1. Introduction
Lesions of the upper airways are not common, but their management is complex, especially when the digestive tract is involved. Restoring the continuity of the airway and digestive tract when necessary is not always possible without the need of complex reconstructions, especially when there is a severe tissue loss. The literature contains many examples of maxillofacial reconstructive surgery using sternocleidomastoid muscle flaps to reconstruct tissue loss in oral cavity or pharyngeal surgery, or even for the closure of pharyngocutaneous fistulas [
1]. In this paper, we aim to present our experience in using vascularized sternocleidomastoid flaps to restore the upper airway in complex situations involving severe tissue loss. Our technique involves the preparation and rotation of the sternal head of the SCM, preserving its vascularization, to cover the airway defect. The vascular anatomy of the SCM and the surgical technique will be thoroughly explained in the specific sections of this article.
2. Materials and Methods
We retrospectively collected data from five patients who were referred to us for complex airway lesions between 2011 and 2023. The medical history data of these patients are shown in
Table 1. The study was conducted in accordance with the Declaration of Helsinki.
The inclusion criteria for the study were (1) tissue loss of the trachea exceeding 50% of its length or the presence of a tracheoesophageal fistula extending over 30% of the tracheal length, (2) complete hospitalization and follow-up data. The exclusion criteria were (1) incomplete clinical or follow-up documentation, (2) patients with airway lesions treated with direct repair or other techniques without use of SCM flap and (3) patients who have undergone previous neck surgery.
Informed consent was obtained from all subjects involved in the study.
Of these five patients, two presented airway lesions only. The first had an extensive lesion of the anterior wall of the trachea due to trauma from a chainsaw accident. The defect extended over 50% of the total length of the trachea. The second patient had necrosis of the anterior wall of the trachea due to descending mediastinitis; in this case as well, the defect extended over 50% of the total length of the trachea. In both cases, the tissue loss was in the proximal half of the trachea.
The remaining three patients had a tracheoesophageal fistula measuring over 4.5 cm or more than one-third of the total length of the trachea. In two cases, the fistula was secondary to the creation of a tracheostomy; in the last patient, the fistula developed after an Ivor Lewis esophagectomy.
All patients underwent follow-up with fiberoptic bronchoscopy and CT scans of the neck and chest at 15 and 28 days, and at 3 and 6 months after the surgery.
Statistical analysis was conducted using descriptive methods, given the small sample size of patients.
3. Results
The sternocleidomastoid muscle, due to its anatomy, has the potential to be pedicled and transposed in various ways [
2]. In our technique, the criteria we considered necessary are (1) the muscle flap must be long enough to cover the defect without tension and (2) the muscle flap must maintain the best possible vascularization to prevent necrosis.
3.1. SCM Anatomy
The sternocleidomastoid muscle consists of two superficial heads, the clavicular and sternal heads, both of which insert into the mastoid process of the temporal bone. It also has a deep head extending from the inner third of the clavicle to the mastoid process. Its blood supply is divided into three main levels: (1) superior, originating from the occipital artery and its branches; (2) middle, supplied by the superior thyroid artery; and (3) inferior, fed by the suprascapular artery. In the first two cases, the blood supply comes directly from the external carotid artery, while in the third case, it comes from branches of the subclavian artery. This complex and interconnected vascularization makes the SCM an excellent muscle for being pedicled and used in reconstructive surgery.
