Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study
Abstract
1. Introduction
2. Subjects and Methods
- -
- Radial strain (RS), representing thickening/thinning of the LV;
- -
- Circumferential strain (CS), representing narrowing/widening of the LV;
- -
- Longitudinal strain (LS), representing shortening/lengthening of the LV.
3. Results
4. Discussion
- -
- The image quality of 3DSTE is worse than that of 2D echocardiography, which may have affected our results.
- -
- Although the rotational mechanics of the LV can be determined using the same LV cast, this was not considered the purpose of the present investigation. However, results on this from the MAGYAR-Healthy Study have already been published in detail. Moreover, the subject population used for this manuscript is partly the same [24].
- -
- Although detailed analysis of other heart cavities can be performed in 3DSTE analysis, this study did not consider this to be its goal.
- -
- As determining all LV strains by 3DSTE has been validated, this paper did not aim to do so.
- -
- The body mass index of some subjects was above 25 kg/m2, meaning they were overweighted, which may have partly influenced our results. Although all the parameters tested in the present study were within the normal range, being overweight can have many effects, e.g., it can raise pulmonary artery pressure.
- -
- Diastolic data on the LV or 3DSTE-derived parameters for the RV would enable an even more detailed analysis, which could be the topic of future investigations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, Part I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92 (Suppl. S1), i2–i13. [Google Scholar] [CrossRef]
- Ghio, S.; Recusani, F.; Klersy, C.; Sebastiani, R.; Laudisa, M.L.; Campana, C.; Gavazzi, A.; Tavazzi, L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am. J. Cardiol. 2000, 85, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Pepi, M.; Galli, C.A.; Maltagliati, A.; Celeste, F.; Muratori, M.; Rezvanieh, S.; Veglia, F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int. J. Cardiol. 2007, 115, 86–89. [Google Scholar] [CrossRef]
