Changes in Serum Bone Metabolism Markers after Living Donor Liver Transplantation (LDLT) and Their Association with Fracture Occurrences
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, H.; Cano, E.J.; Khan, M.Q.; Yetmar, Z.A.; Smith, B.; Rizza, S.A.; Badley, A.D.; Mahmood, M.; Leise, M.D.; Cummins, N.W. Changing Landscape of Liver Transplantation in the Post-DAA and Contemporary ART Era. Life 2022, 12, 1755. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.H.; Han, S.; Ko, J.S.; Gwak, M.S.; Kim, G.S. Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation. J. Pers. Med. 2022, 12, 1352. [Google Scholar] [CrossRef]
- Lin, T.S.; Chen, C.L.; Concejero, A.M.; Yap, A.Q.; Lin, Y.H.; Liu, C.Y.; Chiang, Y.C.; Wang, C.C.; Wang, S.H.; Lin, C.C.; et al. Early and long-term results of routine microsurgical biliary reconstruction in living donor liver transplantation. Liver Transplant. 2013, 19, 207–214. [Google Scholar] [CrossRef]
- Sierra, L.; Barba, R.; Ferrigno, B.; Goyes, D.; Diaz, W.; Patwardhan, V.R.; Saberi, B.; Bonder, A. Living-Donor Liver Transplant and Improved Post-Transplant Survival in Patients with Primary Sclerosing Cholangitis. J. Clin. Med. 2023, 12, 2807. [Google Scholar] [CrossRef]
- Parente, A.; Cho, H.-D.; Kim, K.-H.; Schlegel, A. Association between Hepatocellular Carcinoma Recurrence and Graft Size in Living Donor Liver Transplantation: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6224. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.-K. Emergency, ABO-Incompatible Living Donor Liver Re-Transplantation for Graft Failure Complicated by Pneumonia-Associated Sepsis. J. Clin. Med. 2023, 12, 1110. [Google Scholar] [CrossRef]
