Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction
Abstract
1. Introduction
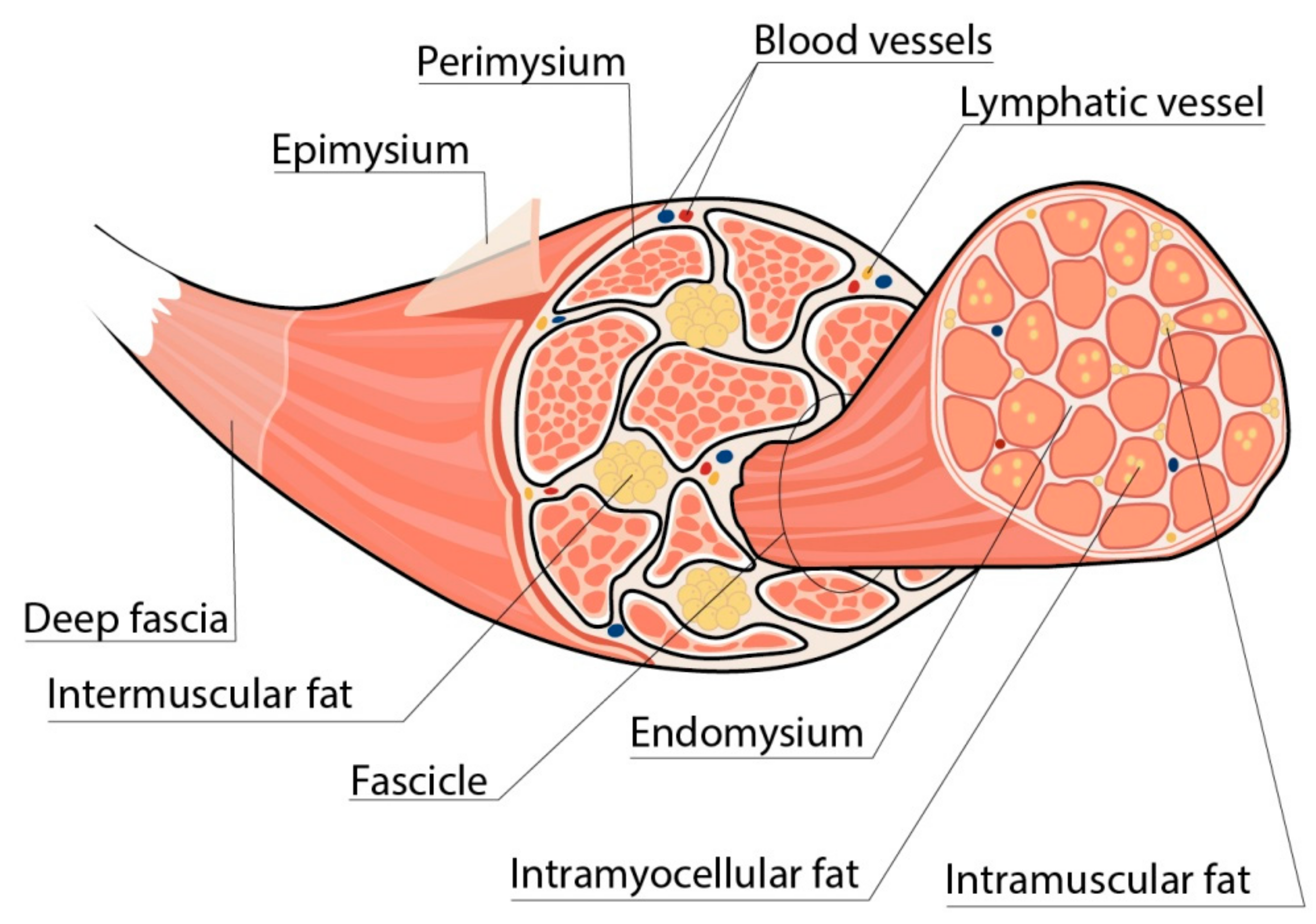
2. Skeletal Muscles in Obesity
2.1. Metabolic Properties of INTM Fat
2.1.1. Fibro-Adipogenic Progenitors
2.1.2. Other Cell Types as Possible Source of INTM Fat
