Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver?
Abstract
1. Introduction
2. Literature Sources and Search Strategy
3. Bariatric Surgery: Overview
4. Epidemiology
5. Pathophysiological Changes after Bariatric Surgery
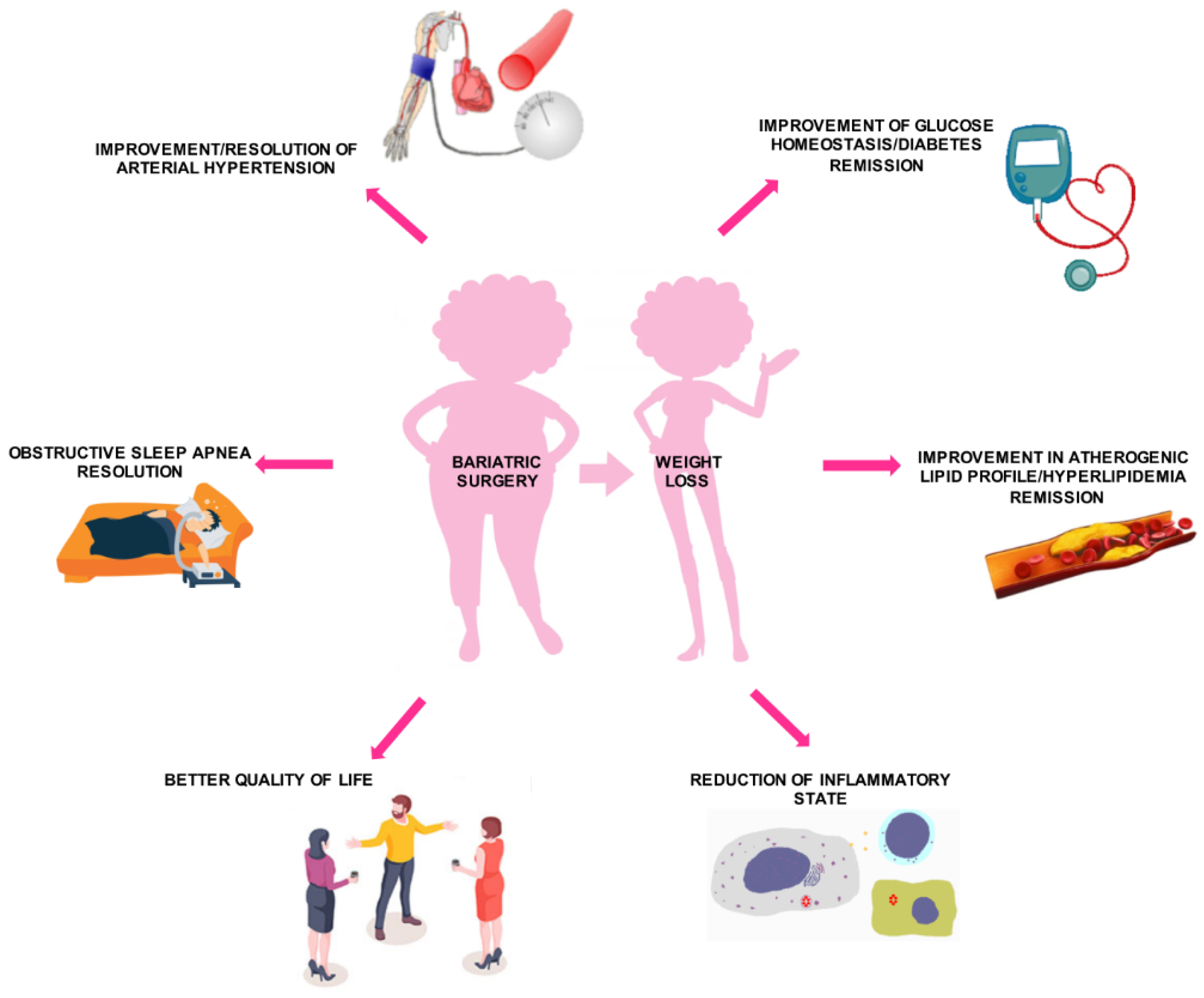
6. Effects of Bariatric Surgery on Diabetes Mellitus and Other Cardiovascular Risk Factors
6.1. T2DM
6.2. Systemic Arterial HTN
6.3. Dyslipidemia
6.4. Inflammation
6.5. Health-Related Quality of Life
7. Long-Term Impact of Bariatric Surgery on Metabolic Profile and Cardiovascular System
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Clark, J.M.; Garvey, W.T.; Niswender, K.D.; Schmidt, A.M.; Ahima, R.S.; Aleman, J.O.; Battarbee, A.N.; Beckman, J.; Bennett, W.L.; Brown, N.J.; et al. Obesity and Overweight: Probing Causes, Consequences, and Novel Therapeutic Approaches Through the American Heart Association’s Strategically Focused Research Network. J. Am. Heart Assoc. 2023, 12, e027693. [Google Scholar] [CrossRef]
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Santos, F.G.C.D.; Godoy-Leite, M.; Penido, E.A.R.; Ribeiro, K.A.; da Gloria Rodrigues-Machado, M.; Rezende, B.A. Eating behaviour, quality of life and cardiovascular risk in obese and overweight children and adolescents: A cross-sectional study. BMC Pediatr. 2023, 23, 299. [Google Scholar] [CrossRef]
- Krzysztoszek, J.; Laudańska-Krzemińska, I.; Bronikowski, M. Assessment of epidemiological obesity among adults in EU countries. Ann. Agric. Environ. Med. 2019, 26, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef]
- Lambert, G.W.; Schlaich, M.P.; Eikelis, N.; Lambert, E.A. Sympathetic activity in obesity: A brief review of methods and supportive data. Ann. N. Y. Acad. Sci. 2019, 1454, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Cirillo, P.; Ziviello, F.; Pellegrino, G.; Conte, S.; Cimmino, G.; Giaquinto, A.; Pacifico, F.; Leonardi, A.; Golino, P.; Trimarco, B. The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb. Haemost. 2015, 113, 363–372. [Google Scholar] [CrossRef]
- Cirillo, P.; Di Palma, V.; Maresca, F.; Pacifico, F.; Ziviello, F.; Bevilacqua, M.; Trimarco, B.; Leonardi, A.; Chiariello, M. The adipokine visfatin induces tissue factor expression in human coronary artery endothelial cells: Another piece in the adipokines puzzle. Thromb. Res. 2012, 130, 403–408. [Google Scholar] [CrossRef]
- Calabro, P.; Cirillo, P.; Limongelli, G.; Maddaloni, V.; Riegler, L.; Palmieri, R.; Pacileo, G.; De Rosa, S.; Pacileo, M.; De Palma, R.; et al. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-kB-dependent pathway. J. Vasc. Res. 2011, 48, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol. 2020, 18, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Almanza, M.; Camara-Gomez, R.; Hervas-Marin, D.; Ponce-Marco, J.L.; Merino-Torres, J.F. Cardiovascular risk reduction over time in patients with diabetes or pre-diabetes undergoing bariatric surgery: Data from a single-center retrospective observational study. BMC Endocr. Disord. 2018, 18, 90. [Google Scholar] [CrossRef]
- McGrice, M.; Don Paul, K. Interventions to improve long-term weight loss in patients following bariatric surgery: Challenges and solutions. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 263–274. [Google Scholar] [CrossRef]
- 2004 ASBS Consensus Conference on Surgery for Severe Obesity. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2005, 1, 297–381. [CrossRef]
- Coutinho, T.; Goel, K.; Correa de Sa, D.; Carter, R.E.; Hodge, D.O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J. Am. Coll. Cardiol. 2013, 61, 553–560. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eisenberg, D.; Azagury, D.; Rogers, A.; Campos, G.M. American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2016, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Pradhan, A.; Khan, M.A.; Anderson, S.G.; Keavney, B.D.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Bariatric surgery and its impact on cardiovascular disease and mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, V. Can Small Bowel Resection Be Defended as Therapy for Obesity? Obes. Surg. 1994, 4, 54. [Google Scholar] [CrossRef]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, A.; Bouchard, C.; Carlsson, B.; Karason, K.; Lonroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, J.; Li, L.; Gloy, V.L.; Nordmann, A.; Tiboni, M.; Li, Y.; Sun, X. Effects of Bariatric Surgery on Mortality, Cardiovascular Events, and Cancer Outcomes in Obese Patients: Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 2590–2601. [Google Scholar] [CrossRef]
