Infantile Nystagmus Syndrome—Associated Inherited Retinal Diseases: Perspectives from Gene Therapy Clinical Trials
Abstract
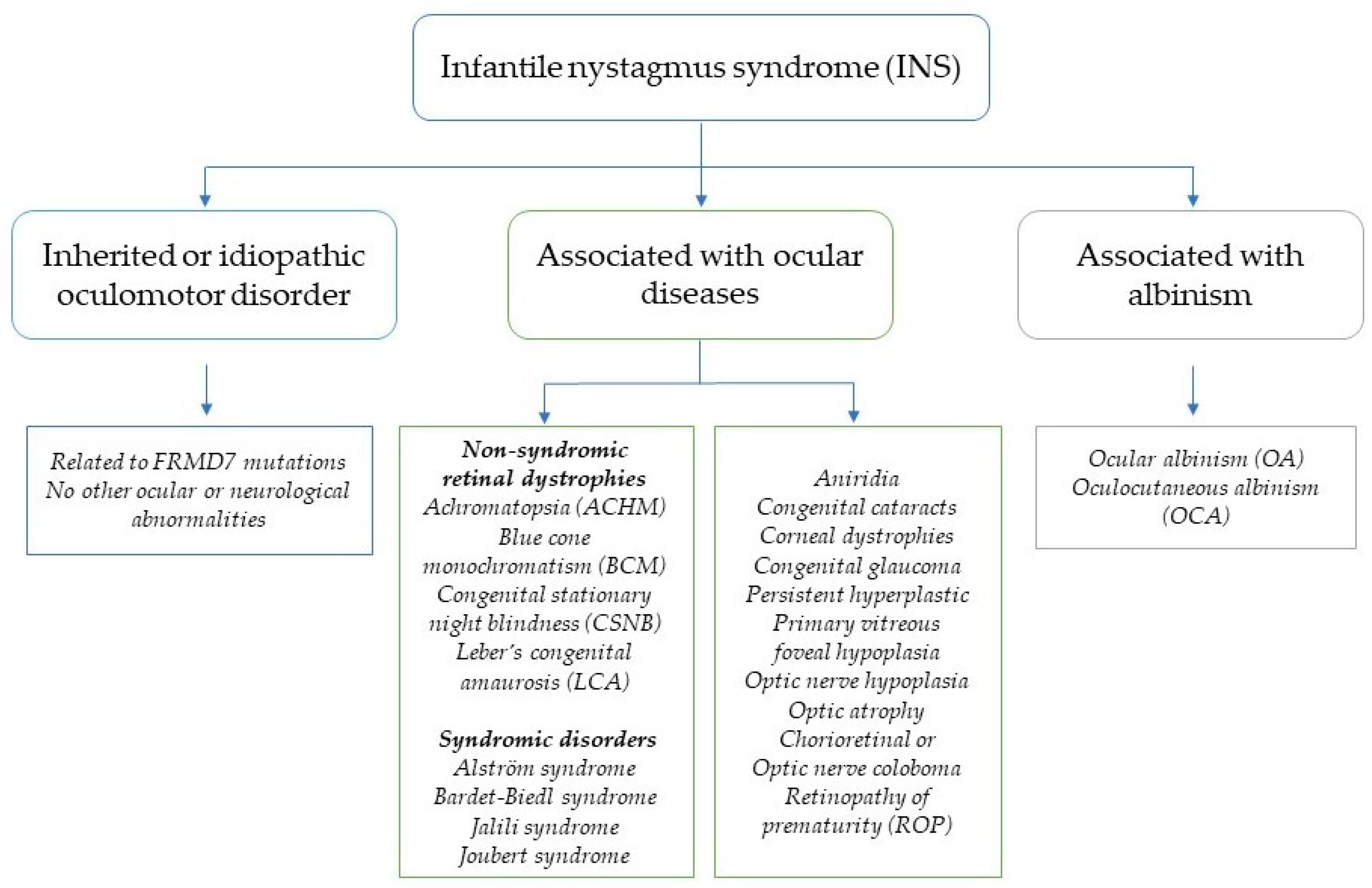
1. Introduction
2. Phenotypic–Genotypic Complexity of IRDs in Children with INS
2.1. The Phenotypic–Genotypic Spectrum of Leber Congenital Amaurosis (LCA)
2.2. Achromatopsia (ACHM)
2.3. Congenital Stationary Night Blindness (CSNB)
2.4. Retinitis Pigmentosa (RP)
2.5. X-Linked Retinoschisis (XL-RS)
3. AAV-Based Gene Therapy for INS-Associated IRDs
3.1. AAV-Based Gene Augmentation Therapy for INS-Associated IRDs in Clinical Trials
3.1.1. AAV-Based Gene Augmentation Therapy for Achromatopsia
3.1.2. AAV-Based Gene Augmentation Therapy for X-Linked Retinoschisis
3.1.3. AAV-Based Gene Augmentation Therapy for Leber Congenital Amaurosis
3.1.4. AAV-Based Gene Augmentation Therapy for X-Linked Retinitis Pigmentosa
3.1.5. AAV-Based Gene Therapy for MERTK-, RLBP1-, and PDE6B-Associated RP
3.2. AAV-Based Gene Silencing for INS-Associated IRDs
3.3. AAV-Based Gene Editing for INS-Associated IRDs
4. Challenges and Prospectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, M.D.; Wong, A. Infantile nystagmus syndrome: Clinical characteristics, current theories of pathogenesis, diagnosis, and management. Can. J. Ophthalmol. 2015, 50, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sarvananthan, N.; Surendran, M.; Roberts, E.O.; Jain, S.; Thomas, S.; Shah, N.; Proudlock, F.A.; Thompson, J.R.; McLean, R.J.; Degg, C.; et al. The prevalence of nystagmus: The Leicestershire nystagmus survey. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.L.; Diehl, N.N.; Mohney, B.G. Incidence and Types of Pediatric Nystagmus. Am. J. Ophthalmol. 2017, 182, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.; McLean, R.J.; Gottlob, I. Nystagmus in childhood. Pediatr. Neonatol. 2014, 55, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, M.; Floyd, M.; Kehoe, T.; Pfeifer, W.; Drack, A.V. The clinical evaluation of infantile nystagmus: What to do first and why. Ophthalmic Genet. 2017, 38, 22–33. [Google Scholar] [CrossRef]
- Weiss, A.H.; Biersdorf, W.R. Visual sensory disorders in congenital nystagmus. Ophthalmology 1989, 96, 517–523. [Google Scholar] [CrossRef]
- Rim, J.H.; Lee, S.T.; Gee, H.Y.; Lee, B.J.; Choi, J.R.; Park, H.W.; Han, S.H.; Han, J. Accuracy of Next-Generation Sequencing for Molecular Diagnosis in Patients With Infantile Nystagmus Syndrome. JAMA Ophthalmol. 2017, 135, 1376–1385. [Google Scholar] [CrossRef]
- Gottlob, I. Nystagmus. Curr. Opin. Ophthalmol. 2000, 11, 330–335. [Google Scholar] [CrossRef]
- Cavuoto, K.M.; Binenbaum, G.; Chang, M.Y.; Heidary, G.; Morrison, D.G.; Trivedi, R.H.; Kim, S.J.; Pineles, S.L. Genetic testing for infantile nystagmus syndrome with or without associated findings. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2023, 27, 259–264. [Google Scholar] [CrossRef]
- Brodsky, M.C.; Dell’Osso, L.F. A unifying neurologic mechanism for infantile nystagmus. JAMA Ophthalmol. 2014, 132, 761–768. [Google Scholar] [CrossRef]
- Hertle, R.W. A Story of Discovery and Change: What We Learned from Studying Nystagmus in Infancy and Childhood. J. Binocul. Vis. Ocul. Motil. 2022, 72, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; Pierce, E.A.; Laster, A.M.; Daiger, S.P.; Birch, D.G.; Ash, J.D.; Iannaccone, A.; Flannery, J.G.; Sahel, J.A.; Zack, D.J.; et al. Inherited Retinal Degenerations: Current Landscape and Knowledge Gaps. Transl. Vis. Sci. Technol. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Kloeckener-Gruissem, B.; Neidhardt, J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010, 29, 335–375. [Google Scholar] [CrossRef] [PubMed]
- Self, J.E.; Lee, H. Novel therapeutics in nystagmus: What has the genetics taught us so far? Ther. Adv. Rare Dis. 2021, 2, 2633004021998714. [Google Scholar] [CrossRef]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha, A., Jr.; Woo, S.J.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef]
- Moon, D.; Park, H.W.; Surl, D.; Won, D.; Lee, S.T.; Shin, S.; Choi, J.R.; Han, J. Precision Medicine through Next-Generation Sequencing in Inherited Eye Diseases in a Korean Cohort. Genes 2021, 13, 27. [Google Scholar] [CrossRef]
- He, X.; Fu, Y.; Ma, L.; Yao, Y.; Ge, S.; Yang, Z.; Fan, X. AAV for Gene Therapy in Ocular Diseases: Progress and Prospects. Research 2023, 6, 0291. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Akhavanrezayat, A.; Karaca, I.; Yavari, N.; Lajevardi, S.; Hwang, J.; Regenold, J.; Matsumiya, W.; Pham, B.; Zaidi, M.; et al. Ocular Gene Therapy: A Literature Review with Special Focus on Immune and Inflammatory Responses. Clin. Ophthalmol. 2022, 16, 1753–1771. [Google Scholar] [CrossRef]
