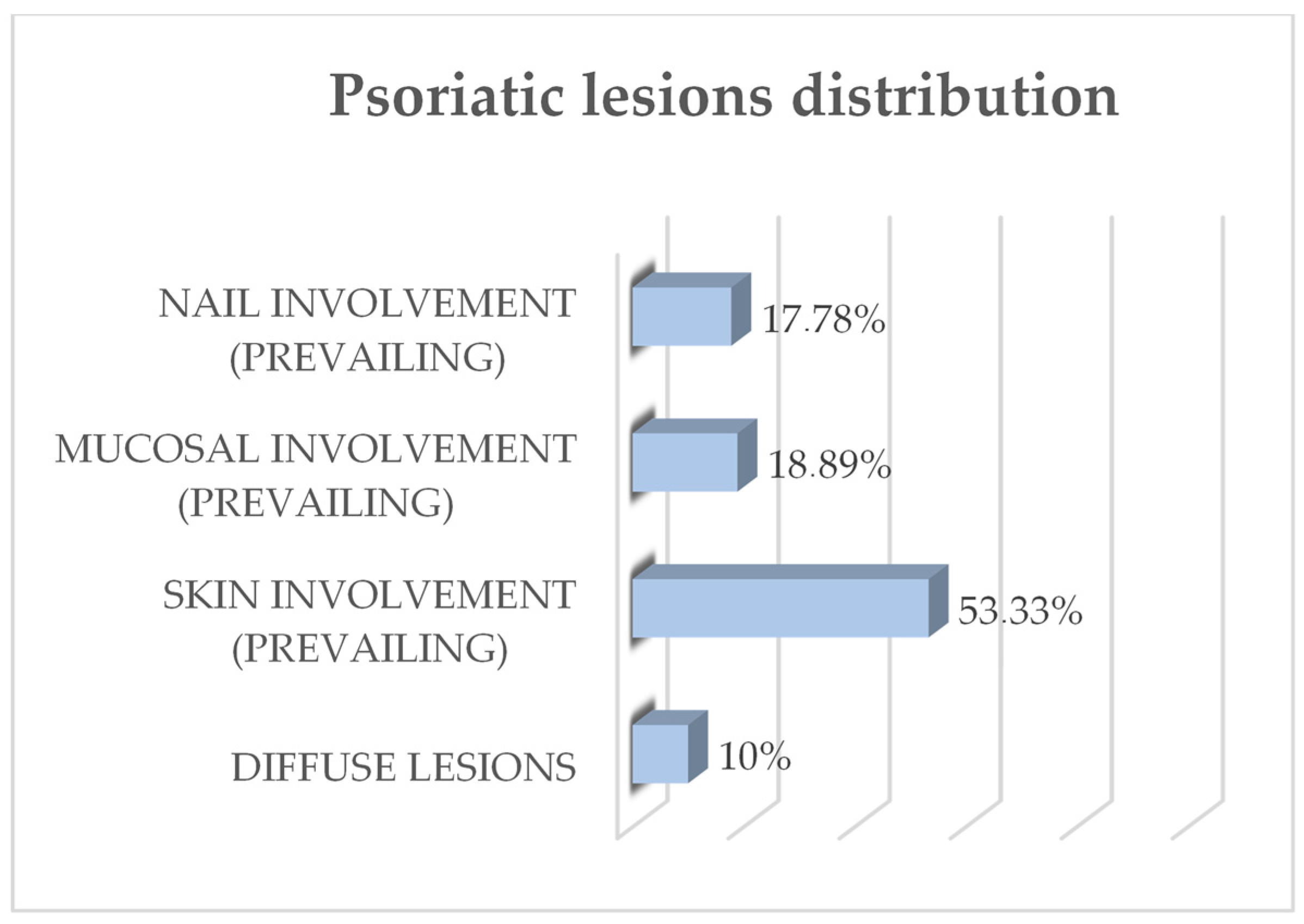
3.1.3. Outcome Variables: Oral Lesions and OHQRoL in Psoriasis Subjects
3.2. Variables Correlations with DLQI and OHIP-14 Scores
3.3. Variables Differences Related to DLQI and OHIP
4. Discussion
4.1. Outcome Variables: Oral Lesions and OHQRoL in Psoriasis Subjects
4.1.1. Psoriasis-Specific and Nonspecific Oral Lesions in Adult Untreated Psoriasis Subjects
4.1.2. Oral Health-Related Quality of Life (OHRQoL) in Adult Untreated Psoriasis Subjects
4.2. Correlation of DLQI and OHIP-14 Scores with Sample and Psoriasis Descriptive Variables in Adult Untreated Psoriasis Subjects
4.3. Comparison of Sample Characteristics, Psoriasis Descriptive Variables by DLQI and OHQRoL
Furthermore, Oral Lesions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, A.A.; Cota, L.O.M.; Mendes, V.S.; Oliveira, A.M.S.D.; Cyrino, R.M.; Costa, F.O. Periodontitis and the Impact of Oral Health on the Quality of Life of Psoriatic Individuals: A Case-Control Study. Clin. Oral Investig. 2021, 25, 2827–2836. [Google Scholar] [CrossRef]
- Ungprasert, P.; Wijarnpreecha, K.; Wetter, D.A. Periodontitis and Risk of Psoriasis: A Systematic Review and Meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 857–862. [Google Scholar] [CrossRef]
- Olejnik, M.; Adamski, Z.; Osmola-Mankowska, A.; Nijakowski, K.; Dorocka-Bobkowska, B. Oral Health Status and Dental Treatment Needs of Psoriatic Patients with Different Therapy Regimes. Aust. Dent. J. 2021, 66, S42–S47. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.K. Psoriasis: A Review of Systemic Comorbidities and Dental Management Considerations. Quintessence Int. 2018, 49, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Psoriasis—Epidemiology and Clinical Spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of Treatment Goals for Moderate to Severe Psoriasis: A European Consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Both, H.; Essink-Bot, M.-L.; Busschbach, J.; Nijsten, T. Critical Review of Generic and Dermatology-Specific Health-Related Quality of Life Instruments. J. Investig. Dermatol. 2007, 127, 2726–2739. [Google Scholar] [CrossRef]
- FINLAY, A.Y.; KHAN, G.K. Dermatology Life Quality Index (DLQI)-a Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, D.; Pietrzak, A.; Krasowska, D.; Borzęcki, A.; Franciszkiewicz-Pietrzak, K.; Polkowska-Pruszyńska, B.; Baranowska, M.; Reich, K. Digestive System in Psoriasis: An Update. Arch. Dermatol. Res. 2017, 309, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.B. Psoriasis: Epidemiology, Clinical Features, and Quality of Life. Ann. Rheum. Dis. 2005, 64, ii18–ii23. [Google Scholar] [CrossRef]