- Benotti, P.N.; Wood, G.C.; Carey, D.J.; Mehra, V.C.; Mirshahi, T.; Lent, M.R.; Petrick, A.T.; Still, C.; Gerhard, G.S.; Hirsch, A.G. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. J. Am. Heart Assoc. 2017, 6, e005126. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Nissen, S.; Schauer, P.R. Gastrointestinal surgery for obesity and diabetes: Weight loss and control of hyperglycemia. Curr. Atheroscler. Rep. 2012, 14, 579–587. [Google Scholar] [CrossRef]
- Miras, A.D.; le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef]
- Jaunoo, S.S.; Southall, P.J. Bariatric surgery. Int. J. Surg. 2010, 8, 86–89. [Google Scholar] [CrossRef]
- Baker, M.T. The history and evolution of bariatric surgical procedures. Surg. Clin. N. Am. 2011, 91, 1181–1201. [Google Scholar] [CrossRef]
- Phillips, B.T.; Shikora, S.A. The history of metabolic and bariatric surgery: Development of standards for patient safety and efficacy. Metab. Clin. Exp. 2018, 79, 97–107. [Google Scholar] [CrossRef]
- Furbetta, N.; Cervelli, R.; Furbetta, F. Laparoscopic adjustable gastric banding, the past, the present and the future. Ann. Transl. Med. 2020, 8, S4. [Google Scholar] [CrossRef]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T.K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: A prospective, double blind study. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Zundel, N.; Buchwald, H.; Scopinaro, N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 2017, 27, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Scopinaro, N.; Marinari, G.M.; Camerini, G. Laparoscopic standard biliopancreatic diversion: Technique and preliminary results. Obes. Surg. 2002, 12, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Biertho, L.; Lebel, S.; Marceau, S.; Hould, F.S.; Julien, F.; Biron, S. Biliopancreatic Diversion with Duodenal Switch: Surgical Technique and Perioperative Care. Surg. Clin. N. Am. 2016, 96, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R. Open and laparoscopic surgical modalities for the management of obesity. J. Gastrointest. Surg. 2003, 7, 468–475. [Google Scholar] [CrossRef]
- Rutledge, R. The mini-gastric bypass: Experience with the first 1,274 cases. Obes. Surg. 2001, 11, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Kular, K.S.; Parmar, C.; Van den Bossche, M.; Graham, Y.; Carr, W.R.J.; Madhok, B.; Magee, C.; Purkayastha, S.; Small, P.K. Perioperative Practices Concerning One Anastomosis (Mini) Gastric Bypass: A Survey of 210 Surgeons. Obes. Surg. 2018, 28, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Chevallier, J.M.; Mahawar, K.; Brown, W.; Kow, L.; White, K.P.; Shikora, S.; Contributors, I.C.C. IFSO (International Federation for Surgery of Obesity and Metabolic Disorders) Consensus Conference Statement on One-Anastomosis Gastric Bypass (OAGB-MGB): Results of a Modified Delphi Study. Obes. Surg. 2020, 30, 1625–1634. [Google Scholar] [CrossRef]
- Deitel, M. Consensus survey on mini-gastric bypass and one-anastomosis gastric bypass. Ann. Bariatr. Metab. Surg. 2018, 1, 1001. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Nautiyal, H.K.; Kosta, S.; Mathur, W.; Fobi, M. Comparison of one-anastomosis gastric bypass and Roux-en-Y gastric bypass for treatment of obesity: A 5-year study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 2038–2044. [Google Scholar] [CrossRef]