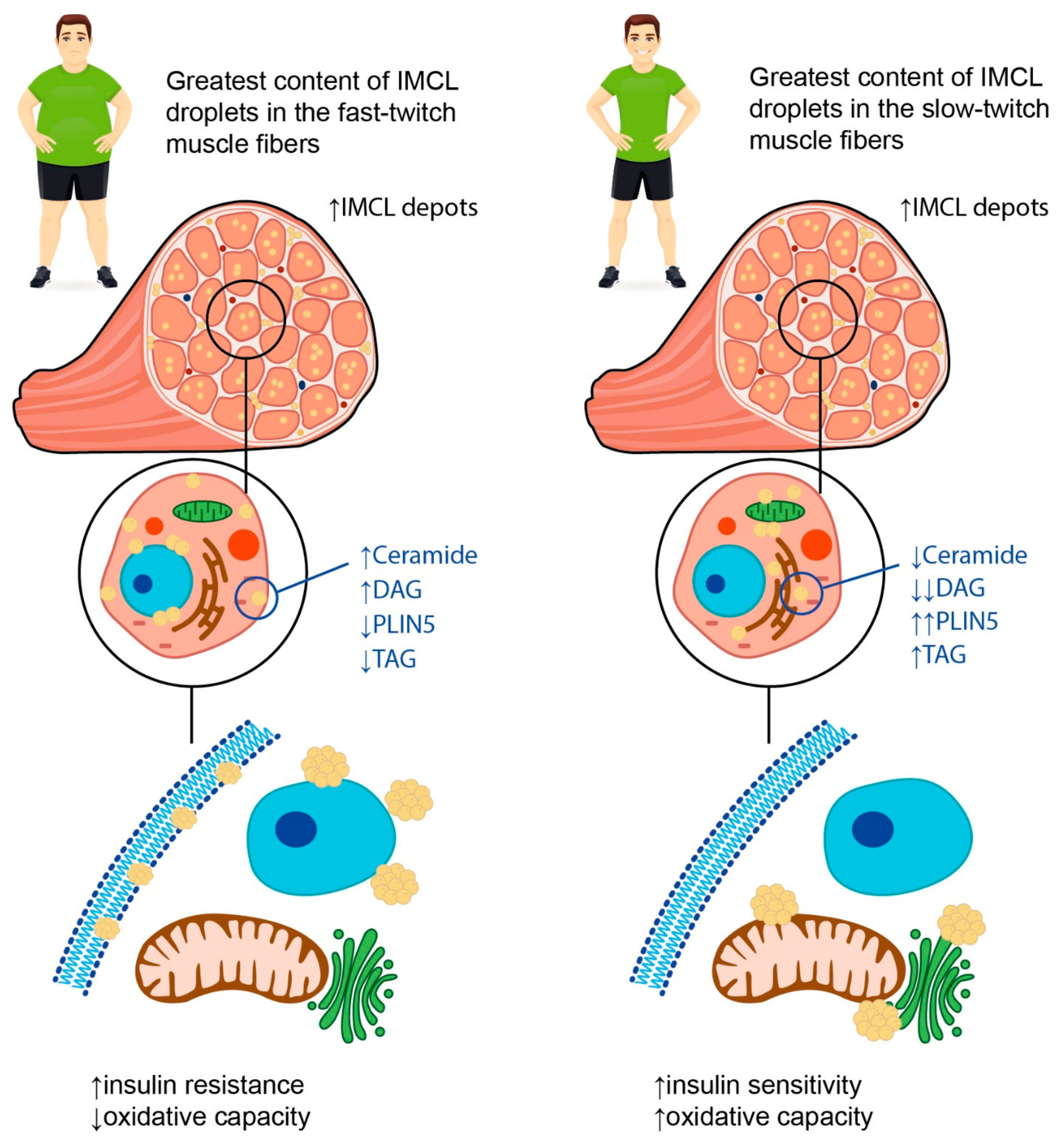
2.2. Intramyocellular Fat Depots
2.3. Skeletal Muscle Composition and Metabolic Flexibility in Obesity
2.4. Myokines in Obesity
2.4.1. Irisin
2.4.2. Myostatin
3. Steroids, Obesity, and Skeletal Muscles
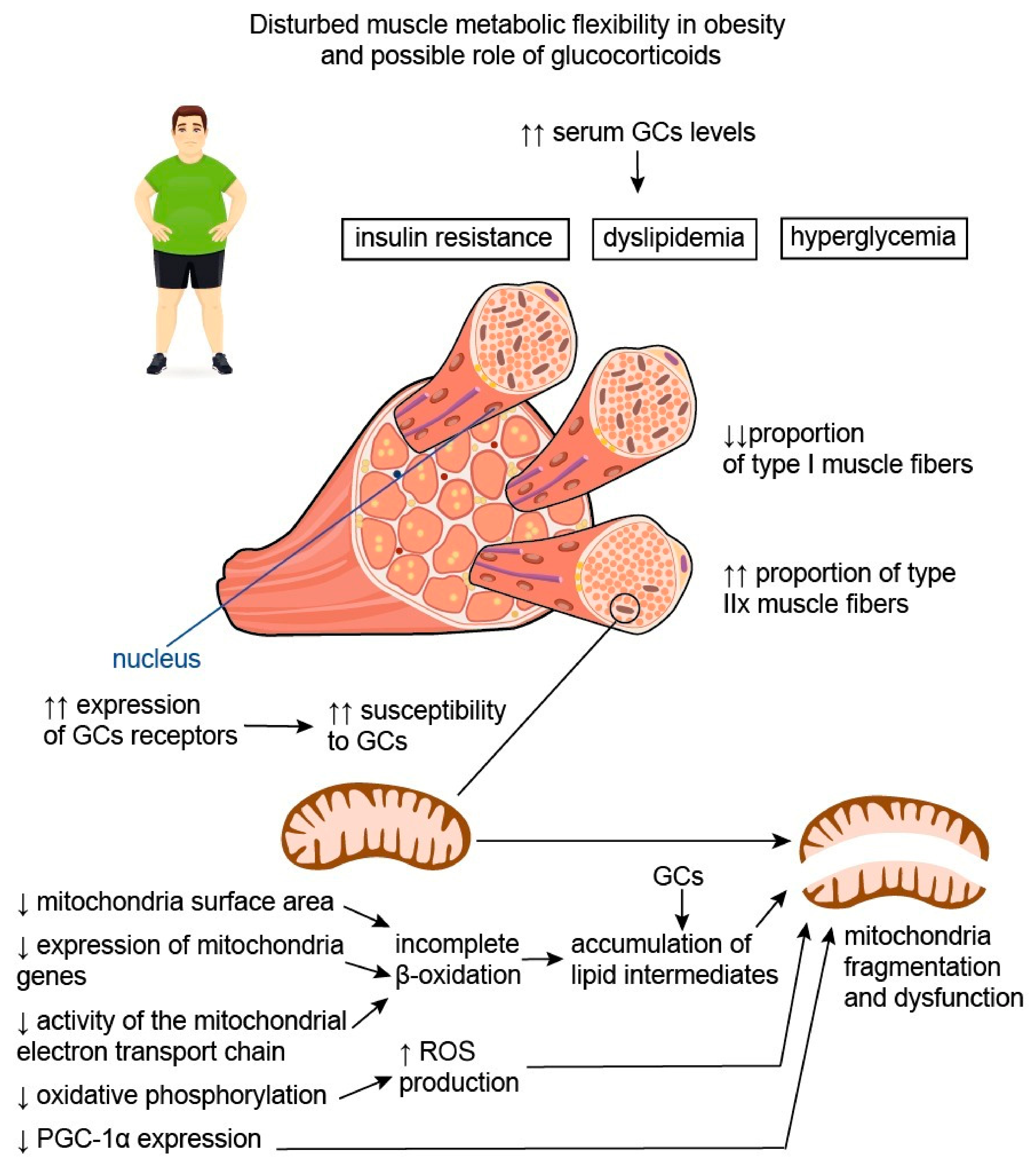