- Altemir, A.; Melé-Ninot, G.; Lázaro-Simó, A.I.; Iglesias-Sancho, M.; Quintana-Codina, M.; Arandes, J.; Carrera-Morodo, M.; Salleras-Redonnet, M. Manifestaciones Orales En Pacientes Con Psoriasis. Prevalencia y Asociación Con Sus Características Clínicas y Epidemiológicas. Actas Dermo-Sifiliográficas 2022, 113, 459–466. [Google Scholar] [CrossRef]
- Di Spirito, F.; Caggiano, M.; Di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Martina, S.; Amato, A. Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl. Sci. 2022, 12, 8995. [Google Scholar] [CrossRef]
- Venugopal, D.C.; Sankarapandian, S.; Narasimhan, M. A Rare Case of Intraoral Psoriasis. Cureus 2019, 11, e5204. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Oral Psoriasis: An Overlooked Enigma. Dermatology 2016, 232, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dua, A.B.; Touma, Z.; Toloza, S.; Jolly, M. Top 10 Recent Developments in Health-Related Quality of Life in Patients with Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2013, 15, 380. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.; Allen, P.F.; Abu-bakr, N.H.; Abdel-Rahman, M.E. Psychometric Properties and Performance of the Oral Health Impact Profile (OHIP-14s-Ar) among Sudanese Adults. J. Oral Sci. 2013, 55, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, D.-Y.; Chen, Y.-M.; Lai, K.-L. Health-Related Quality of Life and Utility: Comparison of Ankylosing Spondylitis, Rheumatoid Arthritis, and Systemic Lupus Erythematosus Patients in Taiwan. Clin. Rheumatol. 2017, 36, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Braga, M.; Cornaby, C.; Cortez, A.; Bernardes, M.; Terroso, G.; Figueiredo, M.; Mesquita, C.D.S.; Costa, L.; Poole, B.D. Depression and Anxiety in Systemic Lupus Erythematosus. Medicine 2018, 97, e11376. [Google Scholar] [CrossRef]
- Isik, A.; Koca, S.S.; Ozturk, A.; Mermi, O. Anxiety and Depression in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2007, 26, 872–878. [Google Scholar] [CrossRef]
- Russell, A.S. Quality-of-Life Assessment in Rheumatoid Arthritis. Pharmacoeconomics 2008, 26, 831–846. [Google Scholar] [CrossRef]
- de Oliveira, B.H.; Nadanovsky, P. Psychometric Properties of the Brazilian Version of the Oral Health Impact Profile–Short Form. Community Dent. Oral Epidemiol. 2005, 33, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chamani, G.; Shakibi, M.; Zarei, M.; Rad, M.; Pouyafard, A.; Parhizkar, A.; Mansoori, M. Assessment of Relationship between Xerostomia and Oral Health-related Quality of Life in Patients with Rheumatoid Arthritis. Oral Dis. 2017, 23, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Blaizot, A.; Monsarrat, P.; Constantin, A.; Vergnes, J.