- Parmar, C.; Abdelhalim, M.A.; Mahawar, K.K.; Boyle, M.; Carr, W.R.J.; Jennings, N.; Small, P.K. Management of super-super obese patients: Comparison between one anastomosis (mini) gastric bypass and Roux-en-Y gastric bypass. Surg. Endosc. 2017, 31, 3504–3509. [Google Scholar] [CrossRef]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e5. [Google Scholar] [CrossRef]
- Robert, M.; Espalieu, P.; Pelascini, E.; Caiazzo, R.; Sterkers, A.; Khamphommala, L.; Poghosyan, T.; Chevallier, J.M.; Malherbe, V.; Chouillard, E.; et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2019, 393, 1299–1309. [Google Scholar] [CrossRef]
- Haddad, A.; Bashir, A.; Fobi, M.; Higa, K.; Herrera, M.F.; Torres, A.J.; Himpens, J.; Shikora, S.; Ramos, A.C.; Kow, L.; et al. The IFSO Worldwide One Anastomosis Gastric Bypass Survey: Techniques and Outcomes? Obes. Surg. 2021, 31, 1411–1421. [Google Scholar] [CrossRef]
- Tack, J.; Arts, J.; Caenepeel, P.; De Wulf, D.; Bisschops, R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 583–590. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity 2020, 28, O1–O58. [Google Scholar] [CrossRef]
- Lim, R.; Beekley, A.; Johnson, D.C.; Davis, K.A. Early and late complications of bariatric operation. Trauma Surg. Acute Care Open 2018, 3, e000219. [Google Scholar] [CrossRef] [PubMed]
- Simoens, C.; Verbiest, A.; Van der Borght, W.; Van den Eynde, A.; Brenninkmeijer, K.; Moyson, C.; Matthys, C.; Meulemans, A.; Lannoo, M.; Van der Schueren, B.; et al. Nonsurgical complications after bariatric surgery. Proc. Nutr. Soc. 2020, 79, E681. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, R.; Gupta, P.K.; Mohan, V. Diabetes in South Asians: Phenotype, Clinical Presentation, and Natural History. Curr. Diabetes Rep. 2018, 18, 30. [Google Scholar] [CrossRef]
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620. [Google Scholar] [CrossRef]
- Sheng, B.; Truong, K.; Spitler, H.; Zhang, L.; Tong, X.; Chen, L. The Long-Term Effects of Bariatric Surgery on Type 2 Diabetes Remission, Microvascular and Macrovascular Complications, and Mortality: A Systematic Review and Meta-Analysis. Obes. Surg. 2017, 27, 2724–2732. [Google Scholar] [CrossRef]
- Alkharaiji, M.; Anyanwagu, U.; Donnelly, R.; Idris, I. Effect of Bariatric Surgery on Cardiovascular Events and Metabolic Outcomes in Obese Patients with Insulin-Treated Type 2 Diabetes: A Retrospective Cohort Study. Obes. Surg. 2019, 29, 3154–3164. [Google Scholar] [CrossRef]
- van Veldhuisen, S.L.; Gorter, T.M.; van Woerden, G.; de Boer, R.A.; Rienstra, M.; Hazebroek, E.J.; van Veldhuisen, D.J. Bariatric surgery and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.P.; Johnson, E.; Haneuse, S.; Arterburn, D.; Coleman, K.J.; O’Connor, P.J.; O’Brien, R.; Bogart, A.; Theis, M.K.; Anau, J.; et al. Association Between Bariatric Surgery and Macrovascular Disease Outcomes in Patients With Type 2 Diabetes and Severe Obesity. JAMA 2018, 320, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease:: A Population-Based Retrospective Cohort Study. Circulation 2021, 143, 1468–1480. [Google Scholar] [CrossRef]
- Domenech-Ximenos, B.; Cuba, V.; Daunis, I.E.P.; Thio-Henestrosa, S.; Jaldo, F.; Biarnes, C.; Molina, X.; Xifra, G.; Ricart, W.; Bardera, A.; et al. Bariatric Surgery-Induced Changes in Intima-Media Thickness and Cardiovascular Risk Factors in Class 3 Obesity: A 3-Year Follow-Up Study. Obesity 2020, 28, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Aleassa, E.M.; Bhatt, D.L.; Tu, C.; Khorgami, Z.; Schauer, P.R.; Brethauer, S.A.; Daigle, C.R. Bariatric surgery is associated with a lower rate of death after myocardial infarction and stroke: A nationwide study. Diabetes Obes. Metab. 2019, 21, 2058–2067. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Fruhbeck, G.; International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yin, L. Circadian rhythms in liver physiology and liver diseases. Compr. Physiol. 2013, 3, 917–940. [Google Scholar] [CrossRef]