- Sato, T.; Tsujino, I.; Ohira, H.; Oyama-Manabe, N.; Yamada, A.; Ito, Y.M.; Goto, C.; Watanabe, T.; Sakaue, S.; Nishimura, M. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J. Am. Soc. Echocardiogr. 2012, 25, 280–286. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Atsumi, A.; Kawamura, R.; Aonuma, K. Three-dimensional speckle tracking echocardiography. Circ. J. 2014, 78, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.A.; Brindise, M.C.; Kutty, S.; Vlachos, P.P. A method for direct estimation of left ventricular global longitudinal strain rate from echocardiograms. Sci. Rep. 2022, 12, 4008. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Edvardsen, T.; Klaeboe, L.G. Imaging and heart failure: Myocardial strain. Curr. Opin. Cardiol. 2019, 34, 490–494. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Seo, Y.; Takeuchi, M. A review of current trends in three-dimensional analysis of left ventricular myocardial strain. Cardiovasc. Ultrasound 2020, 18, 23. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Brouwer, W.P.; Aly, M.F.A.; Russel, I.K.; de Roest, G.J.; Beek, A.M.; van Rossum, A.C.; Kamp, O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 834–839. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 159–168. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Ambrus, N.; Lengyel, C. Normal reference values of left ventricular strain parameters in healthy adults: Real-life experience from the single-center three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 368–377. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Pandian, N.G.; Thomas, J.D.; Perez de Isla, L.; Kamp, O.; Zuber, M.; Nihoyannopoulos, P.; Forster, T.; Nesser, H.J.; Geibel, A.; et al. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: Results from a multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Complexity of left ventricular strains in response to elevated volumes in healthy adults—Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Int. J. Cardiol. Heart Vasc. 2023, 47, 101236. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Right Ventricular Longitudinal Shortening is not Associated with Left Ventricular Rotational Mechanics in Healthy Adults—Insights from the Three-dimensional Speckle-tracking Echocardiographic MAGYAR-Healthy Study. Rev. Cardiovasc. Med. 2024, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- Putthapiban, P.; Amini, M.R.; Abudayyeh, I. Anatomy of the Tricuspid Valve and Pathophysiology of Tricuspid Regurgitation. Interv. Cardiol. Clin. 2022, 11, 1–9. [Google Scholar] [CrossRef]