- Compston, J.E. Osteoporosis after liver transplantation. Liver Transpl. 2003, 9, 321–330. [Google Scholar] [CrossRef]
- Kuo, S.-J.; Siu, K.-K.; Wu, K.-T.; Ko, J.-Y.; Wang, F.-S. The Differential Systemic Biological Effects between Computer Navigation and Conventional Total Knee Arthroplasty (TKA) Surgeries: A Prospective Study. J. Pers. Med. 2022, 12, 1835. [Google Scholar] [CrossRef]
- Ying, M.; Mao, J.; Sheng, L.; Wu, H.; Bai, G.; Zhong, Z.; Pan, Z. Biomarkers for Prostate Cancer Bone Metastasis Detection and Prediction. J. Pers. Med. 2023, 13, 705. [Google Scholar] [CrossRef]
- Sakr, H.; Khired, Z.; Moqadass, M. In Rats, Whole and Refined Grains Decrease Bone Mineral Density and Content through Modulating Osteoprotegerin and Receptor Activator of Nuclear Factor Kappa B. Biomedicines 2023, 11, 1686. [Google Scholar] [CrossRef]
- Maffei, F.; Masini, A.; Marini, S.; Buffa, A.; Malavolta, N.; Maietta Latessa, P.; Dallolio, L. The Impact of an Adapted Physical Activity Program on Bone Turnover, Physical Performance and Fear of Falling in Osteoporotic Women with Vertebral Fractures: A Quasi-Experimental Pilot Study. Biomedicines 2022, 10, 2467. [Google Scholar] [CrossRef]
- Saeki, C.; Oikawa, T.; Ueda, K.; Nakano, M.; Torisu, Y.; Saruta, M.; Tsubota, A. Serum Insulin-Like Growth Factor 1 Levels, Facture Risk Assessment Tool Scores and Bone Disorders in Patients with Primary Biliary Cholangitis. Diagnostics 2022, 12, 1957. [Google Scholar] [CrossRef]