3.1. Glucocorticoids
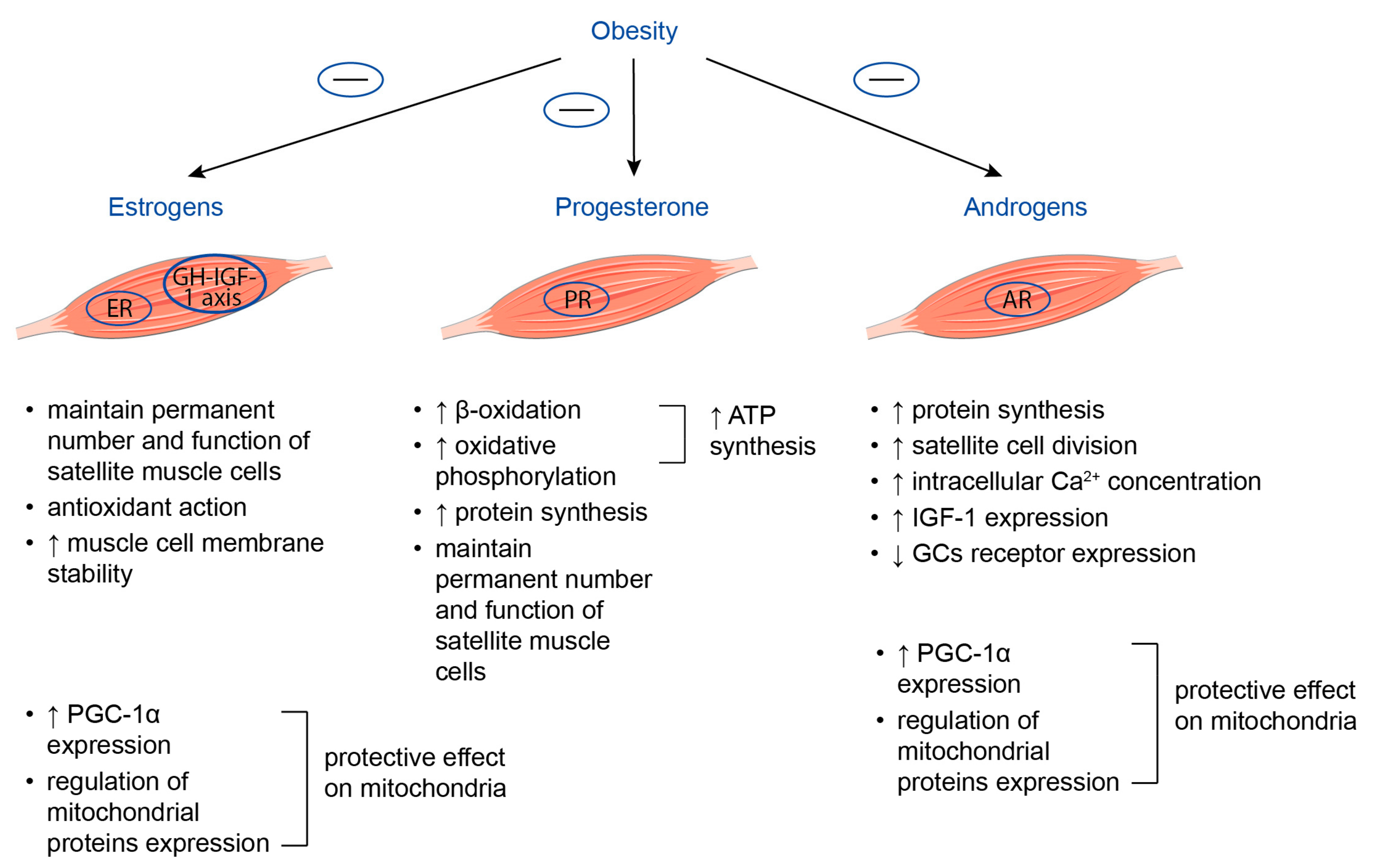
3.2. Sex Steroid Hormones
3.2.1. Estrogens
3.2.2. Progesterone
3.2.3. Androgens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Factsheets. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 April 2023).