3.2. Surgical Technique
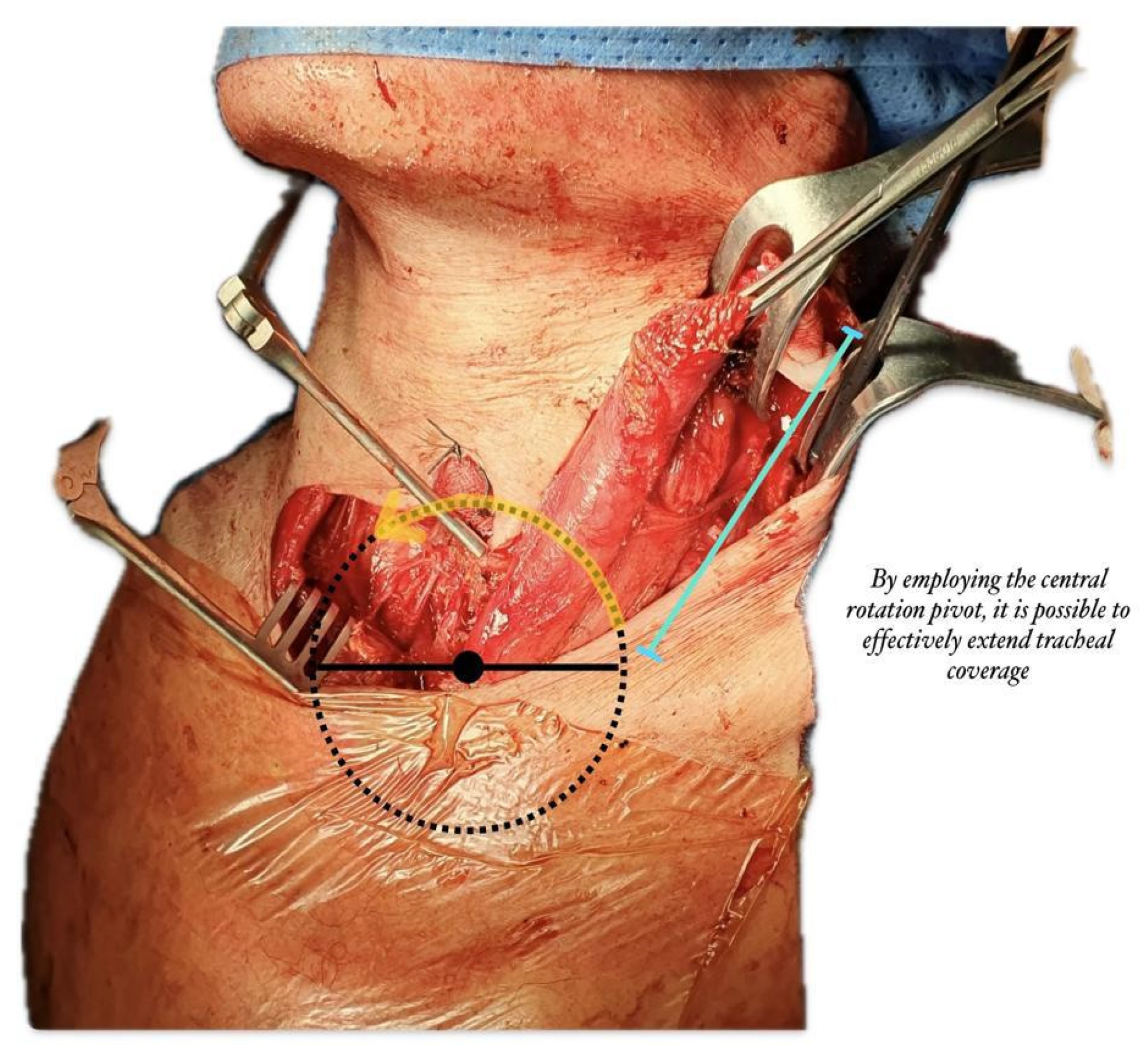
Our technique allows rotation on the sternal head of the sternocleidomastoid muscle with the lowest rotation radius, pedicled to the sternal origin and detached from the mastoid process and superior nuchal line, thus providing optimal vascularization from the superior thyroid artery or external carotid artery and accessory vasculature from the suprascapular artery (
Figure 1).
In case of direct repair, the SCM flap was used to replace the loss tissue loss, restoring the physiological continuity of the airway. Therefore, in the first two patients, the sternal head of the sternocleidomastoid muscle was pedicled, maintaining its vascularization, and rotated medially by approximately 45 degrees. Then, it was used to support the reconstruction of the cartilaginous part of the trachea.