| Data | Measures |
|---|---|
| Clinical data | |
| n | 79 |
| Mean age (years) | 28.1 ± 6.3 |
| Males (%) | 33 (42) |
| Systolic blood pressure (mmHg) | 121.5 ± 3.6 |
| Diastolic blood pressure (mmHg) | 77.9 ± 2.7 |
| Heart rate (1/s) | 70.2 ± 2.0 |
| Weight (kg) | 73.1 ± 14.8 |
| Height (cm) | 168.6 ± 10.2 |
| Body surface area (m2) | 1.85 ± 0.35 |
| Body mass index (kg/m2) | 25.6 ± 1.8 |
| Estimated pulmonary artery pressure (mmHg) | 20.3 ± 2.8 |
| Two-dimensional echocardiographic data | |
| LA diameter (mm) | 36.9 ± 3.3 |
| LV end-diastolic diameter (mm) | 48.2 ± 3.6 |
| LV end-systolic diameter (mm) | 32.5 ± 3.4 |
| LV end-diastolic volume (mL) | 106.1 ± 23.8 |
| LV end-systolic volume (mL) | 38.5 ± 9.4 |
| Interventricular septum (mm) | 9.1 ± 1.2 |
| LV posterior wall (mm) | 9.2 ± 1.3 |
| LV ejection fraction (%) | 64.4 ± 4.2 |
| Early diastolic mitral inflow velocity—E (cm/s) | 82.9 ± 15.1 |
| Late diastolic mitral inflow velocity—A (cm/s) | 55.3 ± 10.4 |
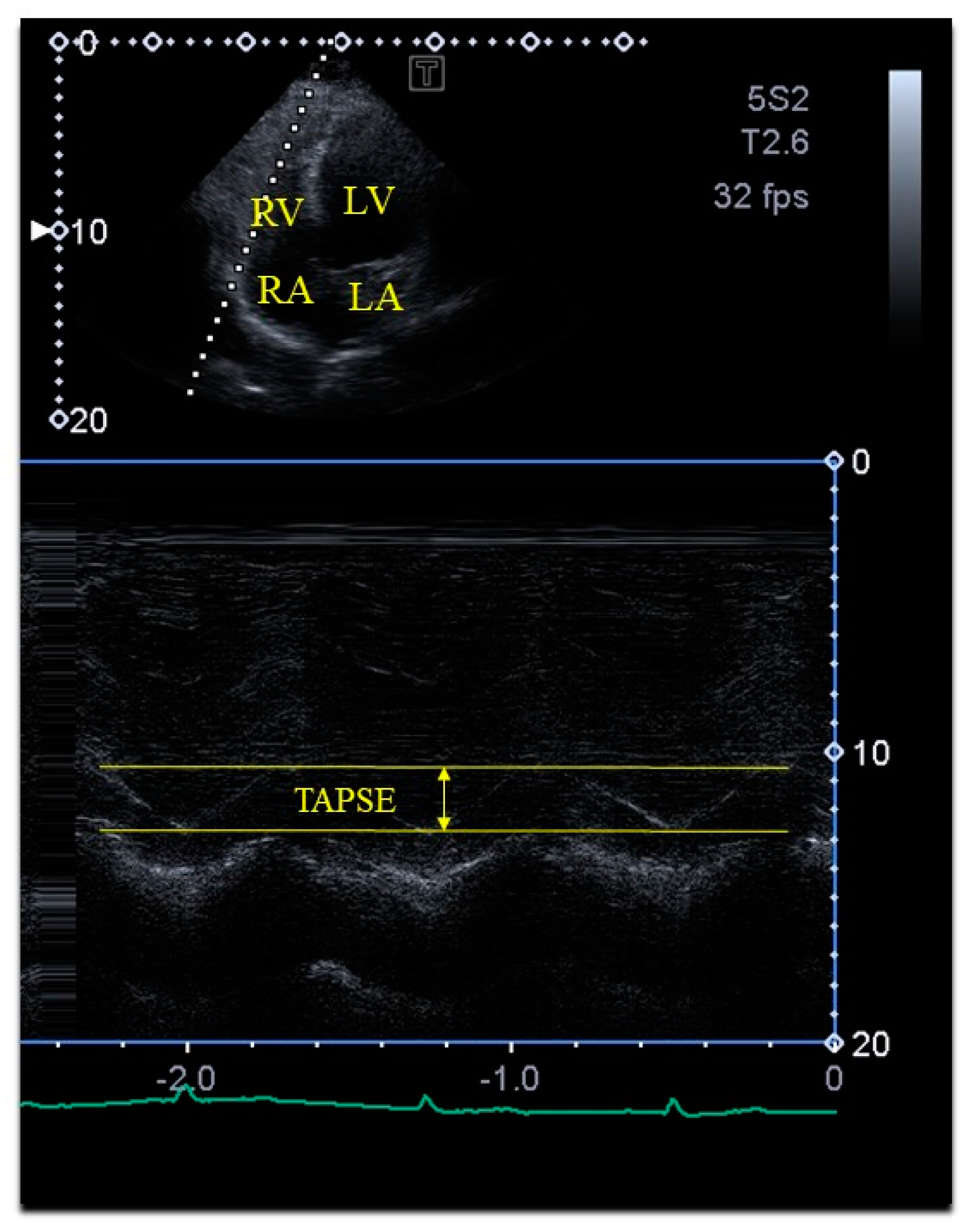
| Tricuspid annular plane systolic excursion (mm) | 23.7 ± 2.9 |
| All Subjects (n = 79) | TAPSE ≤ 21 mm (n = 20) | 21 mm < TAPSE < 27 mm (n = 44) | 27 mm ≤ TAPSE (n = 15) | |
|---|---|---|---|---|
| LV-EDV (mL) | 85.7 ± 20.7 | 82.3 ± 23.8 | 82.8 ± 17.4 | 98.9 ± 19.8 *† |
| LV-ESV (mL) | 36.2 ± 10.2 | 34.4 ± 12.2 | 35.0 ± 8.8 | 42.1 ± 8.5 *† |
| LV-EF (%) | 57.9 ± 5.8 | 58.8 ± 6.6 | 57.8 ± 5.4 | 57.1 ± 5.5 |
| LV mass (g) | 164.1 ± 31.6 | 160.8 ± 28.1 | 164.3 ± 31.4 | 167.8 ± 35.7 |
| global LV-RS (%) | 25.2 ± 10.3 | 29.6 ± 13.0 | 22.7 ± 8.5 * | 26.8 ± 8.6 |
| basal LV-RS (%) | 30.7 ± 13.1 | 35.3 ± 14.9 | 27.5 ± 12.1 * | 34.2 ± 10.4 † |
| global LV-CS (%) | −27.6 ± 5.2 | −27.8 ± 5.6 | −27.7 ± 5.2 | −27.1 ± 4.6 |
| basal LV-CS (%) | −25.1 ± 4.7 | −26.3 ± 5.3 | −24.7 ± 4.5 | −25.0 ± 4.4 |
| global LV-LS (%) | −16.1 ± 2.5 | −16.3 ± 2.9 | −15.5 ± 2.4 | −17.5 ± 1.7 † |
| basal LV-LS (%) | −20.7 ± 4.5 | −21.8 ± 4.9 | −20.4 ± 4.5 | −19.9 ± 3.9 |
| TAPSE (mm) | 23.7 ± 2.9 | 20.3 ± 0.8 | 23.7 ± 1.3 * | 28.5 ± 1.2 *† |