- Ail, D.; Malki, H.; Zin, E.A.; Dalkara, D. Adeno-Associated Virus (AAV)—Based Gene Therapies for Retinal Diseases: Where are We? Appl. Clin. Genet. 2023, 16, 111–130. [Google Scholar] [CrossRef]
- Carss, K.J.; Arno, G.; Erwood, M.; Stephens, J.; Sanchis-Juan, A.; Hull, S.; Megy, K.; Grozeva, D.; Dewhurst, E.; Malka, S.; et al. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am. J. Hum. Genet. 2017, 100, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Allikmets, R. Leber congenital amaurosis: A genetic paradigm. Ophthalmic Genet. 2004, 25, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Loewenstein, J. Leber congenital amaurosis: Disease, genetics and therapy. Semin. Ophthalmol. 2008, 23, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Koenekoop, R.K. An overview of Leber congenital amaurosis: A model to understand human retinal development. Surv. Ophthalmol. 2004, 49, 379–398. [Google Scholar] [CrossRef]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Abu-Amero, K.K. Leber congenital amaurosis: Current genetic basis, scope for genetic testing and personalized medicine. Exp. Eye Res. 2019, 189, 107834. [Google Scholar] [CrossRef]
- Boye, S.E. Leber congenital amaurosis caused by mutations in GUCY2D. Cold Spring Harb. Perspect. Med. 2014, 5, a017350. [Google Scholar] [CrossRef][Green Version]
- Sharon, D.; Wimberg, H.; Kinarty, Y.; Koch, K.W. Genotype-functional-phenotype correlations in photoreceptor guanylate cyclase (GC-E) encoded by GUCY2D. Prog. Retin. Eye Res. 2018, 63, 69–91. [Google Scholar] [CrossRef]
- Bouzia, Z.; Georgiou, M.; Hull, S.; Robson, A.G.; Fujinami, K.; Rotsos, T.; Pontikos, N.; Arno, G.; Webster, A.R.; Hardcastle, A.J.; et al. GUCY2D-Associated Leber Congenital Amaurosis: A Retrospective Natural History Study in Preparation for Trials of Novel Therapies. Am. J. Ophthalmol. 2020, 210, 59–70. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Koenekoop, R.K.; Yzer, S.; Lopez, I.; Arends, M.L.; Voesenek, K.E.; Zonneveld, M.N.; Strom, T.M.; Meitinger, T.; Brunner, H.G.; et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 2006, 79, 556–561. [Google Scholar] [CrossRef]
- Leroy, B.P.; Birch, D.G.; Duncan, J.L.; Lam, B.L.; Koenekoop, R.K.; Porto, F.B.O.; Russell, S.R.; Girach, A. Leber congenital amaurosis due to cep290 mutations—Severe vision impairment with a high unmet medical need: A Review. Retina 2021, 41, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Sheck, L.; Davies, W.I.L.; Moradi, P.; Robson, A.G.; Kumaran, N.; Liasis, A.C.; Webster, A.R.; Moore, A.T.; Michaelides, M. Leber Congenital Amaurosis Associated with Mutations in CEP290, Clinical Phenotype, and Natural History in Preparation for Trials of Novel Therapies. Ophthalmology 2018, 125, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Daich Varela, M.; Georgiou, M.; Alswaiti, Y.; Kabbani, J.; Fujinami, K.; Fujinami-Yokokawa, Y.; Khoda, S.; Mahroo, O.A.; Robson, A.G.; Webster, A.R.; et al. CRB1-Associated Retinal Dystrophies: Genetics, Clinical Characteristics, and Natural History. Am. J. Ophthalmol. 2023, 246, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, M.; Pierce, E.A.; Cox, G.F.; Fulton, A.B. CRB1, one gene, many phenotypes. Semin. Ophthalmol. 2013, 28, 397–405. [Google Scholar] [CrossRef]
- Fahim, A.T.; Bouzia, Z.; Branham, K.H.; Kumaran, N.; Vargas, M.E.; Feathers, K.L.; Perera, N.D.; Young, K.; Khan, N.W.; Heckenlively, J.R.; et al. Detailed clinical characterisation, unique features and natural history of autosomal recessive RDH12-associated retinal degeneration. Br. J. Ophthalmol. 2019, 103, 1789–1796. [Google Scholar] [CrossRef]
- Ba-Abbad, R.; Arno, G.; Robson, A.G.; Bouras, K.; Georgiou, M.; Wright, G.; Webster, A.R.; Michaelides, M. Macula-predominant retinopathy associated with biallelic variants in RDH12. Ophthalmic Genet. 2020, 41, 612–615. [Google Scholar] [CrossRef]
- Falk, M.J.; Zhang, Q.; Nakamaru-Ogiso, E.; Kannabiran, C.; Fonseca-Kelly, Z.; Chakarova, C.; Audo, I.; Mackay, D.S.; Zeitz, C.; Borman, A.D.; et al. NMNAT1 mutations cause Leber congenital amaurosis. Nat. Genet. 2012, 44, 1040–1045. [Google Scholar] [CrossRef]
- Koenekoop, R.K.; Wang, H.; Majewski, J.; Wang, X.; Lopez, I.; Ren, H.; Chen, Y.; Li, Y.; Fishman, G.A.; Genead, M.; et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat. Genet. 2012, 44, 1035–1039. [Google Scholar] [CrossRef]
- Dharmaraj, S.R.; Silva, E.R.; Pina, A.L.; Li, Y.Y.; Yang, J.M.; Carter, C.R.; Loyer, M.K.; El-Hilali, H.K.; Traboulsi, E.K.; Sundin, O.K.; et al. Mutational analysis and clinical correlation in Leber congenital amaurosis. Ophthalmic Genet. 2000, 21, 135–150. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Carroll, J.; Hardcastle, A.J.; Michaelides, M. The cone dysfunction syndromes. Br. J. Ophthalmol. 2016, 100, 115–121. [Google Scholar] [CrossRef]