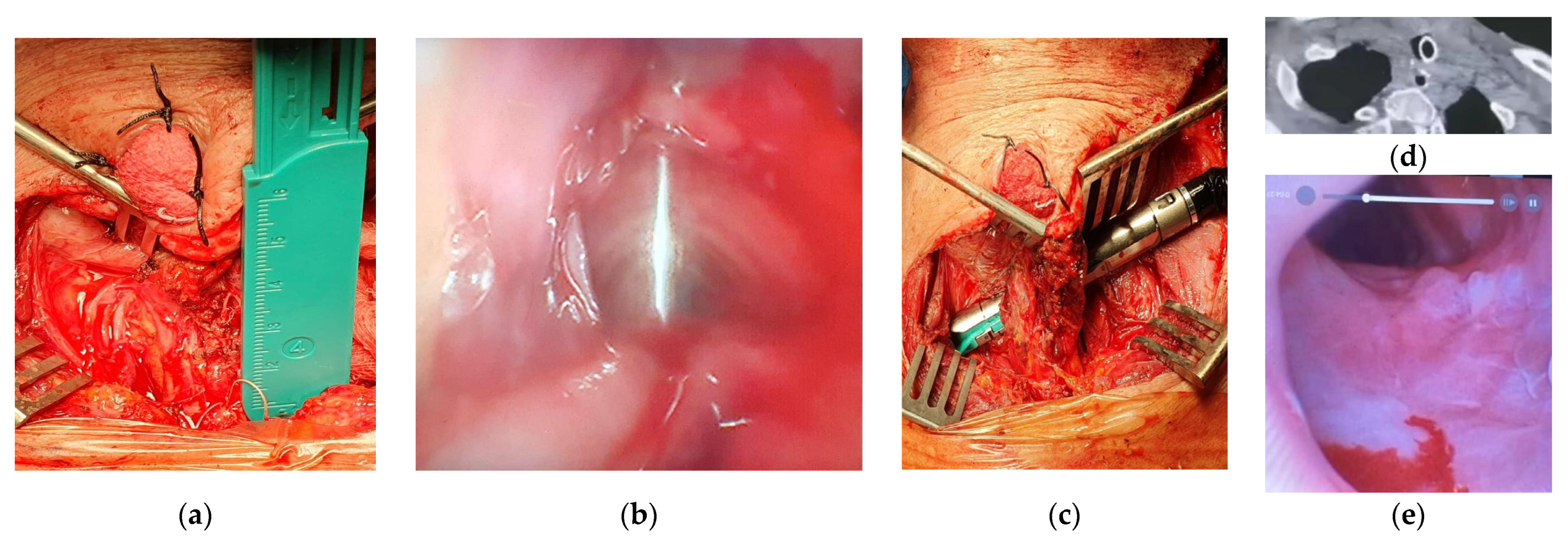
In one patient with a tracheoesophageal fistula clinically not suitable for repair by tracheal resection and anastomosis, the SCM flap was interposed between the two visceral sides of endomechanical sutures to protect them (
Figure 2). In one young patient affected by a TEF and proximal tracheal stenosis clinically suitable for repair by tracheal resection and anastomosis and direct double layer suture of the esophageal defect, we used the SCM flap to cover a left-side lateral defect of the cartilaginous wall of the airway that could not be closed by a tracheo-tracheal anastomosis. Additionally, in one of these patients, the SCM flap allowed the reconstruction of the missing membranous part over a long segment.
All patients enrolled in the study completed the follow-up with CT scans and fibrobronchoscopy at 15 and 28 days after surgery, and at 3 and 9 months. No patient experienced complications of the airway or the upper digestive tract, either in the short term or the long term. The general information regarding surgery and follow-up is presented in
Table 1; a serial number was assigned to each case.
4. Discussion
The use of SCM flaps in reconstructive surgery of the neck and head has been known for over half a century It was first described in 1955 by Owens [
3] for the reconstruction of the oral cavity, and until the 1980s, this type of flap was used exclusively for maxillofacial reconstructive surgery. The anatomy and unique vascularization of the SCM make it a particularly versatile muscle, capable of reaching the structures of the face, oral cavity and neck. After Owens, other authors reported various facial reconstructive techniques using the sternal or clavicular head of the SCM, such as Schottstaedt [
4] in 1955 and Dingman [
5] in 1969. The use of pedicled SCM flaps is also described in patients with various types of neck tumors, where local treatments (surgical and/or radiotherapeutic) make the dissection and preservation of proper flap vascularization difficult. For this reason, studies report total and partial flap loss rates ranging from 10 to 30% [
6]. Subsequently, cases of high esophageal fistula repairs using an SCM flap were described. In 2014, Nakajimaa et al. [
7] presented their experience with the dehiscence of cervical esophagogastric sutures following McKeown procedures; to cover the fistula, they used a vascularized SCM flap with excellent results [
8].
Reconstructive airway surgery is always tricky and challenging [
9]. Tissue loss might not be restorable by direct suture, or the patient could not be clinically suitable for tracheal resection and reconstruction [
10].
The sternocleidomastoid muscle has two main vascular supplies as explained in the text: a dominant one and a secondary one from the superior thyroid artery, which primarily supplies the sternal head of the SCM. In 2021, Srivastava et al. studied and demonstrated that preserving the vascularization from the superior thyroid artery, even in the absence of blood supply from the occipital artery branches, allowed the maintenance of an acceptable flap viability [
11].
The surgical technique we present involves preserving the vascularization from the superior thyroid artery during the dissection of the sternal head of the SCM. Additionally, the rotation of the flap prepared in this manner has a smaller radius, creating less tension. This allows for the use of this robust and well-vascularized flap in airway reconstructions when there is significant tissue loss.