-N.; de Grado, G.F.; Nabet, C.; Cantagrel, A.; Sixou, M. Oral Health-Related Quality of Life among Outpatients with Rheumatoid Arthritis. Int. Dent. J. 2013, 63, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Manzano, B.R.; da Silva Santos, P.S.; Bariquelo, M.H.; Merlini, N.R.G.; Honório, H.M.; Rubira, C.M.F. A Case-Control Study of Oral Diseases and Quality of Life in Individuals with Rheumatoid Arthritis and Systemic Lupus Erythematosus. Clin. Oral Investig. 2021, 25, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Caracho, R.A.; Foratori-Junior, G.A.; dos Santos Fusco, N.; Jesuino, B.G.; Missio, A.L.T.; de Carvalho Sales-Peres, S.H. Systemic Conditions and Oral Health-Related Quality of Life of Pregnant Women of Normal Weight and Who Are Overweight. Int. Dent. J. 2020, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y. Current Severe Psoriasis and the Rule of Tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.V. Assessing Quality of Life Using the Oral Health Impact Profile (OHIP-14) in Subjects with and without Orthodontic Treatment Need in Chennai, Tamil Nadu, India. J. Clin. Diagn. Res. 2017, 11, ZC78. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, M.; Giordano, A.; Brigatti, E.; Migliario, M.; Levrini, L.; Ferriero, G. Psychometric Properties of the Italian Version of the Reduced Form of the Oral Health Impact Profile (OHIP-14). G. Ital. Di Med. Del Lav. Ed Ergon. 2010, 32, B71–B78. [Google Scholar]
- Corrêa, J.D.; Branco, L.G.A.; Calderaro, D.C.; Mendonça, S.M.S.; Travassos, D.V.; Ferreira, G.A.; Teixeira, A.L.; Abreu, L.G.; Silva, T.A. Impact of Systemic Lupus Erythematosus on Oral Health-Related Quality of Life. Lupus 2018, 27, 283–289. [Google Scholar] [CrossRef]
- Abrão, A.L.P.; Santana, C.M.; Bezerra, A.C.B.; Amorim, R.F.B.D.; Silva, M.B.D.; Mota, L.M.H.D.; Falcão, D.P. O Que o Reumatologista Deve Saber Sobre as Manifestações Orofaciais Das Doenças Reumáticas Autoimunes. Rev. Bras. Reumatol. 2016, 56, 441–450. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Ruta, D.A.; Ogden, G.R.; Pitts, N.B.; Ogston, S.A. Assessing Oral Health-related Quality of Life in General Dental Practice in Scotland: Validation of the OHIP-14. Community Dent. Oral Epidemiol. 2006, 34, 53–62. [Google Scholar] [CrossRef]
- Aliko, A.; Ciancaglini, R.; Alushi, A.; Tafaj, A.; Ruci, D. Temporomandibular Joint Involvement in Rheumatoid Arthritis, Systemic Lupus Erythematosus and Systemic Sclerosis. Int. J. Oral Maxillofac. Surg. 2011, 40, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, S.; Jäger, J.; Krohn-Grimberghe, B.; Patschan, S.; Mausberg, R.F.; Schmalz, G.; Haak, R.; Ziebolz, D. Oral Health-Related Quality of Life Depending on Oral Health in Patients with Rheumatoid Arthritis. Clin. Oral Investig. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Spencer, A.J. Development and Evaluation of the Oral Health Impact Profile. Community Dent. Health 1994, 11, 3–11. [Google Scholar] [PubMed]