- Hirji, N.; Aboshiha, J.; Georgiou, M.; Bainbridge, J.; Michaelides, M. Achromatopsia: Clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Thiadens, A.A.; Slingerland, N.W.; Roosing, S.; van Schooneveld, M.J.; van Lith-Verhoeven, J.J.; van Moll-Ramirez, N.; van den Born, L.I.; Hoyng, C.B.; Cremers, F.P.; Klaver, C.C. Genetic etiology and clinical consequences of complete and incomplete achromatopsia. Ophthalmology 2009, 116, 1984–1989.e1. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Wilde, C.; Aboshiha, J.; Cowing, J.; Han, C.; Langlo, C.S.; Chana, R.; Davidson, A.E.; Sergouniotis, P.I.; Bainbridge, J.W.; et al. Retinal structure and function in achromatopsia: Implications for gene therapy. Ophthalmology 2014, 121, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Aboshiha, J.; Dubis, A.M.; Cowing, J.; Fahy, R.T.; Sundaram, V.; Bainbridge, J.W.; Ali, R.R.; Dubra, A.; Nardini, M.; Webster, A.R.; et al. A prospective longitudinal study of retinal structure and function in achromatopsia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5733–5743. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Zobor, D.; Chiang, W.C.; Weisschuh, N.; Staller, J.; Gonzalez Menendez, I.; Chang, S.; Beck, S.C.; Garcia Garrido, M.; Sothilingam, V.; et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 2015, 47, 757–765. [Google Scholar] [CrossRef]
- Kohl, S.; Varsanyi, B.; Antunes, G.A.; Baumann, B.; Hoyng, C.B.; Jagle, H.; Rosenberg, T.; Kellner, U.; Lorenz, B.; Salati, R.; et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet. 2005, 13, 302–308. [Google Scholar] [CrossRef]
- Remmer, M.H.; Rastogi, N.; Ranka, M.P.; Ceisler, E.J. Achromatopsia: A review. Curr. Opin. Ophthalmol. 2015, 26, 333–340. [Google Scholar] [CrossRef]
- Burkard, M.; Kohl, S.; Kratzig, T.; Tanimoto, N.; Brennenstuhl, C.; Bausch, A.E.; Junger, K.; Reuter, P.; Sothilingam, V.; Beck, S.C.; et al. Accessory heterozygous mutations in cone photoreceptor CNGA3 exacerbate CNG channel-associated retinopathy. J. Clin. Investig. 2018, 128, 5663–5675. [Google Scholar] [CrossRef]
- Aligianis, I.A.; Forshew, T.; Johnson, S.; Michaelides, M.; Johnson, C.A.; Trembath, R.C.; Hunt, D.M.; Moore, A.T.; Maher, E.R. Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2). J. Med. Genet. 2002, 39, 656–660. [Google Scholar] [CrossRef]
- Thiadens, A.A.; den Hollander, A.I.; Roosing, S.; Nabuurs, S.B.; Zekveld-Vroon, R.C.; Collin, R.W.; De Baere, E.; Koenekoop, R.K.; van Schooneveld, M.J.; Strom, T.M.; et al. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am. J. Hum. Genet. 2009, 85, 240–247. [Google Scholar] [CrossRef]
- Pieh, C.; Simonsz-Toth, B.; Gottlob, I. Nystagmus characteristics in congenital stationary night blindness (CSNB). Br. J. Ophthalmol. 2008, 92, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Sharma, T. Congenital Stationary Night Blindness. Adv. Exp. Med. Biol. 2018, 1085, 61–64. [Google Scholar] [PubMed]
- Zeitz, C.; Robson, A.G.; Audo, I. Congenital stationary night blindness: An analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog. Retin. Eye Res. 2015, 45, 58–110. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Jordan, S.A.; Farrar, G.J.; Kenna, P.; Humphries, M.M.; Sheils, D.M.; Kumar-Singh, R.; Sharp, E.M.; Soriano, N.; Ayuso, C.; Benitez, J.; et al. Localization of an autosomal dominant retinitis pigmentosa gene to chromosome 7q. Nat. Genet. 1993, 4, 54–58. [Google Scholar] [CrossRef]
- Tee, J.J.; Smith, A.J.; Hardcastle, A.J.; Michaelides, M. RPGR-associated retinopathy: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2016, 100, 1022–1027. [Google Scholar] [CrossRef]
- Williams, K.M.; Georgiou, M.; Kalitzeos, A.; Chow, I.; Hysi, P.G.; Robson, A.G.; Lingham, G.; Chen, F.K.; Mackey, D.A.; Webster, A.R.; et al. Axial Length Distributions in Patients With Genetically Confirmed Inherited Retinal Diseases. Investig. Ophthalmol. Vis. Sci. 2022, 63, 15. [Google Scholar] [CrossRef]