- Knechtle, B.; Jastrzębski, Z.; Hill, L.; Nikolaidis, P.T. Vitamin D and Stress Fractures in Sport: Preventive and Therapeutic Measures—A Narrative Review. Medicina 2021, 57, 223. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Papadopoulou, S.K.; Detopoulou, P.; Tsoumana, D.; Giaginis, C.; Kondyli, F.S.; Lymperaki, E.; Pritsa, A. Vitamin D and Calcium in Osteoporosis, and the Role of Bone Turnover Markers: A Narrative Review of Recent Data from RCTs. Diseases 2023, 11, 29. [Google Scholar] [CrossRef]
- Jura-Półtorak, A.; Szeremeta, A.; Olczyk, K.; Zoń-Giebel, A.; Komosińska-Vassev, K. Bone Metabolism and RANKL/OPG Ratio in Rheumatoid Arthritis Women Treated with TNF-α Inhibitors. J. Clin. Med. 2021, 10, 2905. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Wang, F.-S.; Chen, S.-H.; Kuo, S.-J. Micro Ribonucleic Acid−29a (miR−29a) Antagonist Normalizes Bone Metabolism in Osteogenesis Imperfecta (OI) Mice Model. Biomedicines 2023, 11, 465. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, N.; Fu, Z.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Liao, P.-S.; Chou, Y.-T.; Lin, C.-L.; Hung, C.-H.; Lin, C.-C.; Hsu, C.-C.; Hsu, H.-C.; Huang, J.-M.; Wang, Y.-Y.; et al. The Incidence and Risk Factors of Hip Fracture after Liver Transplantation (LT): A Nationwide Population-Based Study. BioMed Res. Int. 2019, 2019, 5845709. [Google Scholar] [CrossRef]
- Bjoro, K.; Brandsaeter, B.; Wiencke, K.; Bjoro, T.; Godang, K.; Bollerslev, J.; Schrumpf, E. Secondary osteoporosis in liver transplant recipients: A longitudinal study in patients with and without cholestatic liver disease. Scand. J. Gastroenterol. 2003, 38, 320–327. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, D.J. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int. J. Mol. Sci. 2021, 22, 2604. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Ko, J.Y.; Yeh, D.W.; Ke, H.C.; Wu, H.L. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 2008, 149, 1793–1801. [Google Scholar] [CrossRef]