- Slade, G.D.; Spencer, A.J.; Locker, D.; Hunt, R.J.; Strauss, R.P.; Beck, J.D. Variations in the Social Impact of Oral Conditions Among Older Adults in South Australia, Ontario, and North Carolina. J. Dent. Res. 1996, 75, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, N.M.; Steele, J.G.; Pine, C.M.; White, D.; Pitts, N.B. The Impact of Oral Health on People in the UK in 1998. Br. Dent. J. 2001, 190, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Krueger, G.G.; Feldman, S.R.; Camisa, C.; Duvic, M.; Elder, J.T.; Gottlieb, A.B.; Koo, J.; Krueger, J.G.; Lebwohl, M.; Lowe, N.; et al. Two Considerations for Patients with Psoriasis and Their Clinicians. J. Am. Acad. Dermatol. 2000, 43, 281–285. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Pujol, R.M.; García-Patos, V.; Bordas, X.; Smandía, J.A. Psoriasis of Early and Late Onset: A Clinical and Epidemiologic Study from Spain. J. Am. Acad. Dermatol. 2002, 46, 867–873. [Google Scholar] [CrossRef]
- Pathirana, D.; Nast, A.; Ormerod, A.; Reytan, N.; Saiag, P.; Smith, C.; Spuls, P.; Rzany, B. On the Development of the European S3 Guidelines on the Systemic Treatment of Psoriasis Vulgaris: Structure and Challenges. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1458–1467. [Google Scholar] [CrossRef]
- Fortune, D.G.; Main, C.J.; O’Sullivan, T.M.; Griffiths, C.E. Quality of Life in Patients with Psoriasis: The Contribution of Clinical Variables and Psoriasis-Specific Stress. Br. J. Dermatol. 1997, 137, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.E.; Cohen, J.M.; Ho, R.S. Psoriasis and Suicidality: A Review of the Literature. Dermatol. Ther. 2019, 32, e12771. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Gupta, A.K.; Haberman, H.F. Psoriasis and Psychiatry: An Update. Gen. Hosp. Psychiatry 1987, 9, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Kelly, S.E. Psoriasis-an Index of Disability. Clin. Exp. Dermatol. 1987, 12, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Sanders, A.E. The Paradox of Better Subjective Oral Health in Older Age. J. Dent. Res. 2011, 90, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.G.; Gibson, B.; Khan, F.A.; Birnbaum, W. Validity of Two Oral Health-related Quality of Life Measures. Community Dent. Oral Epidemiol. 2003, 31, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, S.-M.; Suh, D.; Shin, H.T.; Suh, D.-C. The Association of Socioeconomic and Clinical Characteristics with Health-Related Quality of Life in Patients with Psoriasis: A Cross-Sectional Study. Health Qual. Life Outcomes 2018, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, A.; Munisamy, M.; Menon, V.; Mohan Raj, P.S.; Priyadarshini, G.; Rajappa, M. Stress and Psoriasis: Exploring the Link through the Prism of Hypothalamo-Pituitary-Adrenal Axis and Inflammation. J. Psychosom. Res. 2023, 170, 111350. [Google Scholar] [CrossRef]