- De Silva, S.R.; Arno, G.; Robson, A.G.; Fakin, A.; Pontikos, N.; Mohamed, M.D.; Bird, A.C.; Moore, A.T.; Michaelides, M.; Webster, A.R.; et al. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog. Retin. Eye Res. 2021, 82, 100898. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Jovanovic, K.; Guimaraes, T.A.C.; Ali, N.; Pontikos, N.; Uwaydat, S.H.; Mahroo, O.A.; Cheetham, M.E.; Webster, A.R.; et al. RP2-Associated X-linked Retinopathy: Clinical Findings, Molecular Genetics, and Natural History. Ophthalmology 2023, 130, 413–422. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Murga-Zamalloa, C.A.; Chan, L.; Hitchcock, P.F.; Swaroop, A.; Khanna, H. Human retinopathy-associated ciliary protein retinitis pigmentosa GTPase regulator mediates cilia-dependent vertebrate development. Hum. Mol. Genet. 2010, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, R.; Lennon, A.; Bird, A.C.; Tulloch, B.; Axton, R.; Miano, M.G.; Meindl, A.; Meitinger, T.; Ciccodicola, A.; Wright, A.F. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet. 2000, 25, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; McDowall, E.; Brown, A.F.; Wright, A.F. The human retinitis pigmentosa GTPase regulator gene variant database. Hum. Mutat. 2008, 29, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, V.; Jambou, M.; Delphin, N.; Zinovieva, E.; Stum, M.; Gigarel, N.; Dollfus, H.; Hamel, C.; Toutain, A.; Dufier, J.L.; et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: Genotype-phenotype correlations and impact on genetic counseling. Hum. Mutat. 2007, 28, 81–91. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; Thiadens, A.A.; Fiocco, M.; Wijnholds, J.; Florijn, R.J.; Schalij-Delfos, N.E.; van Genderen, M.M.; Putter, H.; Cremers, F.P.M.; et al. Clinical and genetic characteristics of male patients with RPGR-associated retinal dystrophies: A Long-Term Follow-up Study. Retina 2019, 39, 1186–1199. [Google Scholar] [CrossRef]
- Hadalin, V.; Sustar, M.; Volk, M.; Maver, A.; Sajovic, J.; Jarc-Vidmar, M.; Peterlin, B.; Hawlina, M.; Fakin, A. Cone Dystrophy Associated with a Novel Variant in the Terminal Codon of the RPGR-ORF15. Genes. 2021, 12, 499. [Google Scholar] [CrossRef]
- Yang, L.; Yin, X.; Feng, L.; You, D.; Wu, L.; Chen, N.; Li, A.; Li, G.; Ma, Z. Novel mutations of RPGR in Chinese retinitis pigmentosa patients and the genotype-phenotype correlation. PLoS ONE 2014, 9, e85752. [Google Scholar] [CrossRef]
- Di Iorio, V.; Karali, M.; Melillo, P.; Testa, F.; Brunetti-Pierri, R.; Musacchia, F.; Condroyer, C.; Neidhardt, J.; Audo, I.; Zeitz, C.; et al. Spectrum of Disease Severity in Patients With X-Linked Retinitis Pigmentosa Due to RPGR Mutations. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef]
- Molday, L.L.; Hicks, D.; Sauer, C.G.; Weber, B.H.; Molday, R.S. Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 816–825. [Google Scholar]
- Sikkink, S.K.; Biswas, S.; Parry, N.R.; Stanga, P.E.; Trump, D. X-linked retinoschisis: An update. J. Med. Genet. 2007, 44, 225–232. [Google Scholar] [CrossRef] [PubMed]
- George, N.D.; Yates, J.R.; Bradshaw, K.; Moore, A.T. Infantile presentation of X linked retinoschisis. Br. J. Ophthalmol. 1995, 79, 653–657. [Google Scholar] [CrossRef]
- Hinds, A.M.; Fahim, A.; Moore, A.T.; Wong, S.C.; Michaelides, M. Bullous X linked retinoschisis: Clinical features and prognosis. Br. J. Ophthalmol. 2018, 102, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Peachey, N.S.; Fishman, G.A.; Derlacki, D.J.; Brigell, M.G. Psychophysical and electroretinographic findings in X-linked juvenile retinoschisis. Arch. Ophthalmol. 1987, 105, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Apushkin, M.A.; Fishman, G.A.; Rajagopalan, A.S. Fundus findings and longitudinal study of visual acuity loss in patients with X-linked retinoschisis. Retina 2005, 25, 612–618. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Bordet, T.; Behar-Cohen, F. Ocular gene therapies in clinical practice: Viral vectors and nonviral alternatives. Drug Discov. Today 2019, 24, 1685–1693. [Google Scholar] [CrossRef]
- Flotte, T.R. Gene therapy progress and prospects: Recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2004, 11, 805–810. [Google Scholar] [CrossRef]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef]
- Au, H.K.E.; Isalan, M.; Mielcarek, M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2021, 8, 809118. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef]
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D.; et al. Triple Vectors Expand AAV Transfer Capacity in the Retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef]
- Trapani, I.; Tornabene, P.; Auricchio, A. Large gene delivery to the retina with AAV vectors: Are we there yet? Gene Ther. 2021, 28, 220–222. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Sudharsan, R.; Dufour, V.L.; Massengill, M.T.; Iwabe, S.; Swider, M.; Lisi, B.; Sumaroka, A.; Marinho, L.F.; Appelbaum, T.; et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proc. Natl. Acad. Sci. USA 2018, 115, E8547–E8556. [Google Scholar] [CrossRef]