- Reynolds, E.L.; Watanabe, M.; Banerjee, M.; Chant, E.; Villegas-Umana, E.; Elafros, M.A.; Gardner, T.W.; Pop-Busui, R.; Pennathur, S.; Feldman, E.L.; et al. The effect of surgical weight loss on diabetes complications in individuals with class II/III obesity. Diabetologia 2023, 66, 1192–1207. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The effect of weight loss on C-reactive protein: A systematic review. Arch. Intern. Med. 2007, 167, 31–39. [Google Scholar] [CrossRef]
- Jia, Q.; Carranza Leon, B.G.; Jensen, M.D. Influence of Free Fatty Acid Concentrations and Weight Loss on Adipose Tissue Direct Free Fatty Acid Storage Rates. J. Clin. Endocrinol. Metab. 2021, 106, e5165–e5179. [Google Scholar] [CrossRef]
- Wooten, J.S.; Breden, M.; Hoeg, T.; Smith, B.K. Effects of weight-loss on adipokines, total and regional body composition and markers of metabolic syndrome in women who are overweight and obese. Endocr. Metab. Sci. 2022, 7–8, 100120. [Google Scholar] [CrossRef]
- Hannah, W.N., Jr.; Harrison, S.A. Lifestyle and Dietary Interventions in the Management of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1365–1374. [Google Scholar] [CrossRef]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, S.M.; Patterson, B.W.; Klein, S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef]
- Umeda, L.M.; Pereira, A.Z.; Carneiro, G.; Arasaki, C.H.; Zanella, M.T. Postprandial adiponectin levels are associated with improvements in postprandial triglycerides after Roux-en-Y gastric bypass in type 2 diabetic patients. Metab. Syndr. Relat. Disord. 2013, 11, 343–348. [Google Scholar] [CrossRef]
- Osto, E.; Doytcheva, P.; Corteville, C.; Bueter, M.; Dorig, C.; Stivala, S.; Buhmann, H.; Colin, S.; Rohrer, L.; Hasballa, R.; et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: Role of glucagon-like peptide-1. Circulation 2015, 131, 871–881. [Google Scholar] [CrossRef]
- Sell, H.; Poitou, C.; Habich, C.; Bouillot, J.L.; Eckel, J.; Clement, K. Heat Shock Protein 60 in Obesity: Effect of Bariatric Surgery and its Relation to Inflammation and Cardiovascular Risk. Obesity 2017, 25, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, D.; Climent, E.; Goday, A.; Flores-Le Roux, J.A.; Pedro-Botet, J. Bariatric surgery and hypertension: Implications and perspectives after the GATEWAY randomized trial. Cardiovasc. Diagn. Ther. 2019, 9, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, G.S.; Smastuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofso, D.; Lindberg, M.; Hertel, J.K.; Hjelmesaeth, J. Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018, 319, 291–301. [Google Scholar] [CrossRef]
- Oliveras, A.; Molina, L.; Goday, A.; Sans, L.; Riera, M.; Vazquez, S.; Benaiges, D.; Granados, A.M.; Ramon, J.M.; Pascual, J. Effect of bariatric surgery on cardiac structure and function in obese patients: Role of the renin-angiotensin system. J. Clin. Hypertens. 2021, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Stefater, M.A.; Inge, T.H. Bariatric Surgery for Adolescents with Type 2 Diabetes: An Emerging Therapeutic Strategy. Curr. Diabetes Rep. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Nor Hanipah, Z.; Rubino, F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world’s leading diabetes organizations. Clevel. Clin. J. Med. 2017, 84, S47–S56. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Rullmann, M.; Seyfried, F.; Preusser, S.; Poppitz, S.; Heba, S.; Gousias, K.; Hoyer, J.; Schutz, T.; Dietrich, A.; et al. Roux-en-Y gastric bypass surgery progressively alters radiologic measures of hypothalamic inflammation in obese patients. JCI Insight 2019, 4, e131329. [Google Scholar] [CrossRef] [PubMed]
- Cerit, Z. Bariatric surgery, diabetes mellitus, and epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 581. [Google Scholar] [CrossRef]
- Buchwald, H.; Buchwald, J.N. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care 2019, 42, 331–340. [Google Scholar] [CrossRef]
- Argyropoulos, G. Bariatric surgery: Prevalence, predictors, and mechanisms of diabetes remission. Curr. Diabetes Rep. 2015, 15, 15. [Google Scholar] [CrossRef]