- Iannone, M.; Janowska, A.; Panduri, S.; Morganti, R.; Davini, G.; Romanelli, M.; Dini, V. Impact of Psychiatric Comorbidities in Psoriasis, Hidradenitis Suppurativa and Atopic Dermatitis: The Importance of a Psychodermatological Approach. Exp. Dermatol. 2022, 31, 956–961. [Google Scholar] [CrossRef]
- Spuls, P.I.; Lecluse, L.L.A.; Poulsen, M.-L.N.F.; Bos, J.D.; Stern, R.S.; Nijsten, T. How Good Are Clinical Severity and Outcome Measures for Psoriasis?: Quantitative Evaluation in a Systematic Review. J. Investig. Dermatol. 2010, 130, 933–943. [Google Scholar] [CrossRef]
- Fox, F.E.; Rumsey, N.; Morris, M. “Ur Skin Is the Thing That Everyone Sees and You Cant Change It!”: Exploring the Appearance-Related Concerns of Young People with Psoriasis. Dev. Neurorehabil. 2007, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Basińska, M.A.; Drozdowska, M. Emotional Intelligence as an Indicator of Satisfaction with Life of Patients with Psoriasis. Adv. Dermatol. Allergol. 2013, 6, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Controne, I.; Scoditti, E.; Buja, A.; Pacifico, A.; Kridin, K.; Fabbro, M.D.; Garbarino, S.; Damiani, G. Do Sleep Disorders and Western Diet Influence Psoriasis? A Scoping Review. Nutrients 2022, 14, 4324. [Google Scholar] [CrossRef]
- Holm, J.G.; Thomsen, S.F. Type 2 Diabetes and Psoriasis: Links and Risks. Psoriasis Targets Ther. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA 2006, 296, 1735. [Google Scholar] [CrossRef] [PubMed]
- Davidovici, B.B.; Sattar, N.; Jörg, P.C.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Ståhle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic Links between Skin Disease and Co-Morbid Conditions. J. Investig. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. Angiogenesis and Oxidative Stress: Common Mechanisms Linking Psoriasis with Atherosclerosis. J. Dermatol. Sci. 2011, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Sung, Y.-K.; Kang, C.P.; Choi, C.-B.; Kang, C.; Bae, S.-C. A Regulatory SNP at Position −899 in CDKN1A Is Associated with Systemic Lupus Erythematosus and Lupus Nephritis. Genes Immun. 2009, 10, 482–486. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Rani, P.L.; Fu, X.; Yu, W.; Bao, F.; Yu, G.; Li, J.; Li, L.; Sun, L.; et al. Identification of PTPN22, ST6GAL1 and JAZF1 as Psoriasis Risk Genes Demonstrates Shared Pathogenesis between Psoriasis and Diabetes. Exp. Dermatol. 2017, 26, 1112–1117. [Google Scholar] [CrossRef]