- Lewin, A.S.; Smith, W.C. Gene Therapy for Rhodopsin Mutations. Cold Spring Harb. Perspect. Med. 2022, 12, a041283. [Google Scholar] [CrossRef]
- Leveillard, T.; Fridlich, R.; Clerin, E.; Ait-Ali, N.; Millet-Puel, G.; Jaillard, C.; Yang, Y.; Zack, D.; van-Dorsselaer, A.; Sahel, J.A. Therapeutic strategy for handling inherited retinal degenerations in a gene-independent manner using rod-derived cone viability factors. Comptes Rendus Biol. 2014, 337, 207–213. [Google Scholar] [CrossRef]
- John, M.C.; Quinn, J.; Hu, M.L.; Cehajic-Kapetanovic, J.; Xue, K. Gene-agnostic therapeutic approaches for inherited retinal degenerations. Front. Mol. Neurosci. 2022, 15, 1068185. [Google Scholar] [CrossRef]
- Michalakis, S.; Schon, C.; Becirovic, E.; Biel, M. Gene therapy for achromatopsia. J. Gene Med. 2017, 19, e2944. [Google Scholar] [CrossRef]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Min, S.H.; Chiodo, V.; Pang, J.J.; Zhong, L.; Zolotukhin, S.; Srivastava, A.; Lewin, A.S.; et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009, 17, 463–471. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Yang, P.; Birch, D.G.; Weng, C.Y.; Moore, A.T.; Iannaccone, A.; Comander, J.I.; Jayasundera, T.; Chulay, J.; XLRS-001 Study Group. Intravitreal Delivery of rAAV2tYF-CB-hRS1 Vector for Gene Augmentation Therapy in Patients with X-Linked Retinoschisis: 1-Year Clinical Results. Ophthalmol. Retin. 2022, 6, 1130–1144. [Google Scholar] [CrossRef]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Tzekov, R.T.; Zhu, Y.; Li, W. The effect of human gene therapy for RPE65-associated Leber’s congenital amaurosis on visual function: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 49. [Google Scholar] [CrossRef]
- Testa, F.; Bacci, G.; Falsini, B.; Iarossi, G.; Melillo, P.; Mucciolo, D.P.; Murro, V.; Salvetti, A.P.; Sodi, A.; Staurenghi, G.; et al. Voretigene neparvovec for inherited retinal dystrophy due to RPE65 mutations: A scoping review of eligibility and treatment challenges from clinical trials to real practice. Eye 2024, 38, 2504–2515. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Ho, A.C.; Peshenko, I.V.; Garafalo, A.V.; Roman, A.J.; Sumaroka, A.; Wu, V.; Krishnan, A.K.; Sheplock, R.; et al. Safety and improved efficacy signals following gene therapy in childhood blindness caused by GUCY2D mutations. iScience 2021, 24, 102409. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Ho, A.C.; Roman, A.J.; Wu, V.; Garafalo, A.V.; Sumaroka, A.; Krishnan, A.K.; Swider, M.; Mascio, A.A.; et al. Night vision restored in days after decades of congenital blindness. iScience 2022, 25, 105274. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Martinez-Fernandez De La Camara, C.; Nanda, A.; Salvetti, A.P.; Fischer, M.D.; MacLaren, R.E. Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin. Orphan Drugs 2018, 6, 167–177. [Google Scholar] [CrossRef]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000, 26, 270–271. [Google Scholar] [CrossRef]
- Ghazi, N.G.; Abboud, E.B.; Nowilaty, S.R.; Alkuraya, H.; Alhommadi, A.; Cai, H.; Hou, R.; Deng, W.T.; Boye, S.L.; Almaghamsi, A.; et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: Results of a phase I trial. Hum. Genet. 2016, 135, 327–343. [Google Scholar] [CrossRef]
- Saari, J.C.; Crabb, J.W. Focus on molecules: Cellular retinaldehyde-binding protein (CRALBP). Exp. Eye Res. 2005, 81, 245–246. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Ehrhart, T.L.; Berson, E.L.; Dryja, T.P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 1995, 92, 3249–3253. [Google Scholar] [CrossRef]
- Pichard, V.; Provost, N.; Mendes-Madeira, A.; Libeau, L.; Hulin, P.; Tshilenge, K.T.; Biget, M.; Ameline, B.; Deschamps, J.Y.; Weber, M.; et al. AAV-mediated Gene Therapy Halts Retinal Degeneration in PDE6beta-deficient Dogs. Mol. Ther. 2016, 24, 867–876. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Jacobson, S.G.; Drack, A.V.; Ho, A.C.; Charng, J.; Garafalo, A.V.; Roman, A.J.; Sumaroka, A.; Han, I.C.; Hochstedler, M.D.; et al. Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat. Med. 2019, 25, 225–228. [Google Scholar] [CrossRef]