- Torquati, A.; Shantavasinkul, P.C.; Omotosho, P.; Corsino, L.; Spagnoli, A. Perioperative changes in prouroguanylin hormone response in severely obese subjects after bariatric surgery. Surgery 2019, 166, 456–459. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Guerrero-Perez, F.; Casajoana, A.; Gomez-Vaquero, C.; Virgili, N.; Lopez-Urdiales, R.; Hernandez-Montoliu, L.; Pujol-Gebelli, J.; Osorio, J.; Alves, C.; Perez-Maraver, M.; et al. Changes in Bone Mineral Density in Patients with Type 2 Diabetes After Different Bariatric Surgery Procedures and the Role of Gastrointestinal Hormones. Obes. Surg. 2020, 30, 180–188. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafre, V.; Llaurado, G.; Keiran, N.; Benaiges, E.; Astiarraga, B.; Martinez, L.; Pellitero, S.; Gonzalez-Clemente, J.M.; Rodriguez, A.; Fernandez-Real, J.M.; et al. Preoperative Circulating Succinate Levels as a Biomarker for Diabetes Remission After Bariatric Surgery. Diabetes Care 2019, 42, 1956–1965. [Google Scholar] [CrossRef]
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11, 1582. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Jimenez, A.; Mari, A.; Casamitjana, R.; Lacy, A.; Ferrannini, E.; Vidal, J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes 2014, 63, 3372–3377. [Google Scholar] [CrossRef]
- Jimenez, A.; Casamitjana, R.; Viaplana-Masclans, J.; Lacy, A.; Vidal, J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013, 36, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.B.; Jacobsen, S.H.; Dirksen, C.; Bojsen-Moller, K.N.; Naver, L.; Hvolris, L.; Clausen, T.R.; Wulff, B.S.; Worm, D.; Lindqvist Hansen, D.; et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E122–E131. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Haluzik, M.; Janez, A. Efficacy of GLP-1 RA Approved for Weight Management in Patients With or Without Diabetes: A Narrative Review. Adv. Ther. 2022, 39, 2452–2467. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef]
- Chandarana, K.; Gelegen, C.; Karra, E.; Choudhury, A.I.; Drew, M.E.; Fauveau, V.; Viollet, B.; Andreelli, F.; Withers, D.J.; Batterham, R.L. Diet and gastrointestinal bypass-induced weight loss: The roles of ghrelin and peptide YY. Diabetes 2011, 60, 810–818. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Boey, D.; Sainsbury, A.; Herzog, H. The role of peptide YY in regulating glucose homeostasis. Peptides 2007, 28, 390–395. [Google Scholar] [CrossRef]
- Mattar, S.G.; Velcu, L.M.; Rabinovitz, M.; Demetris, A.J.; Krasinskas, A.M.; Barinas-Mitchell, E.; Eid, G.M.; Ramanathan, R.; Taylor, D.S.; Schauer, P.R. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann. Surg. 2005, 242, 610–617, discussion 618–620. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Kupski, C.; Swarowsky, A.M.; Glock, L.; Duval, V.; da Silva, J.B. Histological behavior of hepatic steatosis in morbidly obese patients after weight loss induced by bariatric surgery. Obes. Surg. 2005, 15, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Moretto, M.; Kupski, C.; da Silva, V.D.; Padoin, A.V.; Mottin, C.C. Effect of bariatric surgery on liver fibrosis. Obes. Surg. 2012, 22, 1044–1049. [Google Scholar] [CrossRef]
- Karcz, W.K.; Krawczykowski, D.; Kuesters, S.; Marjanovic, G.; Kulemann, B.; Grobe, H.; Karcz-Socha, I.; Hopt, U.T.; Bukhari, W.; Grueneberger, J.M. Influence of Sleeve Gastrectomy on NASH and Type 2 Diabetes Mellitus. J. Obes. 2011, 2011, 765473. [Google Scholar] [CrossRef]
- Blauw, L.L.; Li, Z.; Rensen, S.S.; Greve, J.W.M.; Verhoeven, A.; Derks, R.J.; Giera, M.; Wang, Y.; Rensen, P.C.N. Metabolic liver inflammation in obesity does not robustly decrease hepatic and circulating CETP. Atherosclerosis 2018, 275, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Leonetti, F.; Capoccia, D.; Di Cristofano, C.; Silecchia, G.; Orho-Melander, M.; Melander, O.; Cavallo, M.G. Increased Plasma Proneurotensin Levels Identify NAFLD in Adults With and Without Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2253–2260. [Google Scholar] [CrossRef]