- Frühbeck, G.; Yumuk, V. Obesity: A Gateway Disease with a Rising Prevalence. Obes. Facts 2014, 7, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Can. J. Kidney Health Dis. 2017, 4, 2054358117698669. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- Onyemaechi, N.; Anyanwu, G.; Obikili, E.; Onwuasoigwe, O.; Nwankwo, O. Impact of overweight and obesity on the musculoskeletal system using lumbosacral angles. Patient Prefer. Adherence 2016, 10, 291–296. [Google Scholar] [CrossRef]
- Blasco, B.V.; García-Jiménez, J.; Bodoano, I.; Gutiérrez-Rojas, L. Obesity and Depression: Its Prevalence and Influence as a Prognostic Factor: A Systematic Review. Psychiatry Investig. 2020, 17, 715–724. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- da Silva, T.L.; Nogueira, V.d.S.C.S.; Mulder, A.P. Sarcopenia and poor muscle quality associated with severe obesity in young adults and middle-aged adults. Clin. Nutr. ESPEN 2021, 45, 299–305. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. J. Nutr. Health Aging 2015, 20, 958–963. [Google Scholar] [CrossRef]
- Bauer, J.M.; Cruz-Jentoft, A.J.; Fielding, R.A.; Kanis, J.A.; Reginster, J.-Y.; Bruyère, O.; Cesari, M.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; et al. Is There Enough Evidence for Osteosarcopenic Obesity as a Distinct Entity? A Critical Literature Review. Calcif. Tissue Int. 2019, 105, 109–124, 125–126. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Sayın, S.; Kutlu, R.; Kulaksızoğlu, M. The relationship between sex steroids, insulin resistance and body compositions in obese women: A case-control study. J. Med. Biochem. 2020, 39, 25–31. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Guo, Y.; Wang, F.; Zhou, C.-L.; Tang, C.-Y.; Tang, H.-N.; Yan, D.-W.; Zhou, H.-D. Association of Sex Hormones and Fat Distribution in Men with Different Obese and Metabolic Statuses. Int. J. Gen. Med. 2022, 15, 1225–1238. [Google Scholar] [CrossRef]
- Loh, N.Y.; Humphreys, E.; Karpe, F.; Tomlinson, J.W.; Noordam, R.; Christodoulides, C. Sex hormones, adiposity, and metabolic traits in men and women: A Mendelian randomisation study. Eur. J. Endocrinol. 2022, 186, 407–416. [Google Scholar] [CrossRef]
- Chung, H.S.; Choi, K.M. Organokines in disease. Adv. Clin. Chem. 2020, 94, 261–321. [Google Scholar] [CrossRef]
- Lőrincz, H.; Somodi, S.; Ratku, B.; Harangi, M.; Paragh, G. Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity. Metabolites 2023, 13, 270. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Straight, C.R.; Toth, M.J.; Miller, M.S. Current perspectives on obesity and skeletal muscle contractile function in older adults. J. Appl. Physiol. 2021, 130, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Marcus, R.L.; LaStayo, P.C.; Ryan, A.S. Intermuscular Fat: A Review of the Consequences and Causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. A 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Bergman, B.C.; Brennan, A.M.; Sparks, L.M. Intermuscular adipose tissue in metabolic disease. Nat. Rev. Endocrinol. 2023, 19, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Sachs, S.; Zarini, S.; Kahn, D.E.; Harrison, K.A.; Perreault, L.; Phang, T.; Newsom, S.; Strauss, A.; Kerege, A.; Schoen, J.A.; et al. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E866–E879. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, H.; Wang, T.; Xia, Y.; Jin, L.; Jiang, A.; Zhu, L.; Chen, L.; Li, R.; Li, X. Co-methylated Genes in Different Adipose Depots of Pig are Associated with Metabolic, Inflammatory and Immune Processes. Int. J. Biol. Sci. 2012, 8, 831–837. [Google Scholar] [CrossRef]
- Kahn, D.E.; Bergman, B.C. Keeping It Local in Metabolic Disease: Adipose Tissue Paracrine Signaling and Insulin Resistance. Diabetes 2022, 71, 599–609. [Google Scholar] [CrossRef]
- Lyu, K.; Zhang, D.; Song, J.D.; Li, X.; Perry, R.J.; Samuel, V.T.; Shulman, G.I. Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol—PKCε—Insulin receptor T1160 phosphorylation. J. Clin. Investig. 2021, 6, e139946. [Google Scholar] [CrossRef]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’alessandro, A.; Jackman, M.R.; et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. J. Clin. Investig. 2018, 3, e96805. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Giuliani, G.; Vumbaca, S.; Fuoco, C.; Gargioli, C.; Giorda, E.; Massacci, G.; Palma, A.; Reggio, A.; Riccio, F.; Rosina, M.; et al. SCA-1 micro-heterogeneity in the fate decision of dystrophic fibro/adipogenic progenitors. Cell Death Dis. 2021, 12, 122. [Google Scholar] [CrossRef]
- Marinkovic, M.; Fuoco, C.; Sacco, F.; Perpetuini, A.C.; Giuliani, G.; Micarelli, E.; Pavlidou, T.; Petrilli, L.L.; Reggio, A.; Riccio, F.; et al. Fibro-adipogenic progenitors of dystrophic mice are insensitive to NOTCH regulation of adipogenesis. Life Sci. Alliance 2019, 2, e201900437. [Google Scholar] [CrossRef]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; De Bardi, M.; Bicciato, S.; et al. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat. Commun. 2018, 9, 3670. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Chen, Z.; Mitch, W.E.; Zhang, L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int. 2017, 91, 119–128. [Google Scholar] [CrossRef]
- Nachit, M.; Leclercq, I.A. Emerging awareness on the importance of skeletal muscle in liver diseases: Time to dig deeper into mechanisms! Clin. Sci. 2019, 133, 465–481. [Google Scholar] [CrossRef]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.K.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef]
- Uezumi, A.; Ikemoto-Uezumi, M.; Zhou, H.; Kurosawa, T.; Yoshimoto, Y.; Nakatani, M.; Hitachi, K.; Yamaguchi, H.; Wakatsuki, S.; Araki, T.; et al. Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia. J. Clin. Investig. 2021, 131, e139617. [Google Scholar] [CrossRef]