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10, a phase 1b/2 trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Charng, J.; Lu, M.; Choi, W.; Sheplock, R.; Swider, M.; Kosyk, M.S.; et al. Outcome Measures for Clinical Trials of Leber Congenital Amaurosis Caused by the Intronic Mutation in the CEP290 Gene. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Slijkerman, R.W.; Vache, C.; Dona, M.; Garcia-Garcia, G.; Claustres, M.; Hetterschijt, L.; Peters, T.A.; Hartel, B.P.; Pennings, R.J.; Millan, J.M.; et al. Antisense Oligonucleotide-based Splice Correction for USH2A-associated Retinal Degeneration Caused by a Frequent Deep-intronic Mutation. Mol. Ther. Nucleic Acids 2016, 5, e381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Wu, W.H.; Lee, T.T.; Wu, W.P.; Xu, C.L.; Park, K.S.; Cui, X.; Justus, S.; Lin, C.S.; Jauregui, R.; et al. Clustered Regularly Interspaced Short Palindromic Repeats-Based Genome Surgery for the Treatment of Autosomal Dominant Retinitis Pigmentosa. Ophthalmology 2018, 125, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kleinstiver, B.P.; Leon, M.Y.; Prew, M.S.; Navarro-Gomez, D.; Greenwald, S.H.; Pierce, E.A.; Joung, J.K.; Liu, Q. Allele-Specific CRISPR-Cas9 Genome Editing of the Single-Base P23H Mutation for Rhodopsin-Associated Dominant Retinitis Pigmentosa. CRISPR J. 2018, 1, 55–64. [Google Scholar] [CrossRef]
- Ruan, G.X.; Barry, E.; Yu, D.; Lukason, M.; Cheng, S.H.; Scaria, A. CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol. Ther. 2017, 25, 331–341. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.; Li, Z.; Peterson, R.T.; Yeh, J.R.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef]
- Vazquez-Dominguez, I.; Garanto, A.; Collin, R.W.J. Molecular Therapies for Inherited Retinal Diseases-Current Standing, Opportunities and Challenges. Genes 2019, 10, 654. [Google Scholar] [CrossRef]
- Cai, Y.; Cheng, T.; Yao, Y.; Li, X.; Ma, Y.; Li, L.; Zhao, H.; Bao, J.; Zhang, M.; Qiu, Z.; et al. In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway. Sci. Adv. 2019, 5, eaav3335. [Google Scholar] [CrossRef]
- Bohrer, L.R.; Wiley, L.A.; Burnight, E.R.; Cooke, J.A.; Giacalone, J.C.; Anfinson, K.R.; Andorf, J.L.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes 2019, 10, 278. [Google Scholar] [CrossRef]
- Jo, D.H.; Song, D.W.; Cho, C.S.; Kim, U.G.; Lee, K.J.; Lee, K.; Park, S.W.; Kim, D.; Kim, J.H.; Kim, J.S.; et al. CRISPR-Cas9-mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. Sci. Adv. 2019, 5, eaax1210. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Aleman, T.S.; Jayasundera, K.T.; Ashimatey, B.S.; Kim, K.; Rashid, A.; Jaskolka, M.C.; Myers, R.L.; Lam, B.L.; Bailey, S.T.; et al. Gene Editing for CEP290-Associated Retinal Degeneration. N. Engl. J. Med. 2024, 390, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; Musa, A.; Kantor, A.; McClements, M.E.; Cehajic-Kapetanovic, J.; MacLaren, R.E.; Xue, K. Genome-Editing Strategies for Treating Human Retinal Degenerations. Hum. Gene Ther. 2021, 32, 247–259. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Huang, T.P.; Newby, G.A.; Liu, D.R. Precision genome editing using cytosine and adenine base editors in mammalian cells. Nat. Protoc. 2021, 16, 1089–1128. [Google Scholar] [CrossRef]
- Levy, J.M.; Yeh, W.H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, X.; Zhao, D.; Chen, X.; Wang, Y.; Tang, X.; Li, J.; Li, S.; Sun, X.; Bi, C.; et al. AAV-mediated base-editing therapy ameliorates the disease phenotypes in a mouse model of retinitis pigmentosa. Nat. Commun. 2023, 14, 4923. [Google Scholar] [CrossRef]
- Su, J.; She, K.; Song, L.; Jin, X.; Li, R.; Zhao, Q.; Xiao, J.; Chen, D.; Cheng, H.; Lu, F.; et al. In vivo base editing rescues photoreceptors in a mouse model of retinitis pigmentosa. Mol. Ther. Nucleic Acids 2023, 31, 596–609. [Google Scholar] [CrossRef]
- Davis, J.R.; Wang, X.; Witte, I.P.; Huang, T.P.; Levy, J.M.; Raguram, A.; Banskota, S.; Seidah, N.G.; Musunuru, K.; Liu, D.R. Efficient in vivo base editing via single adeno-associated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng. 2022, 6, 1272–1283. [Google Scholar] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [PubMed]
- Zhi, S.; Chen, Y.; Wu, G.; Wen, J.; Wu, J.; Liu, Q.; Li, Y.; Kang, R.; Hu, S.; Wang, J.; et al. Dual-AAV delivering split prime editor system for in vivo genome editing. Mol. Ther. 2022, 30, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Jo, D.H.; Cho, C.S.; Shin, J.H.; Seo, J.H.; Yu, G.; Gopalappa, R.; Kim, D.; Cho, S.R.; Kim, J.H.; et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat. Biomed. Eng. 2022, 6, 181–194. [Google Scholar] [CrossRef] [PubMed]
- She, K.; Liu, Y.; Zhao, Q.; Jin, X.; Yang, Y.; Su, J.; Li, R.; Song, L.; Xiao, J.; Yao, S.; et al. Dual-AAV split prime editor corrects the mutation and phenotype in mice with inherited retinal degeneration. Signal Transduct. Target. Ther. 2023, 8, 57. [Google Scholar] [CrossRef]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef]
- Kabra, M.; Shahi, P.K.; Wang, Y.; Sinha, D.; Spillane, A.; Newby, G.A.; Saxena, S.; Tong, Y.; Chang, Y.; Abdeen, A.A.; et al. Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy. J. Clin. Investig. 2023, 133, e171356. [Google Scholar] [CrossRef]