- Mehta, N.N.; Li, R.; Krishnamoorthy, P.; Yu, Y.; Farver, W.; Rodrigues, A.; Raper, A.; Wilcox, M.; Baer, A.; DerOhannesian, S.; et al. Abnormal Lipoprotein Particles and Cholesterol Efflux Capacity in Patients with Psoriasis. Atherosclerosis 2012, 224, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Ruschitzka, F. Psoriasis and Atherosclerosis: Two Plaques, One Syndrome? Eur. Heart J. 2012, 33, 1989–1991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rebelo, M.A.B.; de Castro, P.H.D.; Rebelo Vieira, J.M.; Robinson, P.G.; Vettore, M.V. Low Social Position, Periodontal Disease, and Poor Oral Health-Related Quality of Life in Adults with Systemic Arterial Hypertension. J. Periodontol. 2016, 87, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Vu, G.T.; Little, B.B.; Esterhay, R.J.; Jennings, J.A.; Creel, L.; Gettleman, L. Oral Health-related Quality of Life in US Adults with Type 2 Diabetes. J. Public Health Dent. 2022, 82, 79–87. [Google Scholar] [CrossRef]


| DLQI | |||
|---|---|---|---|
| A | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | 8.873 | 1.902, 15.844 | 0.013 |
| PASI | 0.362 | 0.146, 0.578 | 0.001 * |
| BSA | 0.105 | −0.015, 0.226 | 0.085 |
| Age | −0.002 | −0.118, 0.115 | 0.97 |
| Gender | |||
| Female | — | — | |
| Male | −0.691 | −3.930, 2.548 | 0.67 |
| Years since diagnosis | −0.080 | −0.203, 0.042 | 0.19 |
| B | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | 7.041 | 3.926, 10.155 | <0.001 |
| PASI | 0.274 | 0.055, 0.493 | 0.015 * |
| BSA | 0.132 | 0.018, 0.246 | 0.023 * |
| IMID | |||
| No | — | — | |
| Yes | −3.520 | −9.219, 2.179 | 0.22 |
| Hypertension | |||
| No | — | — | |
| Yes | −0.345 | −4.046, 3.356 | 0.85 |
| Dyslipidemia | |||
| No | — | — | |
| Yes | 1.326 | −2.780, 5.431 | 0.52 |
| Diabetes | |||
| No | — | — | |
| Yes | −1.667 | −6.734, 3.400 | 0.51 |
| CVD | |||
| No | — | — | |
| Yes | 2.894 | −1.762, 7.550 | 0.22 |
| C | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | 7.491 | 3.674, 11.308 | <0.001 |
| PASI | 0.376 | 0.156, 0.597 | 0.001 * |
| BSA | 0.130 | 0.012, 0.248 | 0.032 * |
| Skin Involvement | |||
| No | — | — | |
| Yes | −0.502 | −4.351, 3.347 | 0.80 |
| Nail Involvement | |||
| No | — | — | |
| Yes | −2.028 | −6.389, 2.333 | 0.36 |
| Mucosal Involvement | |||
| No | — | — | |
| Yes | −1.070 | −5.391, 3.252 | 0.62 |
| Diffuse Lesions | |||
| No | — | — | |
| Yes | −2.530 | −8.565, 3.505 | 0.41 |
| OHIP | |||
|---|---|---|---|
| A | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | −10.467 | −21.110, 0.177 | 0.054 |
| PASI | 0.027 | −0.303, 0.357 | 0.87 |
| BSA | 0.081 | −0.102, 0.265 | 0.38 |
| Age | 0.318 | 0.140, 0.496 | <0.001 * |
| Gender | |||
| Female | — | — | |
| Male | 1.819 | −3.127, 6.765 | 0.47 |
| Years since diagnosis | −0.031 | −0.218, 0.155 | 0.74 |
| B | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | 1.238 | −3.180, 5.656 | 0.58 |
| PASI | 0.213 | −0.098, 0.523 | 0.18 |