- Collao, N.; Farup, J.; De Lisio, M. Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors. Front. Cell Dev. Biol. 2020, 8, 9. [Google Scholar] [CrossRef]
- Kaur, G.; Davies, M.R.; Liu, X.; Feeley, B.T. The Role of Fibro-Adipogenic Progenitors in Musculoskeletal Disease. Muscles Ligaments Tendons J. 2021, 11, 201–214. [Google Scholar] [CrossRef]
- Moratal, C.; Raffort, J.; Arrighi, N.; Rekima, S.; Schaub, S.; Dechesne, C.A.; Chinetti, G.; Dani, C. IL-1β- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci. Rep. 2018, 8, 17005. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, N.; Moratal, C.; Clément, N.; Giorgetti-Peraldi, S.; Peraldi, P.; Loubat, A.; Kurzenne, J.-Y.; Dani, C.; Chopard, A.; Dechesne, A.C. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015, 6, e1733. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Rudnicki, M.A.; Komaki, M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Wada, M.R.; Inagawa-Ogashiwa, M.; Shimizu, S.; Yasumoto, S.; Hashimoto, N. Generation of different fates from multipotent muscle stem cells. Development 2002, 129, 2987–2995. [Google Scholar] [CrossRef]
- Guo, L.; Cui, H.; Zhao, G.; Liu, R.; Li, Q.; Zheng, M.; Guo, Y.; Wen, J. Intramuscular preadipocytes impede differentiation and promote lipid deposition of muscle satellite cells in chickens. BMC Genom. 2018, 19, 838. [Google Scholar] [CrossRef]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Majka, S.M.; Miller, H.L.; Sullivan, T.; Erickson, P.F.; Kong, R.; Weiser-Evans, M.; Nemenoff, R.; Moldovan, R.; Morandi, S.A.; Davis, J.A.; et al. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte 2012, 1, 215–229. [Google Scholar] [CrossRef]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal Muscle Lipid Content and Insulin Resistance: Evidence for a Paradox in Endurance-Trained Athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Strauss, A.; Bacon, S.; Kerege, A.; Harrison, K.; Brozinick, J.T.; Hunerdosse, D.M.; Playdon, M.C.; Holmes, W.; et al. Intramuscular triglyceride synthesis: Importance in muscle lipid partitioning in humans. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E152–E164. [Google Scholar] [CrossRef]
- Belzunce, M.A.; Henckel, J.; Di Laura, A.; Hart, A. Intramuscular fat in gluteus maximus for different levels of physical activity. Sci. Rep. 2021, 11, 21401. [Google Scholar] [CrossRef] [PubMed]
- Gemmink, A.; Daemen, S.; Brouwers, B.; Huntjens, P.R.; Schaart, G.; Moonen-Kornips, E.; Jörgensen, J.; Hoeks, J.; Schrauwen, P.; Hesselink, M.K.C. Dissociation of intramyocellular lipid storage and insulin resistance in trained athletes and type 2 diabetes patients; involvement of perilipin 5? J. Physiol. 2018, 596, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.; Perreault, L.; Macias, E.; Zarini, S.; Newsom, S.A.; Strauss, A.; Kerege, A.; Harrison, K.; Snell-Bergeon, J.; Bergman, B.C. Subcellular localisation and composition of intramuscular triacylglycerol influence insulin sensitivity in humans. Diabetologia 2021, 64, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Samjoo, I.A.; Hamadeh, M.J.; McCready, C.; Raha, S.; Watt, M.J.; Steinberg, G.R.; Tarnopolsky, M.A. Endurance Training Modulates Intramyocellular Lipid Compartmentalization and Morphology in Skeletal Muscle of Lean and Obese Women. J. Clin. Endocrinol. Metab. 2013, 98, 4852–4862. [Google Scholar] [CrossRef]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice. Bosn. J. Basic Med. Sci. 2021, 21, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Thrush, A.B.; Dent, R.; McPherson, R.; Harper, M.-E. Implications of mitochondrial uncoupling in skeletal muscle in the development and treatment of obesity. FEBS J. 2013, 280, 5015–5029. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Motohashi, N.; Minegishi, K.; Imamura, M.; Aoki, Y. Techniques for Injury, Cell Transplantation, and Histological Analysis in Skeletal Muscle. Methods Mol. Biol. 2023, 2640, 193–205. [Google Scholar] [CrossRef]
- Damer, A.; El Meniawy, S.; McPherson, R.; Wells, G.; Harper, M.; Dent, R. Association of muscle fiber type with measures of obesity: A systematic review. Obes. Rev. 2022, 23, e13444. [Google Scholar] [CrossRef]
- Sung, E.-S.; Han, A.; Hinrichs, T.; Vorgerd, M.; Platen, P. Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women. Int. J. Environ. Res. Public Health 2022, 19, 9789. [Google Scholar] [CrossRef]
- Nomikos, T.; Methenitis, S.; Panagiotakos, D.B. The emerging role of skeletal muscle as a modulator of lipid profile the role of exercise and nutrition. Lipids Health Dis. 2022, 21, 81. [Google Scholar] [CrossRef]
- Reiter, D.A.; Bellissimo, M.P.; Zhou, L.; Boebinger, S.; Wells, G.D.; Jones, D.P.; Ziegler, T.R.; Alvarez, J.A.; Fleischer, C.C. Increased Adiposity is Associated with Altered Skeletal Muscle Energetics. J. Appl. Physiol. 2023, 134, 1083–1092. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Randle, P.; Garland, P.; Hales, C.; Newsholme, E. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Barakati, N.; Bustos, R.Z.; Coletta, D.K.; Langlais, P.R.; Kohler, L.N.; Luo, M.; Funk, J.L.; Willis, W.T.; Mandarino, L.J. Fuel Selection in Skeletal Muscle Exercising at Low Intensity; Reliance on Carbohydrate in Very Sedentary Individuals. Metab. Syndr. Relat. Disord. 2023, 21, 16–24. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Hooks, B.G.; McPherson, R.; Dent, R.R.; Harper, M.-E. Targeting skeletal muscle mitochondrial health in obesity. Clin. Sci. 2022, 136, 1081–1110. [Google Scholar] [CrossRef]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef]