| Global LV-RS ≤ 14.9% (n = 11) | 14.9% < Global LV-RS < 35.5% (n = 56) | 35.5% ≤ Global LV-RS (n = 12) | Global LV-CS ≤ −22.4% (n = 7) | −22.4% < Global LV-CS < 32.8% (n = 62) | −32.8% ≤ Global LV-CS (n = 10) | Global LV-LS ≤ −13.6% (n = 12) | −13.6% < Global LV-LS < −18.6% (n = 53) | −18.6% ≤ Global LV-LS (n = 14) | |
|---|---|---|---|---|---|---|---|---|---|
| LV-EDV (mL) | 71.2 ± 10.8 | 87.8 ± 20.2 * | 89.4 ± 24.1 * | 90.9 ± 18.5 | 85.1 ± 20.9 | 84.1 ± 20.4 | 90.9 ± 25.4 | 85.3 ± 1.95 | 82.9 ± 19.7 |
| LV-ESV (mL) | 33.2 ± 5.4 | 37.1 ± 9.9 | 34.9 ± 13.5 | 44.5 ± 9.0 | 36.6 ± 9.5 † | 27.6 ± 8.8 †/†† | 41.3 ± 13.6 | 35.8 ± 8.6 | 33.4 ± 10.5 |
| LV-EF (%) | 53.2 ± 4.0 | 57.9 ± 4.9 * | 62.1 ± 7.3 */** | 50.7 ± 4.0 | 57.0 ± 3.6 † | 68.1 ± 4.2 †/†† | 54.7 ± 5.4 | 57.8 ± 5.4 | 60.9 ± 5.6 ‡/‡‡ |
| LV mass (g) | 147.1 ± 30.7 | 167.1 ± 31.1 | 165.7 ± 29.3 | 169.0 ± 33.9 | 163.3 ± 31.6 | 165.0 ± 28.9 | 174.6 ± 29.3 | 165.2 ± 33.2 | 150.9 ± 20.9 ‡ |
| global LV-RS (%) | 10.6 ± 3.2 | 24.3 ± 5.0 * | 43.1 ± 7.3 */** | 18.7 ± 9.2 | 24.3 ± 8.5 | 34.2 ± 11.9 †/†† | 28.2 ± 13.0 | 24.4 ± 9.7 | 25.8 ± 9.6 |
| global LV-CS (%) | −24.3 ± 4.9 | −27.5 ± 4.5 * | −31.1 ± 6.2 */** | −19.0 ± 2.5 | −27.0 ± 3.0 † | −26.5 ± 3.3 †/†† | −26.1 ± 5.0 | −27.3 ± 4.8 | −29.8 ± 5.8 |
| global LV-LS (%) | −15.5 ± 2.3 | −16.2 ± 2.4 | −15.9 ± 3.3 | −15.7 ± 2.8 | −16.0 ± 2.5 | −17.2 ± 2.2 | −12.5 ± 1.1 | −15.9 ± 1.4 ‡ | −20.0 ± 1.2 ‡/‡‡ |
| TAPSE (mm) | 23.8 ± 2.4 | 23.9 ± 2.9 | 22.2 ± 3.2 ** | 24.0 ± 2.0 | 23.8 ± 3.0 | 23.2 ± 3.0 | 22.8 ± 1.8 | 24.1 ± 2.9 | 22.8 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Life 2024, 14, 1422. https://doi.org/10.3390/life14111422
Nemes A, Kormányos Á, Ambrus N, Lengyel C. Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Life. 2024; 14(11):1422. https://doi.org/10.3390/life14111422
Chicago/Turabian StyleNemes, Attila, Árpád Kormányos, Nóra Ambrus, and Csaba Lengyel. 2024. "Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study" Life 14, no. 11: 1422. https://doi.org/10.3390/life14111422
APA StyleNemes, A., Kormányos, Á., Ambrus, N., & Lengyel, C. (2024). Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Life, 14(11), 1422. https://doi.org/10.3390/life14111422