- Behari, J. The Wnt/beta-catenin signaling pathway in liver biology and disease. Expert. Rev. Gastroenterol. Hepatol. 2010, 4, 745–756. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zhao, F.; Shen, Q.; Wang, Z.; Lv, X.; Hu, B.; Yu, B.; Fan, J.; Qin, W. Overexpression of Dickkopf-1 predicts poor prognosis for patients with hepatocellular carcinoma after orthotopic liver transplantation by promoting cancer metastasis and recurrence. Med. Oncol. 2014, 31, 966. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Yamashita, T.; Sunagozaka, H.; Okada, H.; Nio, K.; Sakai, Y.; Yamashita, T.; Mizukoshi, E.; Honda, M.; Kaneko, S. Dickkopf-1 Promotes Angiogenesis and is a Biomarker for Hepatic Stem Cell-like Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 2801. [Google Scholar] [CrossRef]
- Amer, O.E.; Wani, K.; Ansari, M.G.A.; Alnaami, A.M.; Aljohani, N.; Abdi, S.; Hussain, S.D.; Al-Daghri, N.M.; Alokail, M.S. Associations of Bone Mineral Density with RANKL and Osteoprotegerin in Arab Postmenopausal Women: A Cross-Sectional Study. Medicina 2022, 58, 976. [Google Scholar] [CrossRef]
- Monegal, A.; Navasa, M.; Peris, P.; Alvarez, L.; Pons, F.; Rodes, J.; Guanabens, N. Serum osteoprotegerin and its ligand in cirrhotic patients referred for orthotopic liver transplantation: Relationship with metabolic bone disease. Liver Int. 2007, 27, 492–497. [Google Scholar] [CrossRef]
- Fabrega, E.; Orive, A.; Garcia-Unzueta, M.; Amado, J.A.; Casafont, F.; Pons-Romero, F. Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand system in the early post-operative period of liver transplantation. Clin. Transplant. 2006, 20, 383–388. [Google Scholar] [CrossRef]