- Livadariu, R.; Timofte, D.; Trifan, A.; Danila, R.; Ionescu, L.; Singeap, A.M.; Ciobanu, D. Vitamin D Deficiency, a Noninvasive Marker of Steatohepatitis in Patients with Obesity and Biopsy Proven Nonalcoholic Fatty Liver Disease. Acta Endocrinol. 2018, 14, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Burguera, B.; Ikramuddin, S.; Cottam, D.; Gourash, W.; Hamad, G.; Eid, G.M.; Mattar, S.; Ramanathan, R.; Barinas-Mitchel, E.; et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 2003, 238, 467–484; discussion 484–465. [Google Scholar] [CrossRef]
- Pournaras, D.J.; Osborne, A.; Hawkins, S.C.; Vincent, R.P.; Mahon, D.; Ewings, P.; Ghatei, M.A.; Bloom, S.R.; Welbourn, R.; le Roux, C.W. Remission of type 2 diabetes after gastric bypass and banding: Mechanisms and 2 year outcomes. Ann. Surg. 2010, 252, 966–971. [Google Scholar] [CrossRef]
- Isbell, J.M.; Tamboli, R.A.; Hansen, E.N.; Saliba, J.; Dunn, J.P.; Phillips, S.E.; Marks-Shulman, P.A.; Abumrad, N.N. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010, 33, 1438–1442. [Google Scholar] [CrossRef]
- Peck, B.C.E.; Seeley, R.J. How does ‘metabolic surgery’ work its magic? New evidence for gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 81–86. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.L.; Zucker, J.D.; Dore, J.; Clement, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra141. [Google Scholar] [CrossRef]
- Nakatani, H.; Kasama, K.; Oshiro, T.; Watanabe, M.; Hirose, H.; Itoh, H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metab. Clin. Exp. 2009, 58, 1400–1407. [Google Scholar] [CrossRef]
- Kohli, R.; Bradley, D.; Setchell, K.D.; Eagon, J.C.; Abumrad, N.; Klein, S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J. Clin. Endocrinol. Metab. 2013, 98, E708–E712. [Google Scholar] [CrossRef]
- Hayoz, C.; Hermann, T.; Raptis, D.A.; Bronnimann, A.; Peterli, R.; Zuber, M. Comparison of metabolic outcomes in patients undergoing laparoscopic roux-en-Y gastric bypass versus sleeve gastrectomy—A systematic review and meta-analysis of randomised controlled trials. Swiss Med. Wkly. 2018, 148, w14633. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef]
- Thaler, J.P.; Cummings, D.E. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009, 150, 2518–2525. [Google Scholar] [CrossRef]
- Patel, R.T.; Shukla, A.P.; Ahn, S.M.; Moreira, M.; Rubino, F. Surgical control of obesity and diabetes: The role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity 2014, 22, 159–169. [Google Scholar] [CrossRef]
- Ricci, C.; Gaeta, M.; Rausa, E.; Macchitella, Y.; Bonavina, L. Early impact of bariatric surgery on type II diabetes, hypertension, and hyperlipidemia: A systematic review, meta-analysis and meta-regression on 6,587 patients. Obes. Surg. 2014, 24, 522–528. [Google Scholar] [CrossRef]
- Ghanim, H.; Monte, S.; Caruana, J.; Green, K.; Abuaysheh, S.; Dandona, P. Decreases in neprilysin and vasoconstrictors and increases in vasodilators following bariatric surgery. Diabetes Obes. Metab. 2018, 20, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.K.; Dubb, S.; Bueter, M.; Seyfried, F.; Patel, K.; Tam, F.W.; Frankel, A.H.; le Roux, C.W. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: A 12-month prospective study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013, 9, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Julia, Z.; Poitou, C.; Bouillot, J.L.; Basdevant, A.; Chapman, M.J.; Clement, K.; Guerin, M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab. 2011, 96, 1151–1159. [Google Scholar] [CrossRef]
- Heffron, S.P.; Parikh, A.; Volodarskiy, A.; Ren-Fielding, C.; Schwartzbard, A.; Nicholson, J.; Bangalore, S. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am. J. Med. 2016, 129, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Lin, B.X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254. [Google Scholar] [CrossRef]