- Zhelankin, A.V.; Iulmetova, L.N.; Ahmetov, I.I.; Generozov, E.V.; Sharova, E.I. Diversity and Differential Expression of MicroRNAs in the Human Skeletal Muscle with Distinct Fiber Type Composition. Life 2023, 13, 659. [Google Scholar] [CrossRef]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef] [PubMed]
- Sarparanta, J.; Garcia-Macia, M.; Singh, R. Autophagy and Mitochondria in Obesity and Type 2 Diabetes. Curr. Diabetes Rev. 2017, 13, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Roszczyc-Owsiejczuk, K.; Zabielski, P. Sphingolipids as a Culprit of Mitochondrial Dysfunction in Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 635175. [Google Scholar] [CrossRef]
- Kobayashi, M.; Deguchi, Y.; Nozaki, Y.; Higami, Y. Contribution of PGC-1α to Obesity- and Caloric Restriction-Related Physiological Changes in White Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 6025. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; LeBrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal Muscle Fiber-type Switching, Exercise Intolerance, and Myopathy in PGC-1α Muscle-specific Knock-out Animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Ritov, V.B.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Coen, P.M.; Goodpaster, B.H. Calorie Restriction-induced Weight Loss and Exercise Have Differential Effects on Skeletal Muscle Mitochondria Despite Similar Effects on Insulin Sensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 81–87. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Wei, W.-P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.-Q. Relationship between circulating irisin levels and overweight/obesity: A meta-analysis. World J. Clin. Cases 2019, 7, 1444–1455. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In Vivo and In Vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Gamas, L.; Matafome, P.; Seiça, R. Irisin and Myonectin Regulation in the Insulin Resistant Muscle: Implications to Adipose Tissue: Muscle Crosstalk. J. Diabetes Res. 2015, 2015, 359159. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar] [CrossRef]
- Yin, J.; Yang, S.; Zha, X.; Miao, Z.; Sheng, C.; Yang, P.; Wang, X.; Qu, S. The Association of Serum Irisin with Impaired Glucose Before and After Laparoscopic Sleeve Gastrectomy in Obesity. Obes. Surg. 2023, 33, 780–788. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Chen, F.; Mitch, E.W.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Salama, A.; Amin, M.M.; Hassan, A. Effects of oleic acid and/or exercise on diet-induced thermogenesis and obesity in rats: Involvement of beige adipocyte differentiation and macrophage M1 inhibition. Res. Pharm. Sci. 2023, 18, 219–230. [Google Scholar] [CrossRef]
- Bonnieu, A.; Carnac, G.; Vernus, B. Myostatin in the Pathophysiology of Skeletal Muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar] [CrossRef]
- Consitt, L.; Clark, B. The vicious cycle of myostatin signaling in sarcopenic obesity: Myostatin role in skeletal muscle growth, insulin signaling and implications for clinical trials. J. Frailty Aging 2017, 7, 21–27. [Google Scholar] [CrossRef]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jürets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum Myostatin is Upregulated in Obesity and Correlates with Insulin Resistance in Humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar] [CrossRef]
- Allen, D.L.; Hittel, D.S.; McPherron, A. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Masuda, S.; Ge, X.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 2015, 55, 183–193, Erratum in: Diabetologia 2015, 58, 643. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin Inhibition in Muscle, but Not Adipose Tissue, Decreases Fat Mass and Improves Insulin Sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Li, G.; Blumenthal, J.; Ortmeyer, H. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity 2012, 21, 1350–1356. [Google Scholar] [CrossRef]
- Perry, C.A.; Van Guilder, G.P.; Butterick, T.A. Decreased myostatin in response to a controlled DASH diet is associated with improved body composition and cardiometabolic biomarkers in older adults: Results from a controlled-feeding diet intervention study. BMC Nutr. 2022, 8, 24. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Cong, L.; Chung, J.P.W.; Li, T.C.; Chan, D.Y.L. Myostatin: A multifunctional role in human female reproduction and fertility—A short review. Reprod. Biol. Endocrinol. 2022, 20, 96. [Google Scholar] [CrossRef]
- Fang, L.; Yan, Y.; Wang, S.; Guo, Y.; Li, Y.; Jia, Q.; Han, X.; Liu, B.; Cheng, J.-C.; Sun, Y.-P. High ovarian GDF-8 levels contribute to elevated estradiol production in ovarian hyperstimulation syndrome by stimulating aromatase expression. Int. J. Biol. Sci. 2021, 17, 2338–2347. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.; Li, Y.; Yu, Y.; Li, Y.; Yan, Y.; Cheng, J.-C.; Sun, Y.-P. High GDF-8 in follicular fluid is associated with a low pregnancy rate in IVF patients with PCOS. Reproduction 2020, 160, 11–19. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef]
- Shirif, A.Z.; Kovačević, S.; Brkljačić, J.; Teofilović, A.; Elaković, I.; Djordjevic, A.; Matić, G. Decreased Glucocorticoid Signaling Potentiates Lipid-Induced Inflammation and Contributes to Insulin Resistance in the Skeletal Muscle of Fructose-Fed Male Rats Exposed to Stress. Int. J. Mol. Sci. 2021, 22, 7206. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, CD003725. [Google Scholar] [CrossRef]