- Ho, O.T.W.; Ng, W.C.A.; Ow, Z.G.W.; Ho, Y.J.; Lim, W.H.; Yong, J.N.; Wang, R.S.; Wong, K.L.; Ng, C.H.; Muthiah, M.D.; et al. Bisphosphonate therapy after liver transplant improves bone mineral density and reduces fracture rates: An updated systematic review and meta-analysis. Transpl. Int. 2021, 34, 1386–1396. [Google Scholar] [CrossRef] [PubMed]



| Sex | Age | Etiology | Fracture after LDLT (within 3 Years) | |
|---|---|---|---|---|
| 1 | F | 32 | PLD | |
| 2 | M | 40 | ALC | |
| 3 | M | 43 | HBV, HCC | T12/L1 VCF (12 months) |
| 4 | F | 43 | HBV | |
| 5 | M | 44 | HCV, HCC | |
| 6 | F | 46 | HBV | |
| 7 | M | 46 | HBV | |
| 8 | M | 47 | HBV, HCC | T12/L1 VCF (12 months) |
| 9 | M | 48 | HBV, HCC | |
| 10 | M | 48 | HCV, ALC | |
| 11 | M | 50 | HBV, HCV | L4 VCF (15 months) |
| 12 | M | 51 | ALC | L5 VCF (24 months) |
| 13 | F | 51 | HBV | |
| 14 | M | 53 | HBV, HCV, HCC | |
| 15 | M | 54 | HCV | |
| 16 | M | 54 | HCV | T12/L1 VCF (15 months) |
| 17 | M | 56 | HCC, ALC | |
| 18 | F | 56 | HBV, HCC | |
| 19 | M | 57 | HBV, HCC | |
| 20 | M | 57 | HBV | T12 VCF (24 months) |
| 21 | M | 60 | HCV, HCC | |
| 22 | M | 58 | HCV | |
| 23 | M | 65 | HBV | |
| 24 | M | 67 | HCV, HCC | |
| 25 | M | 66 | HBV, HCC |
| 0M | 3M | 6M | 12M | p Value | |
|---|---|---|---|---|---|
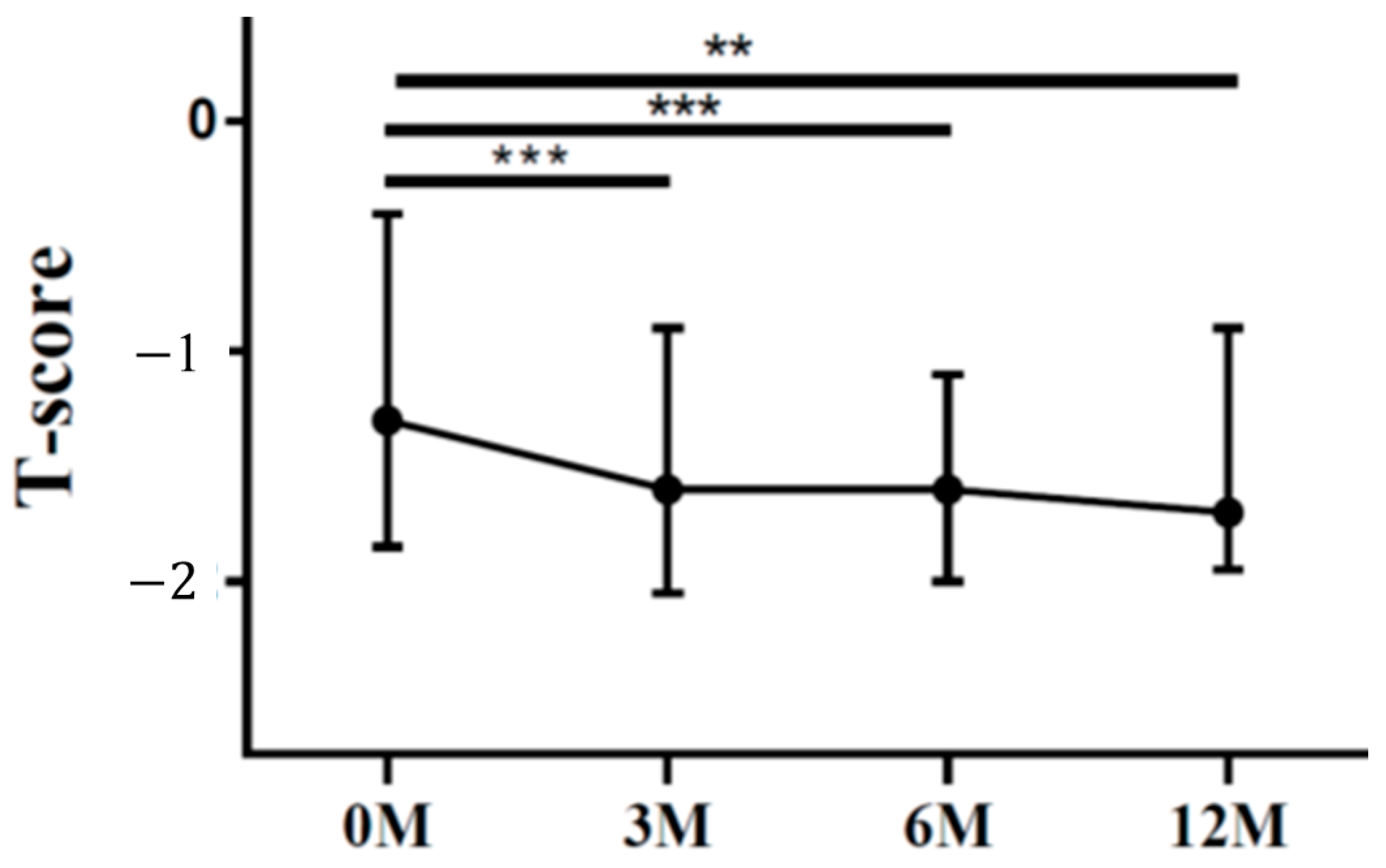
| LS T score | −1.30 (−1.60, −0.80) | −1.20 (−2.00, −0.90) | −1.50 (−2.00, −1.10) | −1.40 (−2.00, −0.90) | 0.024 |
| FN T score | −1.30 (−1.80, −0.50) | −1.60 (−2.00, −0.90) | −1.60 (−1.90, −1.10) | −1.70 (−1.90, −1.00) | <0.001 |
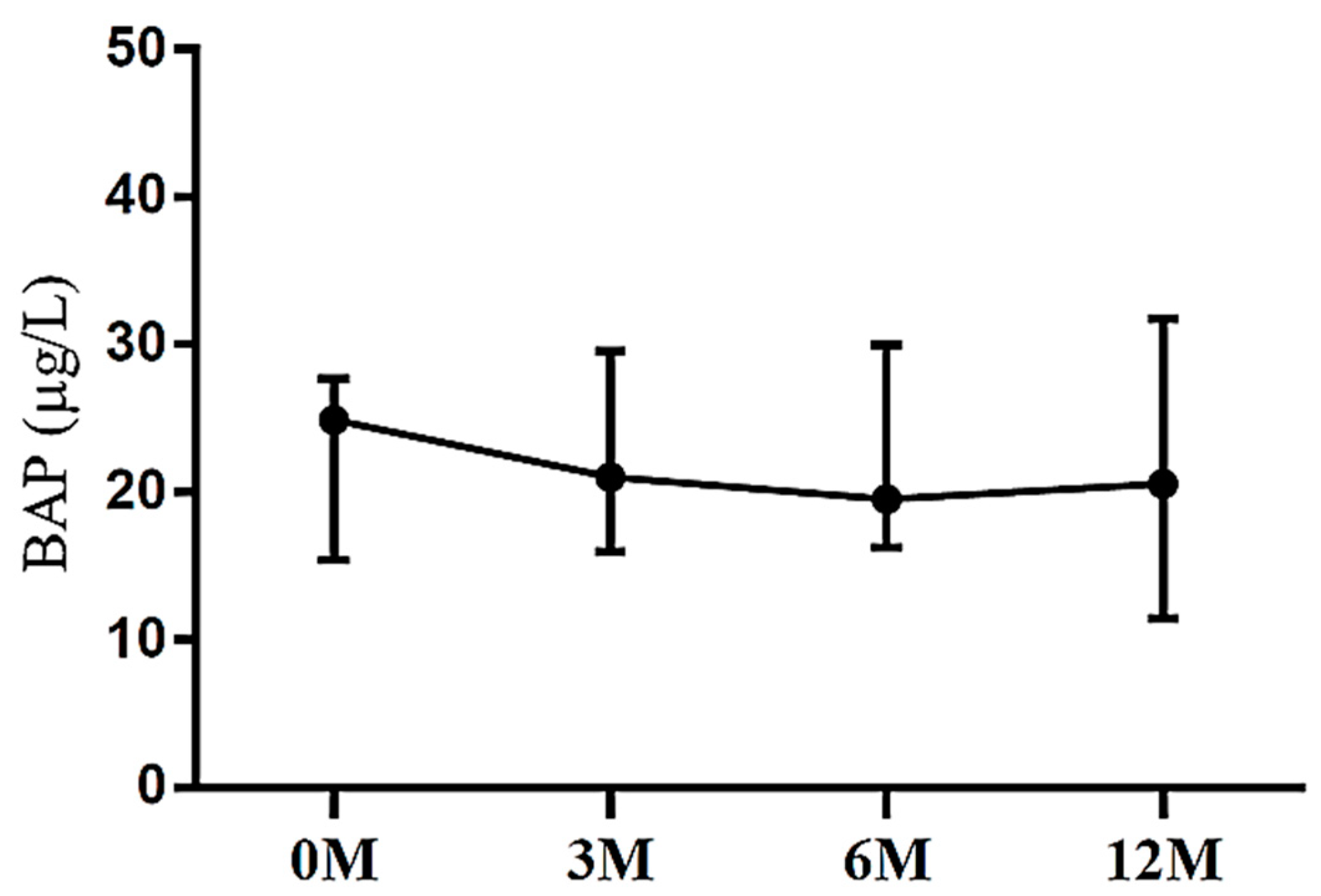
| BAP | 24.86 (15.76, 27.83) | 21.25 (18.39, 27.60) | 21.64 (17.76, 28.48) | 20.55 (12.38, 31.03) | 0.577 |