| BSA | 0.053 | −0.109, 0.215 | 0.52 |
| IMID | |||
| No | — | — | |
| Yes | 11.182 | 3.098, 19.265 | 0.007 * |
| Hypertension | |||
| No | — | — | |
| Yes | 7.722 | 2.472, 12.971 | 0.004 * |
| Dyslipidemia | |||
| No | — | — | |
| Yes | −0.743 | −6.567, 5.080 | 0.80 |
| Diabetes | |||
| No | — | — | |
| Yes | 4.572 | −2.615, 11.758 | 0.21 |
| CVD | |||
| No | — | — | |
| Yes | 1.584 | −5.020, 8.188 | 0.63 |
| C | |||
| Characteristic | Beta | 95% CI 1 | p-Value |
| (Intercept) | 8.084 | 2.039, 14.129 | 0.009 |
| PASI | 0.044 | −0.306, 0.393 | 0.80 |
| BSA | 0.027 | −0.160, 0.214 | 0.77 |
| Skin Involvement | |||
| No | — | — | |
| Yes | −3.493 | −9.588, 2.602 | 0.26 |
| Nail Involvement | |||
| No | — | — | |
| Yes | 1.828 | −5.078, 8.734 | 0.60 |
| Mucosal Involvement | |||
| No | — | — | |
| Yes | 7.257 | 0.413, 14.101 | 0.038 * |
| Diffuse Lesions | |||
| No | — | — | |
| Yes | 3.464 | −6.093, 13.021 | 0.47 |
| Dermatology Life Quality Index | |||
|---|---|---|---|
| Characteristic | DLQI ≤ 10, N = 41 1 | DLQI > 10, N = 48 1 | p-Value 2 |
| Age | 53.00 (13.00) | 53.00 (14.75) | 0.69 |
| Gender | 0.40 | ||
| Female | 20.0 (48.8%) | 19.0 (39.6%) | |
| Male | 21.0 (51.2%) | 29.0 (60.4%) | |
| Years Diagnosis | 15.00 (24.00) | 10.00 (15.00) | 0.20 |
| PASI | 3.00 (5.00) | 11.00 (9.00) | 0.00 * |
| BSA | 15.00 (23.00) | 30.00 (20.00) | 0.00 * |
| IMID | 0.29 | ||
| No | 35.0 (85.4%) | 45.0 (93.8%) | |
| Yes | 6.0 (14.6%) | 3.0 (6.2%) | |
| Hypertension | 0.51 | ||
| No | 25.0 (61.0%) | 33.0 (68.8%) | |
| Yes | 16.0 (39.0%) | 15.0 (31.2%) | |
| Dyslipidemia | 0.29 | ||
| No | 35.0 (85.4%) | 36.0 (75.0%) | |
| Yes | 6.0 (14.6%) | 12.0 (25.0%) | |
| Diabetes (Type 2) | 1.00 | ||
| No | 35.0 (85.4%) | 42.0 (87.5%) | |
| Yes | 6.0 (14.6%) | 6.0 (12.5%) | |
| Cardiovascular disease | 0.27 | ||
| No | 36.0 (87.8%) | 37.0 (77.1%) | |
| Yes | 5.0 (12.2%) | 11.0 (22.9%) | |
| Skin involvement | 0.38 | ||
| No | 17.0 (41.5%) | 15.0 (31.2%) | |
| Yes | 24.0 (58.5%) | 33.0 (68.8%) | |
| Nail involvement | 0.42 | ||
| No | 35.0 (85.4%) | 37.0 (77.1%) | |
| Yes | 6.0 (14.6%) | 11.0 (22.9%) | |
| Mucosal involvement | 0.27 | ||
| No | 36.0 (87.8%) | 37.0 (77.1%) | |
| Yes | 5.0 (12.2%) | 11.0 (22.9%) | |
| Diffuse lesions | 0.73 | ||
| No | 36.0 (87.8%) | 44.0 (91.7%) | |
| Yes | 5.0 (12.2%) | 4.0 (8.3%) | |
| Oral Health-Related Quality of Life | |||||
|---|---|---|---|---|---|
| Characteristic | Low, N = 2 1 | Medium, N = 5 1 | Good, N = 15 1 | Excellent, N = 68 1 | p-Value 2 |
| Age | 65.50 (9.50) | 69.00 (4.00) | 53.00 (19.50) | 52.00 (13.75) | 0.00 * |
| Gender | 0.51 | ||||
| Female | 0.0 (0.0%) | 1.0 (20.0%) | 7.0 (46.7%) | 32.0 (47.1%) | |
| Male | 2.0 (100.0%) | 4.0 (80.0%) | 8.0 (53.3%) | 36.0 (52.9%) | |