In the case of tracheoesophageal fistulas, the presence of the muscle flap, in addition to the previously described advantages, protects the visceral sutures by being interposed between them [
12]. This results in stability of the airway and the replacement of long segment of membranous part. In their study, Alexandre Pl et al. described six patients in whom they used the infrahyoid muscle to close the tracheoesophageal fistula after laryngectomy; in their experience, they proposed this technique as a valid alternative to the SCM flap. However, this can only be applied if the infrahyoid muscle has been previously preserved and in the presence of small fistulas [
13]. The technique we propose by interposition of the SCM flap allows us to address even extremely complex situations with significant tissue loss.
Lurin IA et al. presented the use of an SCM flap in a patient with a laryngopharyngeal injury from a firearm in the context of the war in Ukraine. After removing the metal fragments, the pharynx was repaired with direct suturing, while the destroyed thyroid cartilage was replaced with a SCM flap [
14].
In conclusion, based on our experience, the use of a muscle flap composed by the sternal head of the SCM appears to be an excellent option in situations where restoring the integrity of the airway is complex or impossible due to severe tissue loss. This technique is highly effective, ensuring good vascularization of the flap, providing good airway stability and protecting the visceral sutures when intervention on the digestive tract is necessary.
Author Contributions
Conception and design: S.M., D.G. and M.O.J. Administrative support: S.M. and M.O.J. Provision of study materials or patients: S.M., D.G. and M.O.J. Collection and assembly of data: S.M. and M.O.J. Data analysis and interpretation: S.M., D.G. and M.O.J. Manuscript writing: S.M. Final approval of manuscript: S.M., D.G. and M.O.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study and written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Dataset available on request from the authors. The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fatani, B.; Alabood, A.A.; Alkhayatt, N.M.; Alzahrani, H.H.; Al-Safadi, A. Facial reconstruction using sternocleidomastoid flap: A review of literature. Cereus 2023, 15, e34575. [Google Scholar] [CrossRef] [PubMed]
- Yugueros, P.; Woods, J.E. The sternocleidomastoid myocutaneous flap: A reappraisal. Br. J. Plast. Surg. 1996, 49, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Owens, N. Compound neck pedicle designed for repair of massive facial defects. Plast. Reconstr. Surg. 1955, 15, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Schottstaedt, E.K.; Larsen, L.G.; Bost, T.C. Complete muscle transposition. J. Bone Joint Slug. 1955, 37, 897. [Google Scholar] [CrossRef]
- Dingman, R.O.; Grabb, W.C.; Oneal, R.M.; Ponitz, R.J. Sternocleidomastoid muscle transplant to the masseter area. Plast. Reconstr. Surg. 1969, 43, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.F.; Farrar, E.M.; Roberts, D.J.H.; Moor, J.W. Revisiting the sternocleidomastoid flap as a reconstructive option in head and neck surgery. J. Laryngol. Otol. 2019, 133, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Satomura, H.; Takahashi, M.; Muroi, H.; Kuwano, H.; Kato, H. Effectiveness of sternocleidomastoid Flap repair for cervical anastomotic leakage after esophageal reconstruction. Dig. Surg. 2014, 31, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hong, Z.; Lin, Z.; Chen, M.; Yang, X.; Lin, Y.; Lin, W.; Zhu, J.; Xie, S.; Kang, M.; et al. Efficacy of sternocleidomastoid muscle flap in reducing anastomotic mediastinal/pleural cavity leak. Esophagus 2023, 20, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, M.; Mathisen, D.J. Carinal resection. Thorac. Surg. Clin. 2014, 24, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.; Gullane, P.J. The sternocleidomastoid muscle flap. Head Neck Surg. 1980, 2, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kumar, T.; Pandey, S.K.; Shukla, R.C.; Pai, E.; Pandey, M. Sternocleidomastoid flap for pedicled reconstruction in head & neck surgery-revisiting the anatomy and technique. World J. Surg. Oncol. 2021, 19, 349. [Google Scholar] [PubMed]
- Mastromarino, M.G.; Cardillo, G.; Jaus, M.O. Areverse thymic fat pad flap to cover the anastomosis of an extended tracheal resection following induction chemotherapy: A challenging case report. Surgeries 2022, 3, 271–276. [Google Scholar] [CrossRef]
- Alexandre, P.L.; Silveira, H.; Marques, P.; Moura, C.P. Sternohyoid or sternocleidomastoid muscle flap for tracheoesophageal puncture closure in irradiated patients: A care case series. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2024, 141, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lurin, I.A.; Makarov, V.V.; Khoroshun, E.M.; Nehoduiko, V.V.; Shypilov, S.A.; Smolianyk, K.M. Features of the use of ladder myoplasty of gunshot wound to the laryngopharynx: Case report. Int. J. Surg. Case Rep. 2023, 111, 108875. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).