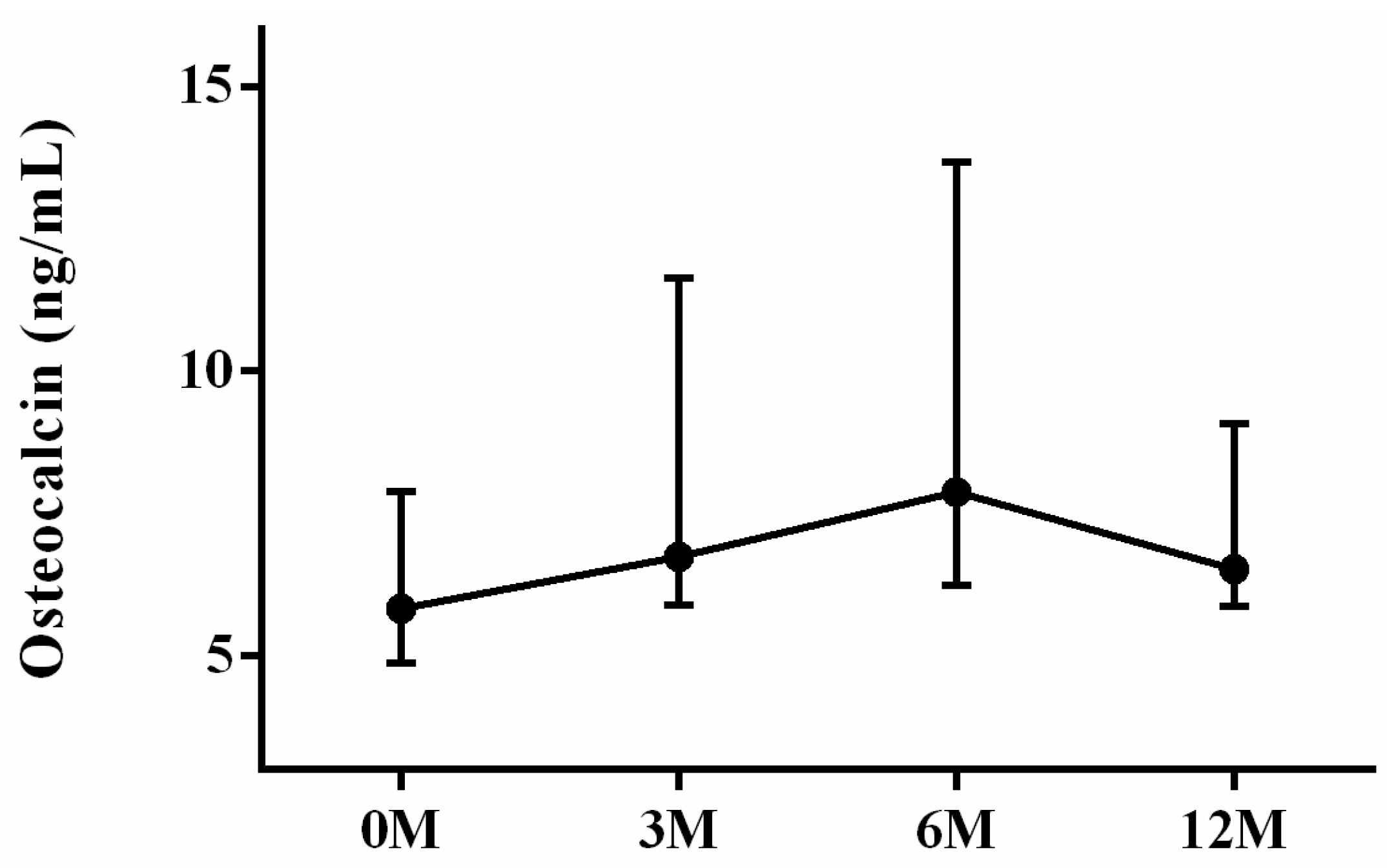
| OCN | 5.83 (5.15, 7.65) | 7.08 (6.06, 11.30) | 7.88 (6.17, 13.32) | 6.52 (6.06, 8.74) | 0.082 |
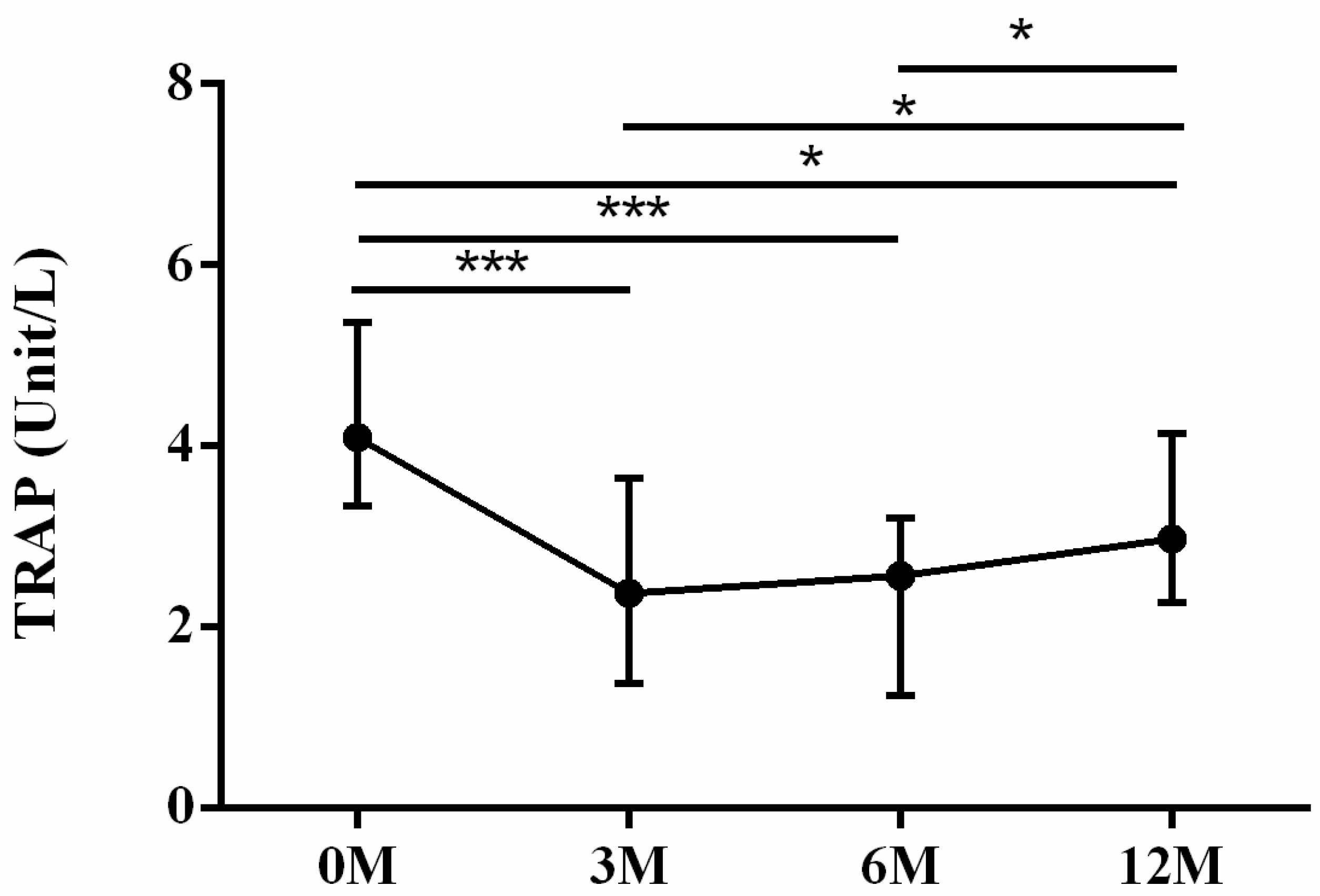
| TRAP | 4.10 (3.31, 5.18) | 2.38 (1.42, 3.57) | 2.57 (1.63, 3.17) | 2.98 (2.31, 4.11) | <0.001 |
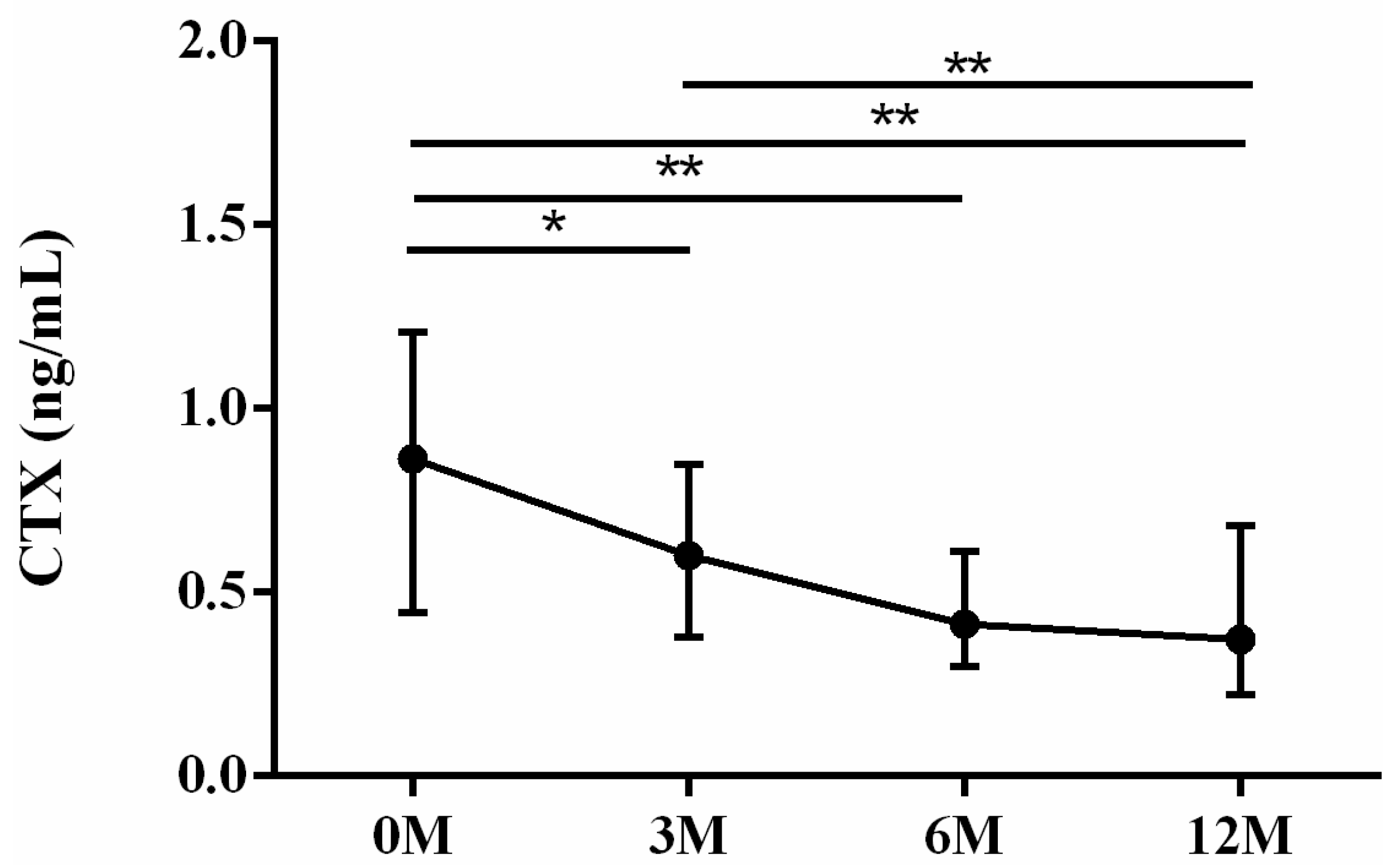
| CTX | 0.76 (0.44, 1.09) | 0.57 (0.34, 0.83) | 0.39 (0.27, 0.59) | 0.37 (0.23, 0.64) | 0.001 |
| IGF-1 | 84.76 (34.52, 107.29) | 243.63 (157.45, 272.90) | 190.07 (165.51, 248.58) | 114.69 (889.30, 149.10) | <0.001 |
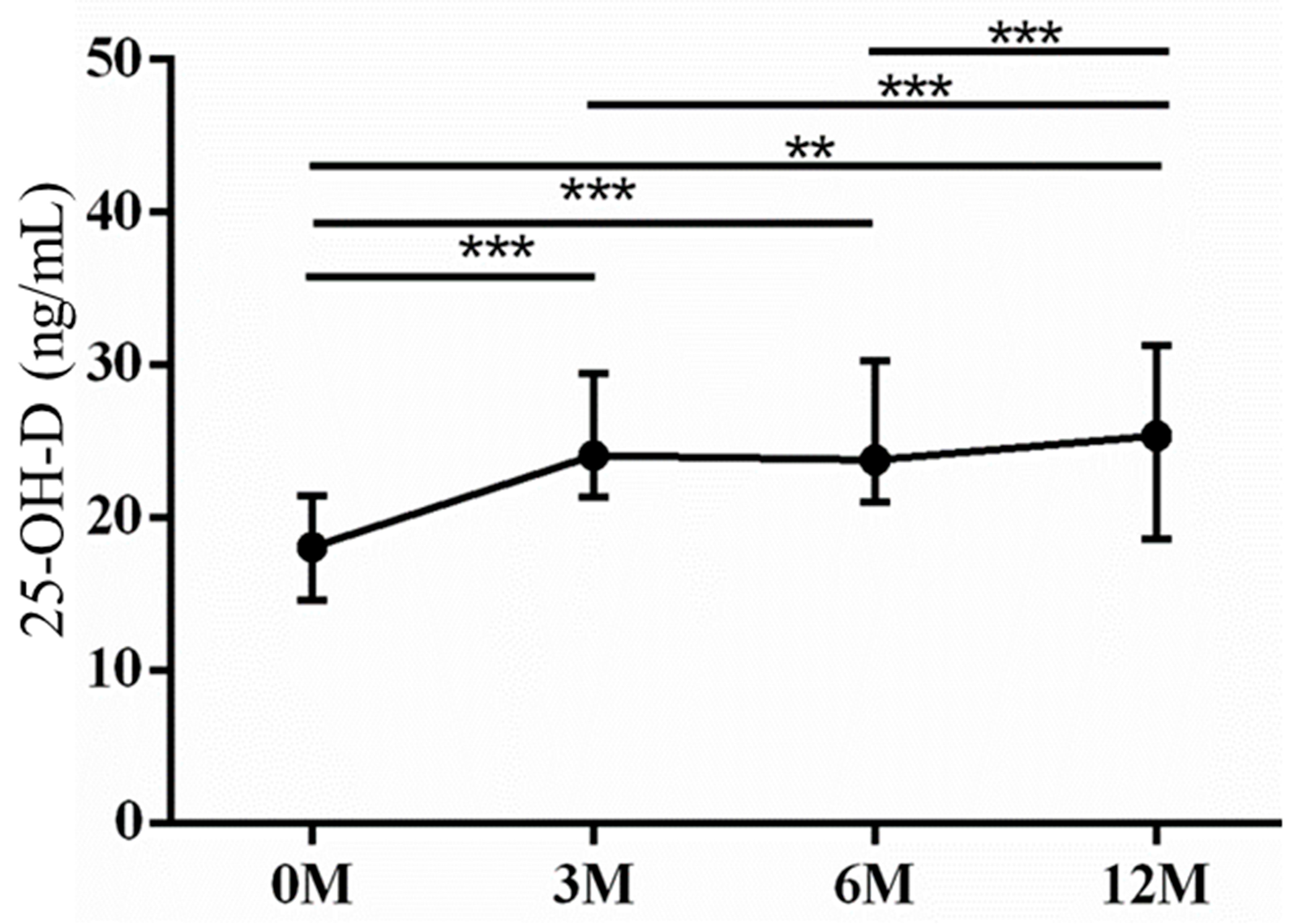
| 25-OH-D | 16.00 (13.60, 20.90) | 24.10 (21.40, 28.10) | 23.80 (21.70, 29.70) | 25.40 (18.80, 29.70) | 0.001 |
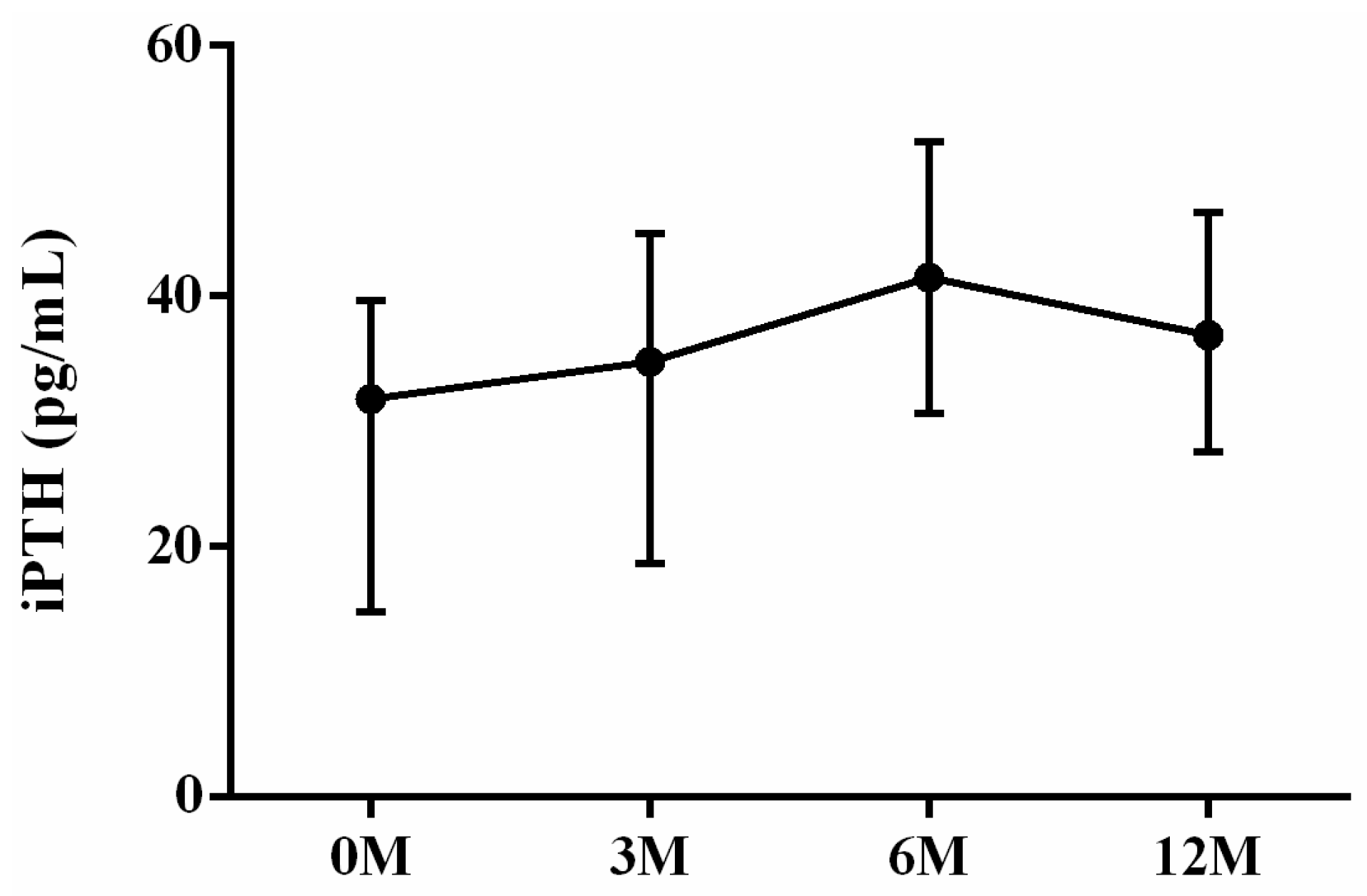
| iPTH | 31.80 (14.80, 39.50) | 34.80 (20.55, 44.65) | 41.50 (30.95, 50.45) | 36.90 (27.65, 46.20) | 0.054 |
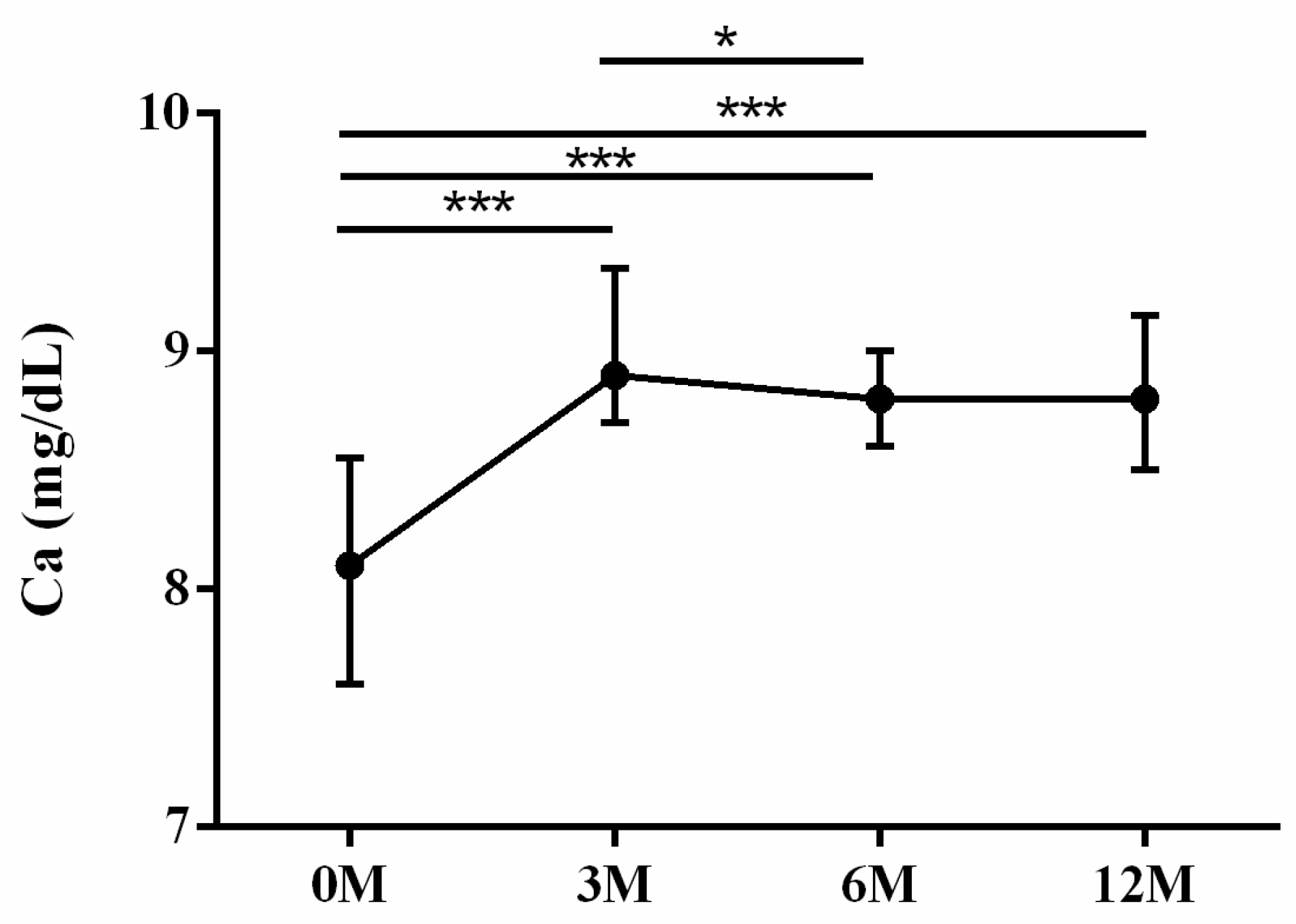
| Ca | 8.10 (7.60, 8.50) | 8.90 (8.70, 9.30) | 8.80 (8.65, 8.95) | 8.80 (8.50, 9.00) | <0.001 |
| P | 3.20 (2.40, 3.90) | 3.50 (3.20, 3.70) | 3.20 (2.75, 3.55) | 2.90 (2.60, 3.50) | 0.045 |
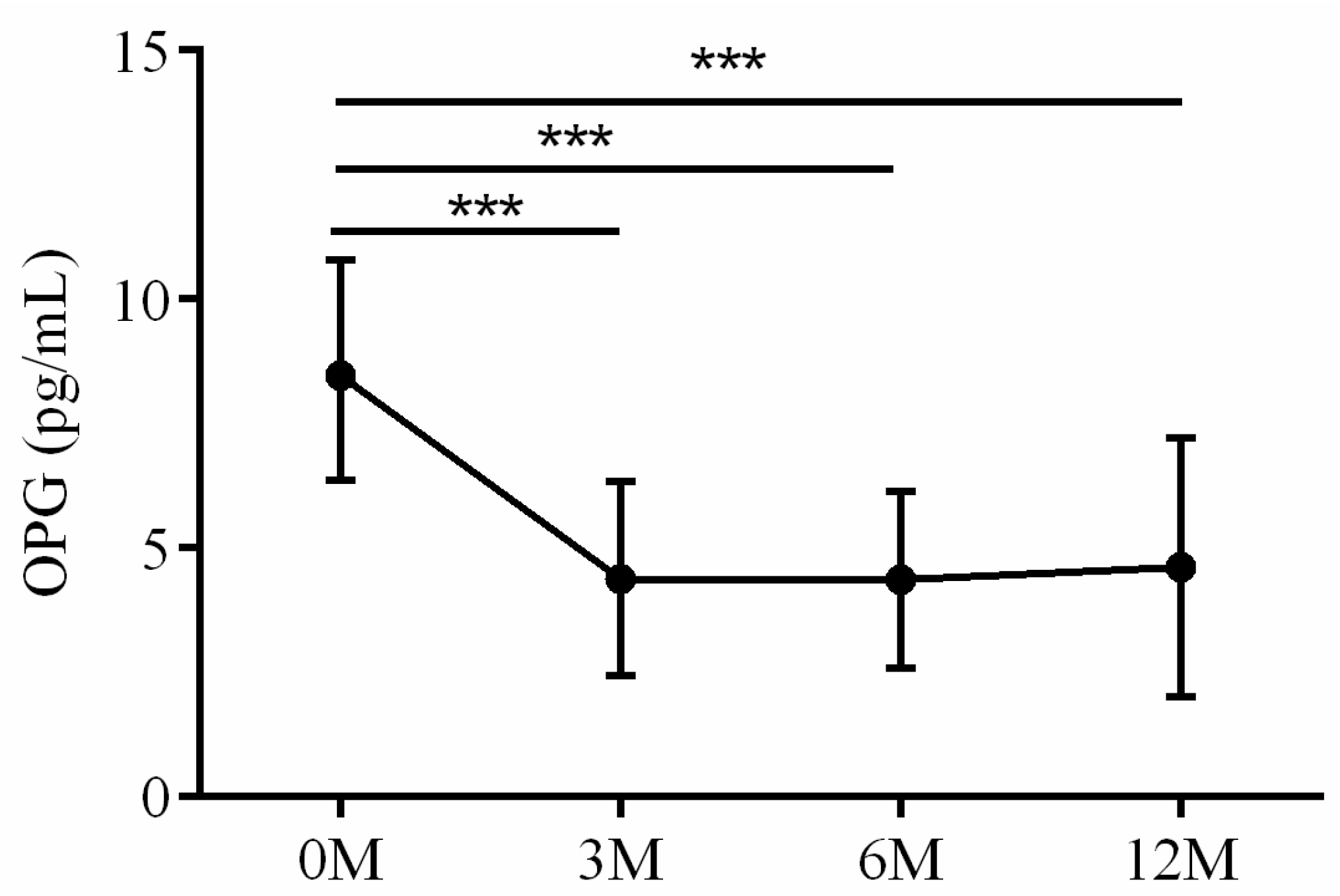
| OPG | 8.47 (6.36, 10.74) | 4.56 (2.87, 5.13) | 4.41 (3.04, 5.61) | 3.98 (2.77, 4.81) | 0.002 |
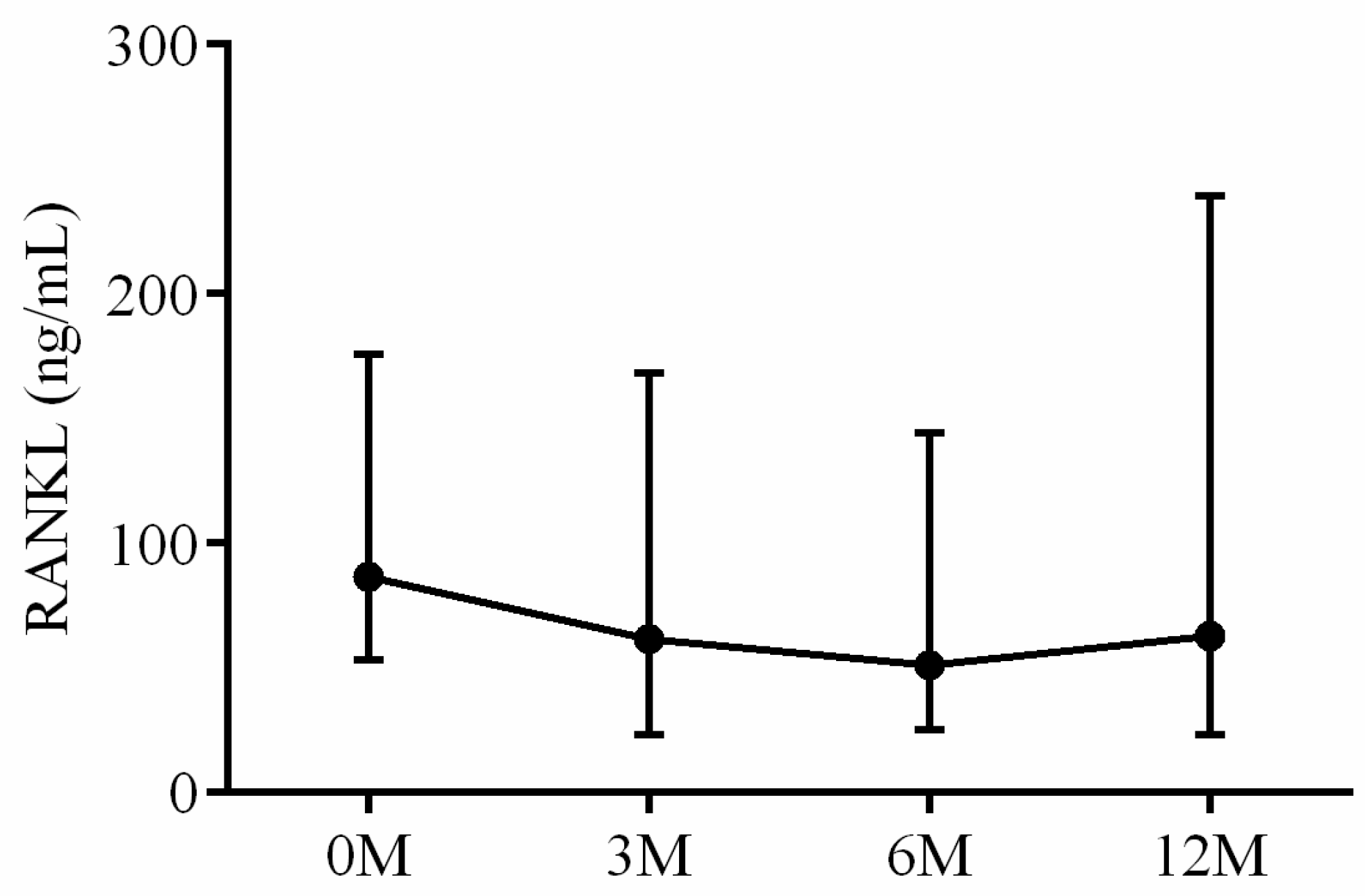
| RANKL | 86.72 (53.54, 163.89) | 61.53 (24.20, 162.53) | 51.19 (28.36, 159.87) | 55.91 (18.02, 206.27) | 0.744 |
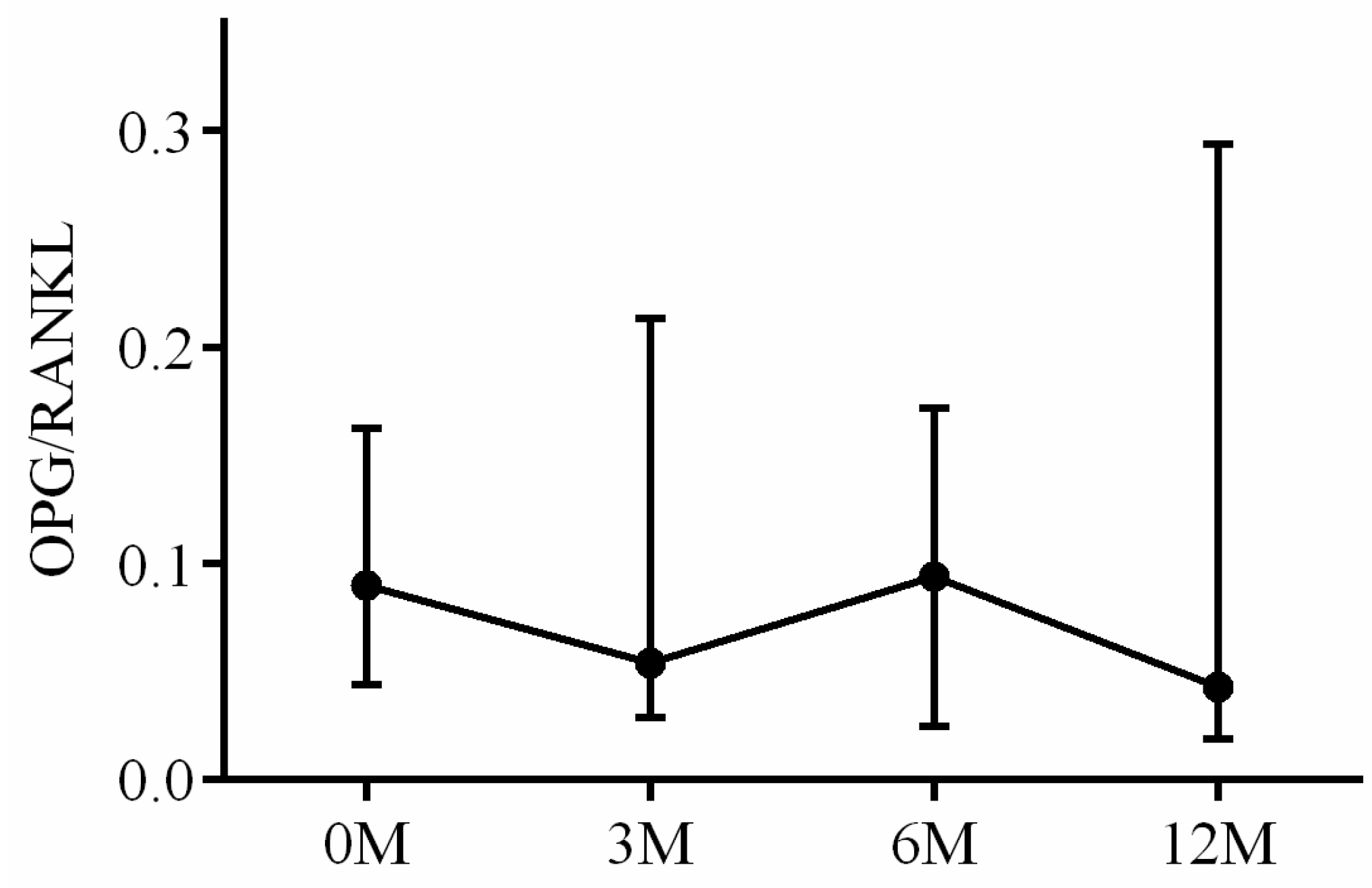
| OPG/RANKL ratio | 0.09 (0.05, 0.16) | 0.05 (0.03, 0.20) | 0.09 (0.03, 0.17) | 0.04 (0.02, 0.29) | 0.491 |
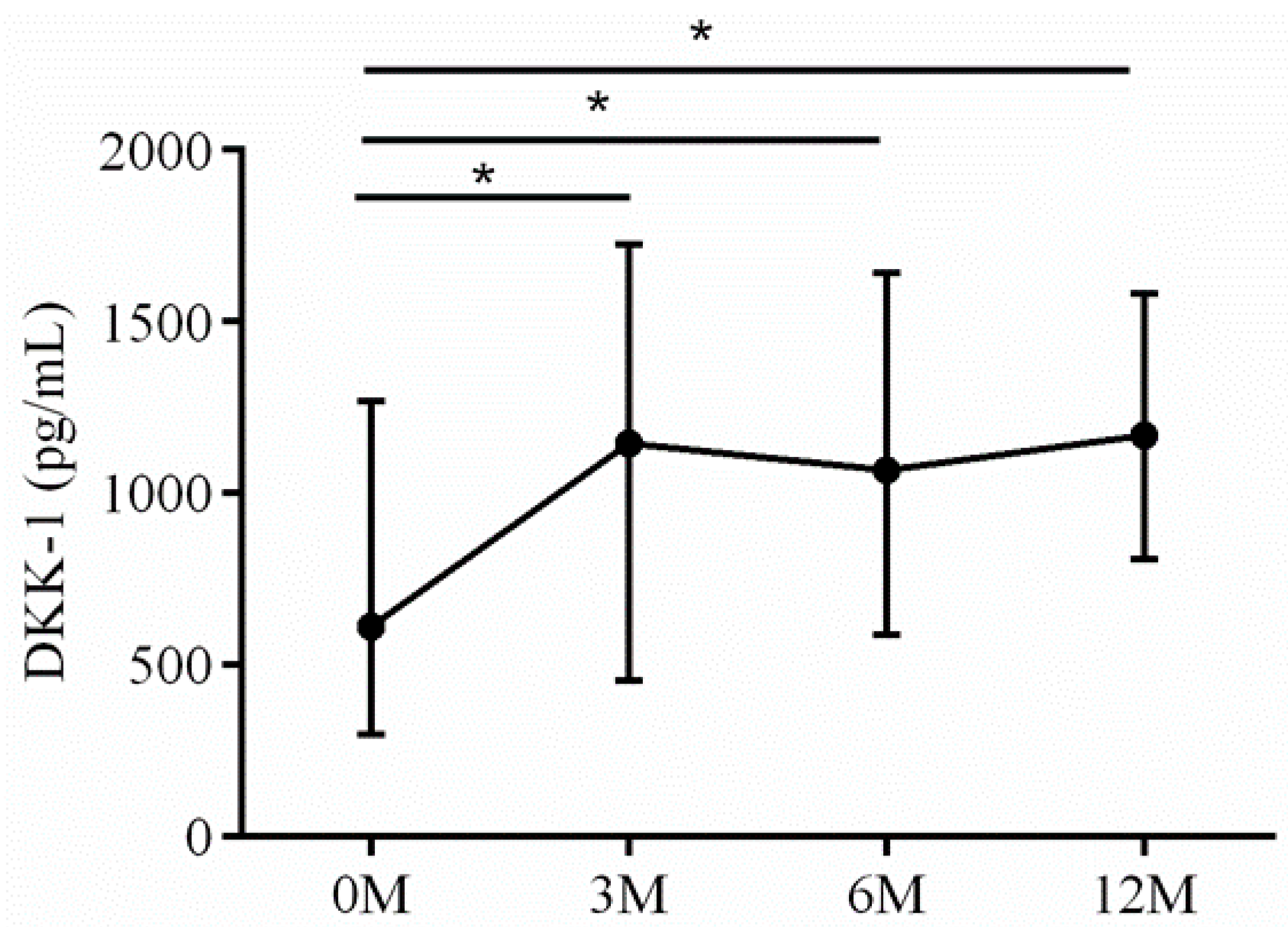
| DKK-1 | 618.37 (300.64, 882.44) | 1262.53 (650.08,1079.65) | 1067.14 (599.35, 1713.41) | 1096.26 (809.19, 1352.73) | 0.006 |
| L-Spine | Femoral Neck | |||