| Years Diagnosis | 8.00 (0.00) | 20.00 (10.00) | 12.00 (29.50) | 11.00 (14.25) | 0.90 |
| PASI | 1.00 (1.00) | 16.00 (20.00) | 4.00 (7.50) | 7.00 (9.00) | 0.01 * |
| BSA | 17.50 (17.50) | 40.00 (25.00) | 17.00 (24.00) | 25.00 (25.00) | 0.11 |
| IMID | 0.02 * | ||||
| No | 0.0 (0.0%) | 5.0 (100.0%) | 13.0 (86.7%) | 63.0 (92.6%) | |
| Yes | 2.0 (100.0%) | 0.0 (0.0%) | 2.0 (13.3%) | 5.0 (7.4%) | |
| Hypertension | 0.00 * | ||||
| No | 0.0 (0.0%) | 1.0 (20.0%) | 7.0 (46.7%) | 51.0 (75.0%) | |
| Yes | 2.0 (100.0%) | 4.0 (80.0%) | 8.0 (53.3%) | 17.0 (25.0%) | |
| Dyslipidemia | 0.13 | ||||
| No | 2.0 (100.0%) | 2.0 (40.0%) | 13.0 (86.7%) | 55.0 (80.9%) | |
| Yes | 0.0 (0.0%) | 3.0 (60.0%) | 2.0 (13.3%) | 13.0 (19.1%) | |
| Diabetes (Type 2) | 0.02 * | ||||
| No | 0.0 (0.0%) | 5.0 (100.0%) | 12.0 (80.0%) | 61.0 (89.7%) | |
| Yes | 2.0 (100.0%) | 0.0 (0.0%) | 3.0 (20.0%) | 7.0 (10.3%) | |
| Cardiovascular disease | 0.01 * | ||||
| No | 2.0 (100.0%) | 1.0 (20.0%) | 12.0 (80.0%) | 59.0 (86.8%) | |
| Yes | 0.0 (0.0%) | 4.0 (80.0%) | 3.0 (20.0%) | 9.0 (13.2%) | |
| Skin involvement | 0.19 | ||||
| No | 0.0 (0.0%) | 3.0 (60.0%) | 8.0 (53.3%) | 21.0 (30.9%) | |
| Yes | 2.0 (100.0%) | 2.0 (40.0%) | 7.0 (46.7%) | 47.0 (69.1%) | |
| Nail involvement | 0.14 | ||||
| No | 2.0 (100.0%) | 5.0 (100.0%) | 9.0 (60.0%) | 57.0 (83.8%) | |
| Yes | 0.0 (0.0%) | 0.0 (0.0%) | 6.0 (40.0%) | 11.0 (16.2%) | |
| Mucosal involvement | 0.03 * | ||||
| No | 0.0 (0.0%) | 3.0 (60.0%) | 13.0 (86.7%) | 57.0 (83.8%) | |
| Yes | 2.0 (100.0%) | 2.0 (40.0%) | 2.0 (13.3%) | 11.0 (16.2%) | |
| Diffuse lesions | 0.13 | ||||
| No | 2.0 (100.0%) | 3.0 (60.0%) | 13.0 (86.7%) | 63.0 (92.6%) | |
| Yes | 0.0 (0.0%) | 2.0 (40.0%) | 2.0 (13.3%) | 5.0 (7.4%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; Raimondo, A.; Di Palo, M.P.; Martina, S.; Fordellone, M.; Rosa, D.; Amato, M.; Lembo, S. Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review. Life 2024, 14, 347. https://doi.org/10.3390/life14030347
Di Spirito F, Raimondo A, Di Palo MP, Martina S, Fordellone M, Rosa D, Amato M, Lembo S. Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review. Life. 2024; 14(3):347. https://doi.org/10.3390/life14030347
Chicago/Turabian StyleDi Spirito, Federica, Annunziata Raimondo, Maria Pia Di Palo, Stefano Martina, Mario Fordellone, Donato Rosa, Massimo Amato, and Serena Lembo. 2024. "Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review" Life 14, no. 3: 347. https://doi.org/10.3390/life14030347
APA StyleDi Spirito, F., Raimondo, A., Di Palo, M. P., Martina, S., Fordellone, M., Rosa, D., Amato, M., & Lembo, S. (2024). Oral Lesions and Oral Health-Related Quality of Life in Adult Patients with Psoriasis: A Retrospective Chart Review. Life, 14(3), 347. https://doi.org/10.3390/life14030347