- Perpetuini, A.C.; Giuliani, G.; Reggio, A.; Cerretani, M.; Santoriello, M.; Stefanelli, R.; Palma, A.; Vumbaca, S.; Harper, S.; Castagnoli, L.; et al. Janus effect of glucocorticoids on differentiation of muscle fibro/adipogenic progenitors. Sci. Rep. 2020, 10, 5363. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Salamone, I.M.; Page, P.G.; Warner, J.L.; Demonbreun, A.R.; McNally, E.M. Intermittent Glucocorticoid Dosing Improves Muscle Repair and Function in Mice with Limb-Girdle Muscular Dystrophy. Am. J. Pathol. 2017, 187, 2520–2535, Erratum in: Am. J. Pathol. 2021, 191, 2039. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Zelikovich, A.S.; Salamone, I.M.; Fischer, J.A.; McNally, E.M. Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, 39–52. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Barefield, D.; Warner, J.L.; Vo, A.H.; Hadhazy, M.; Earley, J.U.; Demonbreun, A.; McNally, E.M. Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J. Clin. Investig. 2017, 127, 2418–2432. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Magdalena, P.A. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef]
- Braun, T.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Webster, J.M.; Waaijenberg, K.; van de Worp, W.R.; Kelders, M.C.; Lambrichts, S.; Martin, C.; Verhaegen, F.; van der Heyden, B.; Smith, C.; Lavery, G.G.; et al. 11β-HSD1 determines the extent of muscle atrophy in a model of acute exacerbation of COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L400–L412. [Google Scholar] [CrossRef]
- Webster, J.M.; Sagmeister, M.S.; Fenton, C.G.; Seabright, A.P.; Lai, Y.-C.; Jones, S.W.; Filer, A.; Cooper, M.S.; Lavery, G.G.; Raza, K.; et al. Global Deletion of 11β-HSD1 Prevents Muscle Wasting Associated with Glucocorticoid Therapy in Polyarthritis. Int. J. Mol. Sci. 2021, 22, 7828. [Google Scholar] [CrossRef]
- Lee, M.-K.; Jeong, H.H.; Kim, M.-J.; Ryu, H.; Baek, J.; Lee, B. Nutrients against Glucocorticoid-Induced Muscle Atrophy. Foods 2022, 11, 687. [Google Scholar] [CrossRef]
- Sato, A.; Richardson, D.; Cregor, M.; Davis, H.M.; Au, E.; McAndrews, K.; Zimmers, T.A.; Organ, J.; Peacock, M.; Plotkin, L.I.; et al. Glucocorticoids Induce Bone and Muscle Atrophy by Tissue-Specific Mechanisms Upstream of E3 Ubiquitin Ligases. Endocrinology 2017, 158, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Salamone, I.M.; Quattrocelli, M.; Barefield, D.Y.; Page, P.G.; Tahtah, I.; Hadhazy, M.; Tomar, G.; McNally, E.M. Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms. J. Clin. Investig. 2022, 132, e149828. [Google Scholar] [CrossRef] [PubMed]
- Zumbaugh, M.D.; Johnson, E.S.; Shi, T.H.; Gerrard, E.D. Molecular and biochemical regulation of skeletal muscle metabolism. J. Anim. Sci. 2022, 100, skac035. [Google Scholar] [CrossRef]
- Maher, A.C.; Fu, M.H.; Isfort, R.J.; Varbanov, A.R.; Qu, X.A.; Tarnopolsky, M.A. Sex Differences in Global mRNA Content of Human Skeletal Muscle. PLoS ONE 2009, 4, e6335. [Google Scholar] [CrossRef]
- Della Peruta, C.; Lozanoska-Ochser, B.; Renzini, A.; Moresi, V.; Riera, C.S.; Bouché, M.; Coletti, D. Sex Differences in Inflammation and Muscle Wasting in Aging and Disease. Int. J. Mol. Sci. 2023, 24, 4651. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Amaral, I.M.; Canas, P.M.; Cunha, R.A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci. Rep. 2018, 8, 10742. [Google Scholar] [CrossRef]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef]
- Manzano, R.; Toivonen, J.M.; Calvo, A.C.; Miana-Mena, F.J.; Zaragoza, P.; Muñoz, M.J.; Montarras, D.; Osta, R. Sex, fiber-type, and age dependent in vitro proliferation of mouse muscle satellite cells. J. Cell. Biochem. 2011, 112, 2825–2836. [Google Scholar] [CrossRef]
- Sesillo, F.B.; Rajesh, V.; Wong, M.; Duran, P.; Rudell, J.B.; Rundio, C.P.; Baynes, B.B.; Laurent, L.C.; Sacco, A.; Christman, K.L.; et al. Muscle stem cells and fibro-adipogenic progenitors in female pelvic floor muscle regeneration following birth injury. npj Regen. Med. 2022, 7, 72. [Google Scholar] [CrossRef]
- Wu, R.-Y.; Sung, W.-H.; Cheng, H.-C.; Yeh, H.-J. Investigating the rate of skeletal muscle atrophy in men and women in the intensive care unit: A prospective observational study. Sci. Rep. 2022, 12, 16629. [Google Scholar] [CrossRef]
- de Jong, J.C.; Attema, B.J.; van der Hoek, M.D.; Verschuren, L.; Caspers, M.P.; Kleemann, R.; van der Leij, F.R.; Hoek, A.M.V.D.; Nieuwenhuizen, A.G.; Keijer, J. Sex differences in skeletal muscle-aging trajectory: Same processes, but with a different ranking. Geroscience 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Shu, Y.; Xia, J.; Yu, Q.; Wang, G.; Zhang, J.; He, J.; Wang, H.; Zhang, L.; Wu, H. Integrated analysis of mRNA and miRNA expression profiles reveals muscle growth differences between adult female and male Chinese concave-eared frogs (Odorrana tormota). Gene 2018, 678, 241–251. [Google Scholar] [CrossRef]
- Ogawa, M.; Kitano, T.; Kawata, N.; Sugihira, T.; Kitakaze, T.; Harada, N.; Yamaji, R. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J. Nutr. Biochem. 2017, 49, 63–70. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef]
- Alexander, S.E.; Pollock, A.C.; Lamon, S. The effect of sex hormones on skeletal muscle adaptation in females. Eur. J. Sport Sci. 2022, 22, 1035–1045. [Google Scholar] [CrossRef]
- Huang, L.-T.; Wang, J.-H. The Therapeutic Intervention of Sex Steroid Hormones for Sarcopenia. Front. Med. 2021, 8, 739251. [Google Scholar] [CrossRef]
- Pellegrino, A.; Tiidus, P.M.; Vandenboom, R. Mechanisms of Estrogen Influence on Skeletal Muscle: Mass, Regeneration, and Mitochondrial Function. Sports Med. 2022, 52, 2853–2869. [Google Scholar] [CrossRef]
- Kamwa, V.; Welch, C.; Hassan-Smith, Z.K. The endocrinology of sarcopenia and frailty. Minerva Endocrinol. 2021, 46, 453–468. [Google Scholar] [CrossRef]