|---|---|---|---|---|
| ρ | p Value | ρ | p Value | |
| BAP | 0.041 | 0.691 | 0.221 | 0.032 |
| Osteocalcin | 0.075 | 0.467 | 0.054 | 0.602 |
| TRAP | 0.102 | 0.325 | 0.133 | 0.196 |
| CTX | 0.164 | 0.110 | 0.446 | <0.001 |
| IGF-1 | −0.020 | 0.848 | −0.005 | 0.956 |
| 25-OH-D | −0.011 | 0.914 | −0.302 | 0.003 |
| iPTH | 0.099 | 0.358 | 0.164 | 0.125 |
| Ca | −0.033 | 0.751 | −0.330 | 0.001 |
| P | −0.165 | 0.283 | 0.122 | 0.242 |
| OPG | 0.050 | 0.655 | −0.027 | 0.810 |
| RANKL | −0.019 | 0.869 | −0.099 | 0.378 |
| OPG/RANKL ratio | 0.014 | 0.898 | 0.066 | 0.562 |
| DKK-1 | 0.057 | 0.589 | −0.162 | 0.122 |
| Fracture (+) | Fracture (−) | p Value | |
|---|---|---|---|
| LS T score | −1.50 (−1.60, −1.10) | −1.30 (−1.55, −0.75) | 0.424 |
| FN T score | −1.75 (−2.10, −0.30) | −1.20 (−1.70, −0.55) | 0.465 |
| BAP | 16.43 (14.44, 24.86) | 25.01 (17.99, 31.98) | 0.134 |
| Osteocalcin | 6.91 (5.38, 8.71) | 5.72 (4.63, 7.11) | 0.238 |
| TRAP | 3.73 (3.38, 4.08) | 4.52 (3.22, 5.62) | 0.194 |
| CTX | 0.55 (0.34, 0.63) | 0.93 (0.52, 1.61) | 0.080 |
| IGF-1 | 80.79 (28.69, 88.42) | 84.76 (35.99, 107.86) | 0.631 |
| 25-OH-D | 18.20 (14.30, 21.50) | 16.00 (12.85, 19.85) | 0.589 |
| iPTH | 38.50 (9.00, 39.90) | 26.30 (14.80, 36.50) | 0.298 |
| Ca | 8.45 (7.70, 8.80) | 8.00 (7.55, 8.25) | 0.171 |
| P | 2.85 (2.20, 3.90) | 3.30 (2.50, 4.10) | 0.749 |
| OPG | 10.88 (9.71, 11.45) | 7.22 (6.31, 9.92) | 0.046 |
| RANKL | 32.27 (29.93, 52.60) | 104.75 (76.34, 189.34) | 0.001 |
| OPG/RANKL ratio | 0.28 (0.24, 0.36) | 0.08 (0.04, 0.11) | <0.001 |
| DKK-1 | 1181.37 (930.30, 1705.89) | 353.29 (260.95, 792.56) | 0.020 |
| Fracture (+) versus Fracture (−) | |||
|---|---|---|---|