- An, M.; Raguram, A.; Du, S.W.; Banskota, S.; Davis, J.R.; Newby, G.A.; Chen, P.Z.; Palczewski, K.; Liu, D.R. Engineered virus-like particles for transient delivery of prime editor ribonucleoprotein complexes in vivo. Nat. Biotechnol. 2024, 42, 1526–1537. [Google Scholar]
| Diseases | Gene | Protein | Function |
|---|---|---|---|
| LCA1 | GUCY2D | Retinal guanylate cyclase-1 | Phototransduction |
| LCA2 | RPE65 | Retinoid isomerohydrolase RPE65 | Vision (retinoid) cycle |
| LCA3 | SPATA7 | Spermatogenesis associated protein 7 | Photoreceptor ciliary transport |
| LCA4 | AIPL1 | Aryl-hydrocarbon interacting protein-like 1 | Phototransduction |
| LCA5 | LCA5 | Lebercilin | Photoreceptor ciliary transport |
| LCA6 | RPGRIP1 | Retinitis pigmentosa GTPase regulator-interacting protein 1 | Photoreceptor ciliary transport |
| LCA7 | CRX | Cone–rod homeobox | Photoreceptor morphogenesis |
| LCA8 | CRB1 | Crumbs homologus 1 | Photoreceptor morphogenesis |
| LCA9 | NMNAT1 | Nicotinamide nucleotide adenyltransferase 1 | Coenzyme NAD biosynthesis |
| LCA10 | CEP290 | Centrosomal protein 290 | Photoreceptor ciliary transport |
| LCA11 | IMPDH1 | Inosine 5′-monophosphate dehydrogenase 1 | Guanine synthesis |
| LCA12 | RD3 | Retinal Degeneration 3 | Photoreceptor ciliary transport |
| LCA13 | RDH12 | Retinol dehydrogenase 12 | Visual (retinoid) cycle |
| LCA14 | LRAT | Lecithin:retinol acyltransferase | Visual (retinoid) cycle |
| LCA15 | TULP1 | Tubby-like protein | Photoreceptor ciliary transport |
| LCA16 | KCNJ13 | Potassium inwardly rectifying channel subfamily J member 13 | Signal transduction |
| LCA17 | CABP4 | Calcium-binding protein 4 | Signal transduction |
| GDF6 | Growth differentiation factor 6 | Photoreceptor morphogenesis | |
| LCA18 | PRPH2 | Peripherin 2 | Photoreceptor morphogenesis |
| LCA19 | USP45 | ubiquitin specific peptidase 45 | deubiquitylation |
| Unclassified | CCT2 | Chaperonin Containing TCP1 Subunit 2 | Photoreceptor ciliary transport |
| CLUAP1 | Clusterin associated protein 1 | Photoreceptor morphogenesis | |
| DTHD1 | Death-domain containing protein 1 | unknown | |
| IQCB1 | IQ motif containing B1 protein | Photoreceptor ciliary transport | |
| OTX2 | Orthodenticle homeobox 2 protein | Photoreceptor morphogenesis |
| Inheritance Pattern | Types | Genes | Gene ID (OMIM#) |
|---|---|---|---|
| Autosomal Dominant | Riggs | RHO | 180380 |
| GNAT1 | 610444 | ||
| PDE6B | 180072 | ||
| Abnormal fundus | SAG | 181031 | |
| GRK1 | 613411 | ||
| RDH5 | 610617 | ||
| RLBP1 | 180090 | ||
| RPE65 | 180069 | ||
| Autosomal Recessive | Complete | GRM6 | 604096 |
| TRPM1 | 603576 | ||
| RIMS2 | 606630 | ||
| GPR179 | 414515 | ||
| GNB3 | 617024 | ||
| LRIT3 | 615004 | ||
| Incomplete | CABP4 | 608965 | |
| CACNA2D4 | 608171 | ||
| Riggs | SLC24A1 | 613830 | |
| GNAT1 | 610444 | ||
| X-linked | Complete | NYX | 300278 |
| Incomplete | CACNA1F | 300110 |
| Gene | Mutation | Treatment (Sponsor) | Agent (Constructs) | Function | Delivery Route | Clinical Trial ID | Trial Phase |
|---|---|---|---|---|---|---|---|
| Leber Congenital Amaurosis (LCA) | |||||||
| GUCY2D | Loss of Function | Atsena Therapeutics (ATSN-101) | AAV8-GRK1-GUCY2D | Restores function | Subretinal | NCT03920007 | I/II |
| CEP290 | c.2991+ 1655A>G | Editas (EDIT-101) | AAV5-GRK1-Cas9 | Gene editing | Subretinal | NCT03872479 BRILLANCE | I/II |
| CEP290 | c.2991+ 1655A>G | ProQR Therapeutics | QR-110 (Sepofarsen) | Normal mRNA (AON) | Intravitreal | NCT03913143NCT04855045 NCT03140969 | II/III II/III I/II |
| LCA5 | Loss of Function | Opus Genetics | AAV8.hLCA5 (OPGx-001) | Restores function | Subretinal | NCT05616793 | I/II |
| RPE65 | Loss of Function | Janssen/MeiraGTx UK II Ltd. | AAV5-PRE65 | Restores function | Subretinal | NTC02781480 | I/II |
| AAV6-RPE65 | NCT02946879 | I/II | |||||
| RPE65 | Loss of Function | HuidaGene Therapeutics | rAAV2-RPE65 rAAV9-RPE65 | Restores function | Subretinal | NCT05906953 NCT06088992 | I/II I |
| RPE65 | Loss of Function | Spark Therapeutics | AAV2-hRPE65v2 | Restores functional protein | Subretinal | NCT00999609 | III |
| AAV2-hRPE65v2 | NCT01208389 | I/II | |||||
| AAV2-hRPE65v2 | NCT03602820 | III | |||||
| RPE65 | Loss of Function | AGTC | rAAV2-CB-hRPE65 | Restores function | Subretinal | NCT00749957 | I/II |
| RPE65 | Loss of Function | Nantes University Hospital | rAAV2/4.hRPE65 | Restores function | Subretinal | NCT01496040 | I/II |
| RPE65 | Loss of Function | University of Pennsylvania | rAAV-CBSB-hRPE65 | Restores function | Subretinal | NCT00481546 | I/II |
| Achromatopsia (ACHM) | |||||||