- Weissberger, A.J.; Ho, K.K.Y.; Lazarus, L. Contrasting Effects of Oral and Transdermal Routes of Estrogen Replacement Therapy on 24-Hour Growth Hormone (GH) Secretion, Insulin-Like Growth Factor I, and GH-Binding Protein in Postmenopausal Women. J. Clin. Endocrinol. Metab. 1991, 72, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.C.; Curran, E.M.; Judy, B.M.; Lubahn, D.B.; Estes, D.M. Estrogen receptor-α deficiency promotes increased TNF-α secretion and bacterial killing by murine macrophages in response to microbial stimuli in vitro. J. Leukoc. Biol. 2004, 75, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Reid, M.B. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1165–R1170. [Google Scholar] [CrossRef]
- Sørensen, M.B.; Rosenfalck, A.M.; Højgaard, L.; Ottesen, B. Obesity and Sarcopenia after Menopause Are Reversed by Sex Hormone Replacement Therapy. Obes. Res. 2001, 9, 622–626. [Google Scholar] [CrossRef]
- Shaia, K.L.; Harris, B.S.; Selter, J.H.; Price, T.M. Reproductive Functions of the Mitochondrial Progesterone Receptor (PR-M). Reprod. Sci. 2023, 30, 1443–1452. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef]
- Smith, G.I.; Yoshino, J.; Reeds, D.N.; Bradley, D.; Burrows, R.E.; Heisey, H.D.; Moseley, A.C.; Mittendorfer, B. Testosterone and Progesterone, But Not Estradiol, Stimulate Muscle Protein Synthesis in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 256–265. [Google Scholar] [CrossRef]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.-K.; Laakkonen, E.K.; Sipilä, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381.e6. [Google Scholar] [CrossRef]
- Whynott, R.M.; Summers, K.M.; Jakubiak, M.; Van Voorhis, B.J.; Mejia, R.B. The effect of weight and body mass index on serum progesterone values and live birth rate in cryopreserved in vitro fertilization cycles. F&S Rep. 2021, 2, 195–200. [Google Scholar] [CrossRef]
- Bellver, J.; Rodríguez-Varela, C.; Brandão, P.; Labarta, E. Serum progesterone concentrations are reduced in obese women on the day of embryo transfer. Reprod. Biomed. Online 2022, 45, 679–687. [Google Scholar] [CrossRef]
- Oh, H.; Wild, R.A.; Manson, J.E.; Bea, J.W.; Shadyab, A.H.; Pfeiffer, R.M.; Saquib, N.; Underland, L.; Anderson, G.L.; Xu, X.; et al. Obesity, Height, and Serum Androgen Metabolism among Postmenopausal Women in the Women’s Health Initiative Observational Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2018–2029. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Tipton, K.D.; Doyle, D.; Phillips, S.M.; Cortiella, J.; Wolfe, R.R.; Rossetti, M.L.; Fukuda, D.H.; Gordon, B.S.; Mumford, P.W.; et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am. J. Physiol. Metab. 1998, 275, E864–E871. [Google Scholar] [CrossRef]
- Deane, C.; Hughes, D.; Sculthorpe, N.; Lewis, M.; Stewart, C.; Sharples, A.P. Impaired hypertrophy in myoblasts is improved with testosterone administration. J. Steroid Biochem. Mol. Biol. 2013, 138, 152–161. [Google Scholar] [CrossRef]
- Zeng, F.; Zhao, H.; Liao, J. Androgen interacts with exercise through the mTOR pathway to induce skeletal muscle hypertrophy. Biol. Sport 2017, 34, 313–321. [Google Scholar] [CrossRef]
- Kochakian, C.D.; Welder, A.A. Anabolic-androgenic steroids: In cell culture. Vitr. Cell. Dev. Biol. Anim. 1993, 29, 433–438. [Google Scholar] [CrossRef]
- Estrada, M.; Espinosa, A.; Müller, M.; Jaimovich, E. Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases Via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Rossetti, M.L.; Steiner, J.L.; Gordon, B.S. Increased mitochondrial turnover in the skeletal muscle of fasted, castrated mice is related to the magnitude of autophagy activation and muscle atrophy. Mol. Cell. Endocrinol. 2018, 473, 178–185. [Google Scholar] [CrossRef]
- Rossetti, M.L.; Gordon, B.S. The role of androgens in the regulation of muscle oxidative capacity following aerobic exercise training. Appl. Physiol. Nutr. Metab. 2017, 42, 1001–1007. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lou, S.; Shi, R. From mitochondria to sarcopenia: Role of 17β-estradiol and testosterone. Front. Endocrinol. 2023, 14, 1156583. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Males | Females | Reference |
|---|---|---|---|
| Muscle fiber type | More glycolytic fibers (fast-twitch) compared to females | More oxidative fibers (low-twitch) compared to males | [118,120] |
| Satellite cells | Greater number of satellite cells and higher proliferation capacity compared to males | Less number of satellite cells and reduced proliferation capacity compared to females | [121,122] |
| Susceptibility to atrophy | More susceptible to inflammation-induced atrophy | More susceptible to disuse-induced atrophy | [123,124] |
| Predominant mechanism of protein degradation | Autophagy | Ubiquitin–proteasome system | [125,126,127] |
| Age-related shift in myofiber types | Towards type 1 (slow-twitch) dominant composition | No shift occurs | [124] |
| Top-ranked differentially expressed process during aging | Oxidative phosphorylation | Cell growth | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheptulina, A.F.; Antyukh, K.Y.; Kiselev, A.R.; Mitkovskaya, N.P.; Drapkina, O.M. Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction. Life 2023, 13, 1415. https://doi.org/10.3390/life13061415
Sheptulina AF, Antyukh KY, Kiselev AR, Mitkovskaya NP, Drapkina OM. Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction. Life. 2023; 13(6):1415. https://doi.org/10.3390/life13061415
Chicago/Turabian StyleSheptulina, Anna F., Karina Yu Antyukh, Anton R. Kiselev, Natalia P. Mitkovskaya, and Oxana M. Drapkina. 2023. "Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction" Life 13, no. 6: 1415. https://doi.org/10.3390/life13061415
APA StyleSheptulina, A. F., Antyukh, K. Y., Kiselev, A. R., Mitkovskaya, N. P., & Drapkina, O. M. (2023). Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction. Life, 13(6), 1415. https://doi.org/10.3390/life13061415