- Puzziferri, N.; Roshek, T.B., 3rd; Mayo, H.G.; Gallagher, R.; Belle, S.H.; Livingston, E.H. Long-term follow-up after bariatric surgery: A systematic review. JAMA 2014, 312, 934–942. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Meron-Eldar, S.; Brethauer, S.A.; Schauer, P.R.; Young, J.B. Effect of bariatric surgery on cardiovascular risk profile. Am. J. Cardiol. 2011, 108, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Nicklas, B.J.; Fernandez, A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2011, 7, 618–624. [Google Scholar] [CrossRef]
- Magallares, A.; Schomerus, G. Mental and physical health-related quality of life in obese patients before and after bariatric surgery: A meta-analysis. Psychol. Health Med. 2015, 20, 165–176. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014, 2014, CD003641. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Thomas, A.J.; Connett, J.E.; Bantle, J.P.; Leslie, D.B.; Wang, Q.; Inabnet, W.B., 3rd; Jeffery, R.W.; et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. JAMA 2018, 319, 266–278. [Google Scholar] [CrossRef]
- Schiavon, C.A.; Bhatt, D.L.; Ikeoka, D.; Santucci, E.V.; Santos, R.N.; Damiani, L.P.; Oliveira, J.D.; Machado, R.H.V.; Halpern, H.; Monteiro, F.L.J.; et al. Three-Year Outcomes of Bariatric Surgery in Patients With Obesity and Hypertension: A Randomized Clinical Trial. Ann. Intern. Med. 2020, 173, 685–693. [Google Scholar] [CrossRef]
- Dixon, J.B.; O’Brien, P.E.; Playfair, J.; Chapman, L.; Schachter, L.M.; Skinner, S.; Proietto, J.; Bailey, M.; Anderson, M. Adjustable gastric banding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA 2008, 299, 316–323. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Playfair, J.; Laurie, C.; Ritchie, M.E.; Brown, W.A.; Burton, P.; Shaw, J.E.; O’Brien, P.E. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 545–552. [Google Scholar] [CrossRef]
- Ding, S.A.; Simonson, D.C.; Wewalka, M.; Halperin, F.; Foster, K.; Goebel-Fabbri, A.; Hamdy, O.; Clancy, K.; Lautz, D.; Vernon, A.; et al. Adjustable Gastric Band Surgery or Medical Management in Patients With Type 2 Diabetes: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2015, 100, 2546–2556. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Q.; Chen, B.; Yu, P.; Zhao, H.; Ouyang, X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: A randomized controlled trial. Diabetes Res. Clin. Pract. 2013, 101, 50–56. [Google Scholar] [CrossRef]
- Simonson, D.C.; Halperin, F.; Foster, K.; Vernon, A.; Goldfine, A.B. Clinical and Patient-Centered Outcomes in Obese Patients With Type 2 Diabetes 3 Years After Randomization to Roux-en-Y Gastric Bypass Surgery Versus Intensive Lifestyle Management: The SLIMM-T2D Study. Diabetes Care 2018, 41, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Arterburn, D.E.; Westbrook, E.O.; Kuzma, J.N.; Stewart, S.D.; Chan, C.P.; Bock, S.N.; Landers, J.T.; Kratz, M.; Foster-Schubert, K.E.; et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: The CROSSROADS randomised controlled trial. Diabetologia 2016, 59, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; Gallagher, J.W.; Neiberg, R.H.; Eagleton, E.B.; DeLany, J.P.; Lang, W.; Punchai, S.; Gourash, W.; Jakicic, J.M. Bariatric Surgery vs Lifestyle Intervention for Diabetes Treatment: 5-Year Outcomes From a Randomized Trial. J. Clin. Endocrinol. Metab. 2020, 105, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; Belle, S.H.; Neiberg, R.H.; Pierson, S.K.; Eagleton, J.K.; Kalarchian, M.A.; DeLany, J.P.; Lang, W.; Jakicic, J.M. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015, 150, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. Jama 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef]
- Christou, N.V.; Sampalis, J.S.; Liberman, M.; Look, D.; Auger, S.; McLean, A.P.; MacLean, L.D. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann. Surg. 2004, 240, 416–423; discussion 423–414. [Google Scholar] [CrossRef]