| GNCA3 | Loss of Function | Janssen/ STZ eyetrial | rAAV2/8.hCNGA3 | Restores function | Subretinal | NCT02610582 | I/II |
| GNCA3 | Loss of Function | AGTC (Beacon Therapeutics) | rAAV2tYF- PR1.7-hCNGA3 | Restores function | Subretinal | NCT02935517 | I/II |
| GNCA3 | Loss of Function | Janssen/MeiraGTx UK II Ltd. | AAV2/8-hG1.7p. coCNGA3 | Restores function | Subretinal | NCT03758404 | I/II |
| GNCB3 | Loss of Function | Janssen/MeiraGTx UK II Ltd. | rAAV2/8.hCNGB3 | Restores function | Subretinal | NCT03758404 | I/II |
| GNCB3 | Loss of Function | AGTC (Beacon Therapeutics) | rAAV2tYF- PR1.7-hCNGB3 | Restores function | Subretinal | NCT02599922 | I/II |
| GNCB3 | Loss of Function | Janssen/MeiraGTx UK II Ltd. | AAV2/8-hG1.7p. coCNGB3 | Restores function | Subretinal | NCT03001310 | I/II |
| X-linked Retinoschisis (XL-RS) | |||||||
| RS1 | Loss of Function | AGTC | rAAV2YF-CB-hRS1 | Restores function | Intravitreal | NCT02416622 | I/II |
| RS1 | Loss of Function | Atsena Therapeutics | ATSN-201 (AAV.SPR.hRS1) | Restores function | Intravitreal | NCT05878860 | I/II |
| RS1 | Loss of Function | InnoVec Biotherapeutics | IVB102 | Restores function | Intravitreal | NCT06289452 | I/II |
| RS1 | Loss of Function | Shanghai General Hospital | LX103 | Restores function | Intravitreal | NCT05814952 | I/II |
| RS1 | Loss of Function | West China Hospital | JWK002 | Restores function | Intravitreal | NCT06345898 | I/II |
| RS1 | Loss of Function | National Eye Institute | AAV8-scRS/ IRBPhRS | Restores function | Intravitreal | NCT02317887 | I/II |
| X-linked Retinitis Pigmentosa (XL-RP) | |||||||
| RPGR | Loss of Function | Janssen | AAV5-hRKp.RPGR | Restores function | Subretinal | NCT04671443 (LUMEOS) | III |
| RPGR | Loss of Function | AGTC (Beacon Therapeutics) | rAAV2tYF-GRK1-RPGRco | Restores function | Subretinal | NCT06333249 (SKYLINE) | II |
| RPGR | Loss of Function | AGTC (Beacon Therapeutics) | rAAV2tYF-GRK1-RPGRco | Restores function | Subretinal | NCT04850118 (VISTA) | II/III |
| RPGR | Loss of Function | 4D Molecular Therapeutics | AAV.R100-hcoRPGR (4D-125) | Restores function | Intravitreal | NCT04517149 (EXCEL) | I/II |
| RPGR | Loss of Function | Janssen | AAV5-hRKp.RPGR | Restores function | Subretinal | NCT05926583 NCT04794101 | III III |
| RPGR | Loss of Function | Frontera Therapeutics | FT-002 | Restores function | Intravitreal | NCT06492850 | I/II |
| RPGR | Loss of Function | Biogen | AAV8-RPGR (BIIB112) | Restores function | Subretinal | NCT03584165 (SOLSTICE) | III |
| Autosomal dominant Retinitis Pigmentosa (adRP) | |||||||
| RHO | P23H | ProQR Therapeutics | QR-1123 | Gene silencing | Intravitreal | NCT04123626 (AURORA) | I/II |
| RHO | Optogenetic | AbbVie | AAV2-ChR2 (RST-001) | Gene agnostic | Intravitreal | NCT02556736 | I/II |
| RHO | Agnostic | SparingVision | AAV-RdCVF-RdCVFL (SPVN06) | Gene agnostic | Subretinal | NCT05748873 | I/II |
| RHO | Genetic modifier | Ocugen | AAV5-NR2E3 (OCU400-301) | Gene agnostic | Subretinal | NCT06388200 | III |
| Autosomal recessive Retinitis Pigmentosa (arRP) | |||||||
| RLBP1 | Loss of Function | Novartis Therapeutics | AAV8-RLBP1 (CPK850) | Restores function | Subretinal | NCT03374657 | I/II |
| USH2A | USH2A (exon 3) | Laboratoires Thea | Ultevursen (QR-421a) | Gene silencing | Intravitreal | NCT05176717 NCT05158296 | II/III |
| PDE6A | Loss of Function | STZ eyetrial | rAAV-hPDE6A | Restores function | Subretinal | NCT04611503 | I/II |
| PDE6B | Loss of Function | Coave Therapeutics | AAV5-hPDE6B | Restore function | Subretinal | NCT03328130 | I/II |
| CNGA1 | Loss of Function | ViGeneron GmbH | AAV2.NN-CNGA1 (VG-901) | Restore function | Intravitreal | NCT06291935 | I |
| MERTK | Loss of Function | Fowzan Alkuraya | AAV2-MERTK | Restores function | Subretinal | NCT01482195 | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Hertle, R.W. Infantile Nystagmus Syndrome—Associated Inherited Retinal Diseases: Perspectives from Gene Therapy Clinical Trials. Life 2024, 14, 1356. https://doi.org/10.3390/life14111356
Gong X, Hertle RW. Infantile Nystagmus Syndrome—Associated Inherited Retinal Diseases: Perspectives from Gene Therapy Clinical Trials. Life. 2024; 14(11):1356. https://doi.org/10.3390/life14111356
Chicago/Turabian StyleGong, Xiaoming, and Richard W. Hertle. 2024. "Infantile Nystagmus Syndrome—Associated Inherited Retinal Diseases: Perspectives from Gene Therapy Clinical Trials" Life 14, no. 11: 1356. https://doi.org/10.3390/life14111356
APA StyleGong, X., & Hertle, R. W. (2024). Infantile Nystagmus Syndrome—Associated Inherited Retinal Diseases: Perspectives from Gene Therapy Clinical Trials. Life, 14(11), 1356. https://doi.org/10.3390/life14111356