| AUC | p Value | Cut-Off Value for Fracture | |
| OPG | 0.78 ± 0.10 | 0.042 | >8.31 |
| RANKL | 0.96 ± 0.04 | <0.001 | <54.47 |
| OPG/RANKL ratio | 1.00 ± 0.00 | <0.001 | >0.17 |
| DKK-1 | 0.82 ± 0.08 | 0.019 | >661.35 |
| Cut-Off Value | 0M | 3M | 6M | 12M | |||||
|---|---|---|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | ||
| OPG | >8.31 | 5/6 | 5/19 | 5/6 | 3/19 | 5/6 | 3/19 | 5/6 | 3/19 |
| RANKL | <54.47 | 5/6 | 2/19 | 6/6 | 2/19 | 6/6 | 3/19 | 6/6 | 3/19 |
| OPG/RANKL ratio | >0.17 | 6/6 | 0/19 | 6/6 | 0/19 | 6/6 | 0/19 | 6/6 | 1/19 |
| DKK-1 | >661.35 | 5/6 | 2/19 | 6/6 | 3/19 | 6/6 | 3/19 | 6/6 | 2/19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, S.-J.; Chen, C.-L.; Chen, S.-H.; Ko, J.-Y. Changes in Serum Bone Metabolism Markers after Living Donor Liver Transplantation (LDLT) and Their Association with Fracture Occurrences. Life 2023, 13, 1438. https://doi.org/10.3390/life13071438
Kuo S-J, Chen C-L, Chen S-H, Ko J-Y. Changes in Serum Bone Metabolism Markers after Living Donor Liver Transplantation (LDLT) and Their Association with Fracture Occurrences. Life. 2023; 13(7):1438. https://doi.org/10.3390/life13071438
Chicago/Turabian StyleKuo, Shu-Jui, Chao-Long Chen, Sung-Hsiung Chen, and Jih-Yang Ko. 2023. "Changes in Serum Bone Metabolism Markers after Living Donor Liver Transplantation (LDLT) and Their Association with Fracture Occurrences" Life 13, no. 7: 1438. https://doi.org/10.3390/life13071438
APA StyleKuo, S.-J., Chen, C.-L., Chen, S.-H., & Ko, J.-Y. (2023). Changes in Serum Bone Metabolism Markers after Living Donor Liver Transplantation (LDLT) and Their Association with Fracture Occurrences. Life, 13(7), 1438. https://doi.org/10.3390/life13071438