- Adams, T.D.; Gress, R.E.; Smith, S.C.; Halverson, R.C.; Simper, S.C.; Rosamond, W.D.; Lamonte, M.J.; Stroup, A.M.; Hunt, S.C. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007, 357, 753–761. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Hong, D.; Lee, Y.; Tarride, J.E.; Paterson, J.M.; Anvari, M. Association Between Bariatric Surgery and All-Cause Mortality: A Population-Based Matched Cohort Study in a Universal Health Care System. Ann. Intern. Med. 2020, 173, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. JAMA 2019, 322, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, Y.; Wang, Y.; Wang, X.; An, Z.; Yu, X. Effect of bariatric surgery on long-term cardiovascular outcomes: A systematic review and meta-analysis of population-based cohort studies. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2022, 18, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
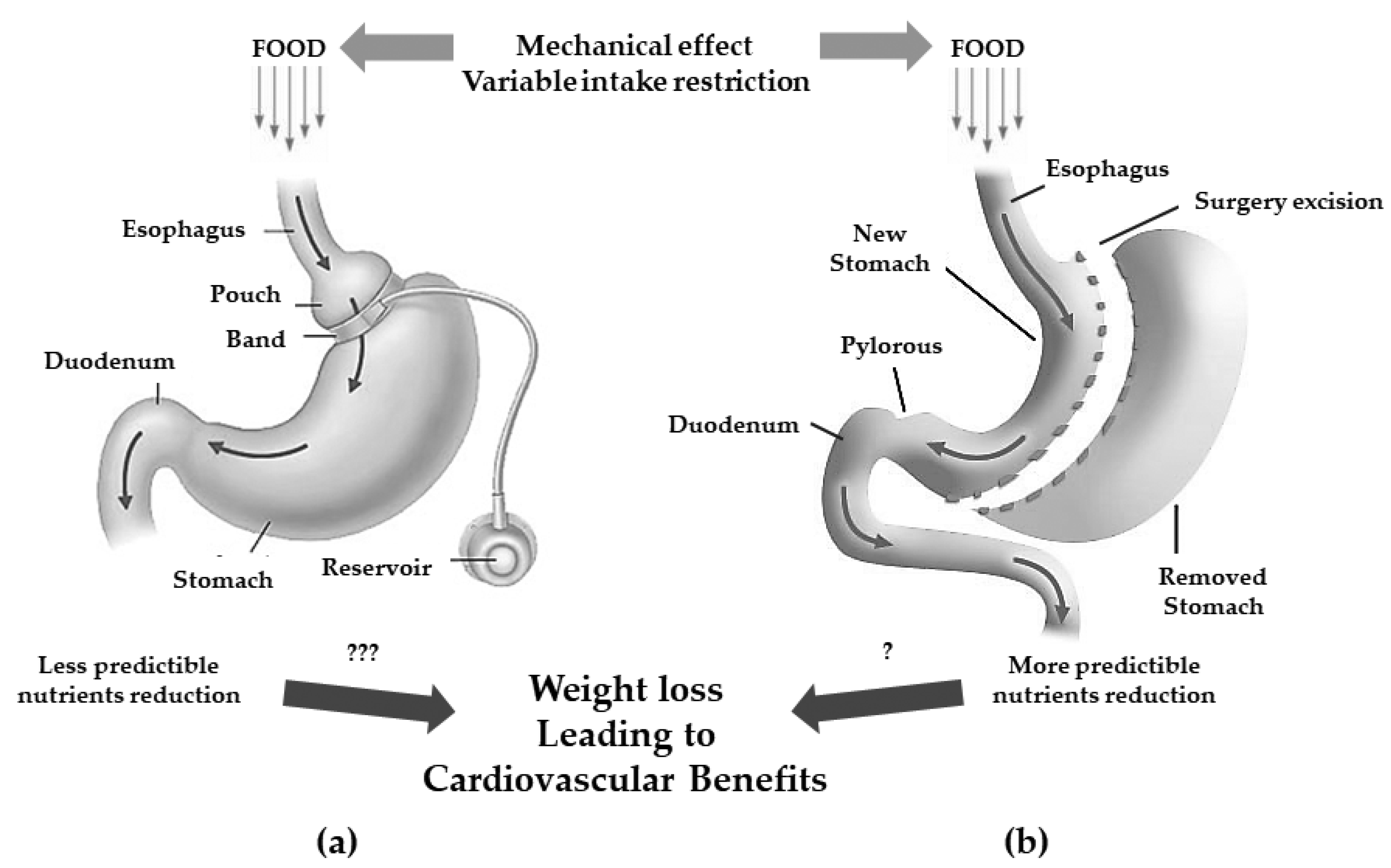
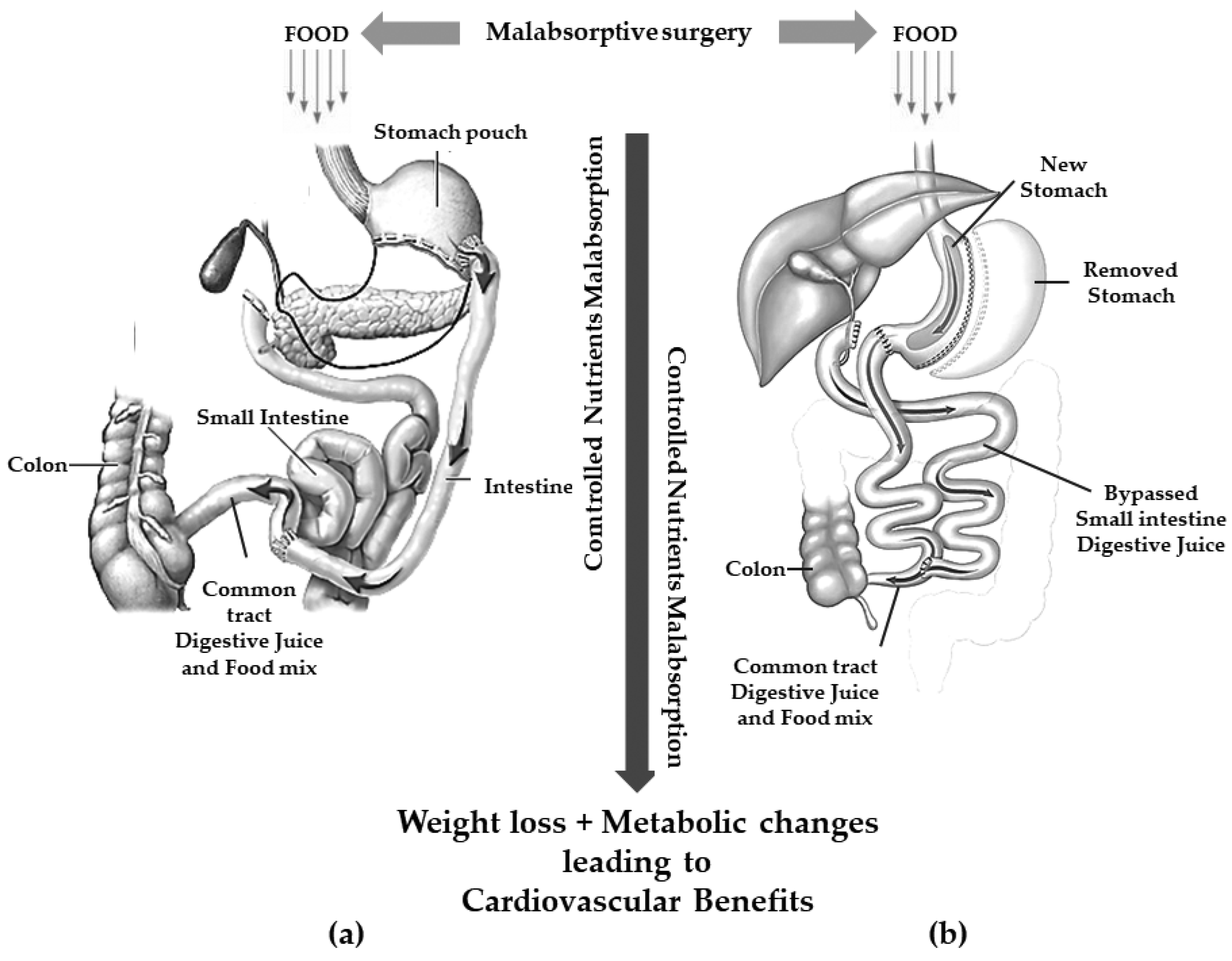
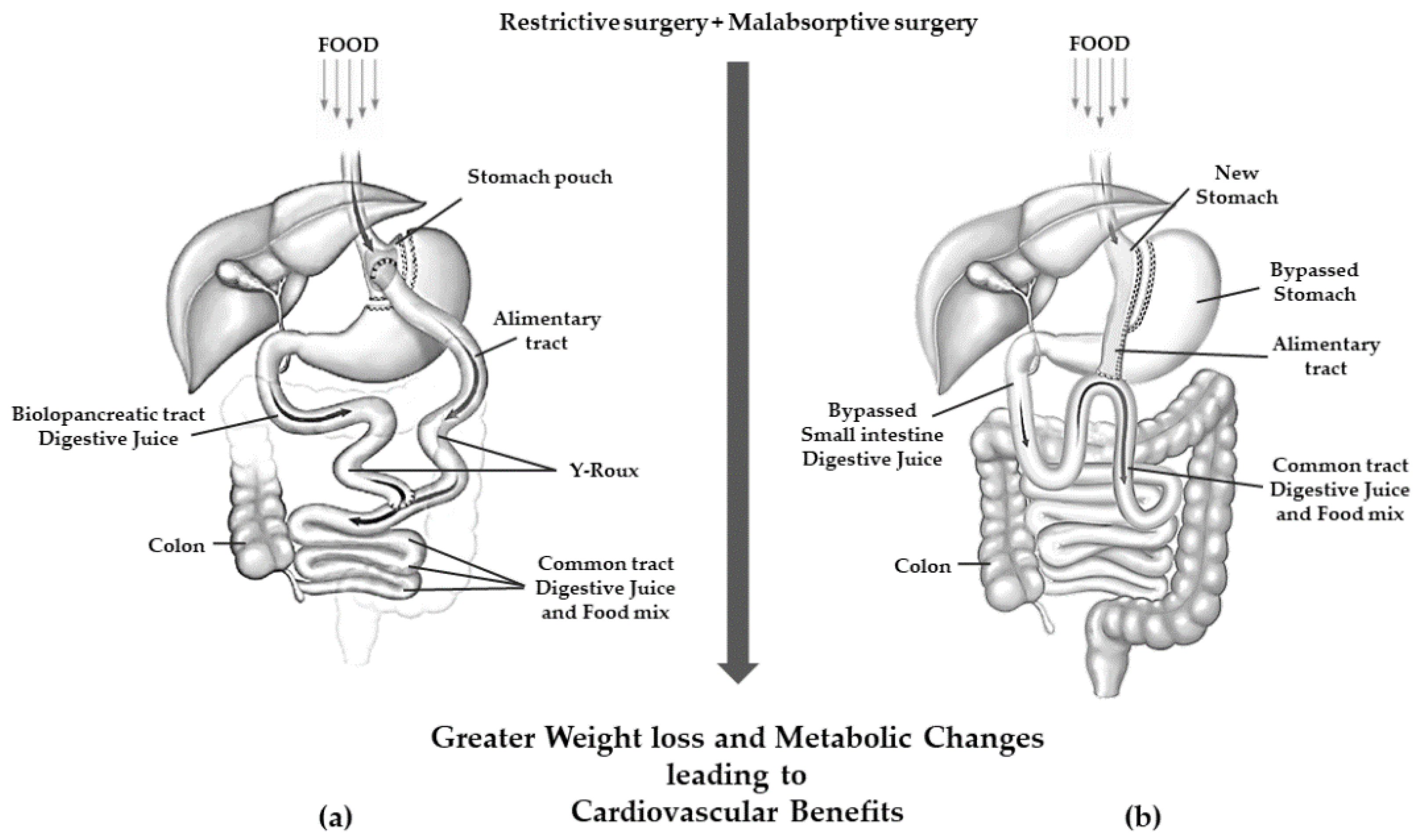
| MBS | Principal Mechanism of Action | Technical Aspects | Expected Weight Loss | Side Effects |
|---|---|---|---|---|
| AGB | Gastric restriction |
| 20–25% |
|
| SG | Gastric restriction |
| 25–30% |
|
| BPD and DS | Mainly malabsorptive |
| 35–45% |
|
| RYGB | Mixed restrictive and malabsorptive |
| 30–35% |
|
| OAGB | Mixed restrictive and malabsorptive |
| 35–45% |
|
| Molecule/Hormone | Change Direction | Final Physiologic Effect | Clinical Changes |
|---|---|---|---|
| Adiponectin | 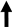 | Reduction in inflammatory state - Changes in total fat mass | Reduced risk of atherosclerosis |
| GLP-1 | 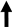 | Improvement of obesity-induced endothelial dysfunction - Restoration of the endothelial protective properties of HDL - Improvement of B-cell function and reduction of insulin resistance - Satiety induction - Inhibition of glucagon release | Decreased incidence of common carotid artery intima-media thickness augmentation - Improvement of T2DM - Facilitation of weight loss |
| Hsp60 | 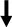 | Reduced inflammation | Reduced CVD risk |
| PRA, aldosterone, ACE activity, endothelin-1 | 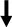 | Reduced vasoconstriction | Improvement in blood pressure control |
| ACE/ACE2 ratio, atrial natriuretic peptide, neprylisin | 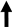 | Augmented vasodilatation | Improvement in blood pressure control |
| Succinate | 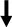 | Limitation of Krebs Cycle with a reduction in glucose production | Better T2DM control |
| PYY | 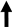 | Regulation of central appetite - Regulation of glucose homeostasis | Improvement of T2DM - Facilitation of weight loss |
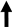 indicates molecule/hormone increase;
indicates molecule/hormone increase; 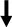 indicates molecule/hormone decrease.
indicates molecule/hormone decrease.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottino, R.; Carbone, A.; Formisano, T.; D’Elia, S.; Orlandi, M.; Sperlongano, S.; Molinari, D.; Castaldo, P.; Palladino, A.; Barbareschi, C.; et al. Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver? Life 2023, 13, 1552. https://doi.org/10.3390/life13071552
Bottino R, Carbone A, Formisano T, D’Elia S, Orlandi M, Sperlongano S, Molinari D, Castaldo P, Palladino A, Barbareschi C, et al. Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver? Life. 2023; 13(7):1552. https://doi.org/10.3390/life13071552
Chicago/Turabian StyleBottino, Roberta, Andreina Carbone, Tiziana Formisano, Saverio D’Elia, Massimiliano Orlandi, Simona Sperlongano, Daniele Molinari, Pasquale Castaldo, Alberto Palladino, Consiglia Barbareschi, and et al. 2023. "Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver?" Life 13, no. 7: 1552. https://doi.org/10.3390/life13071552
APA StyleBottino, R., Carbone, A., Formisano, T., D’Elia, S., Orlandi, M., Sperlongano, S., Molinari, D., Castaldo, P., Palladino, A., Barbareschi, C., Tolone, S., Docimo, L., & Cimmino, G. (2023). Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver? Life, 13(7), 1552. https://doi.org/10.3390/life13071552






