Bioactive Phytoconstituents and Their Therapeutic Potentials in the Treatment of Haematological Cancers: A Review
Abstract
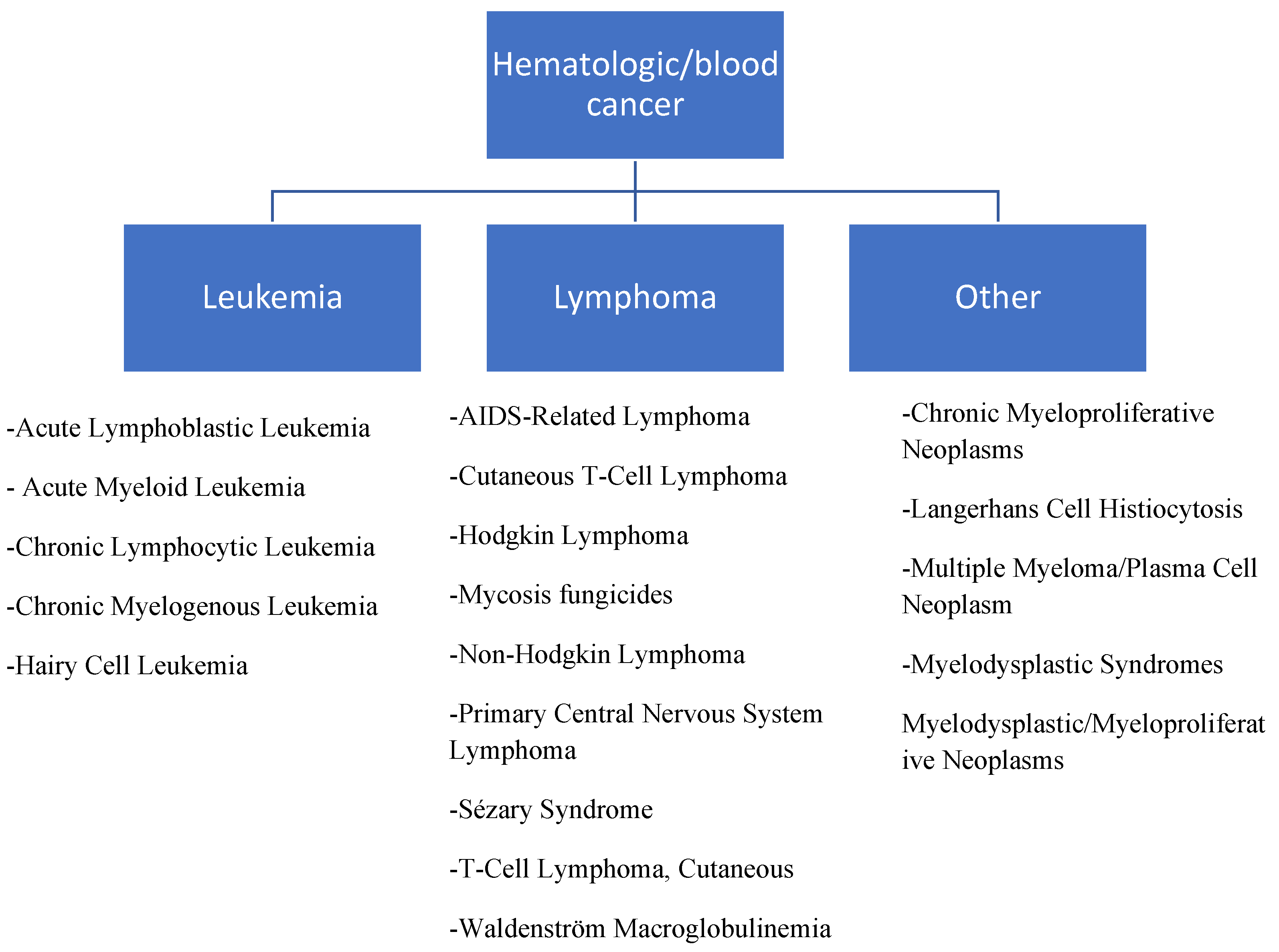
1. Introduction
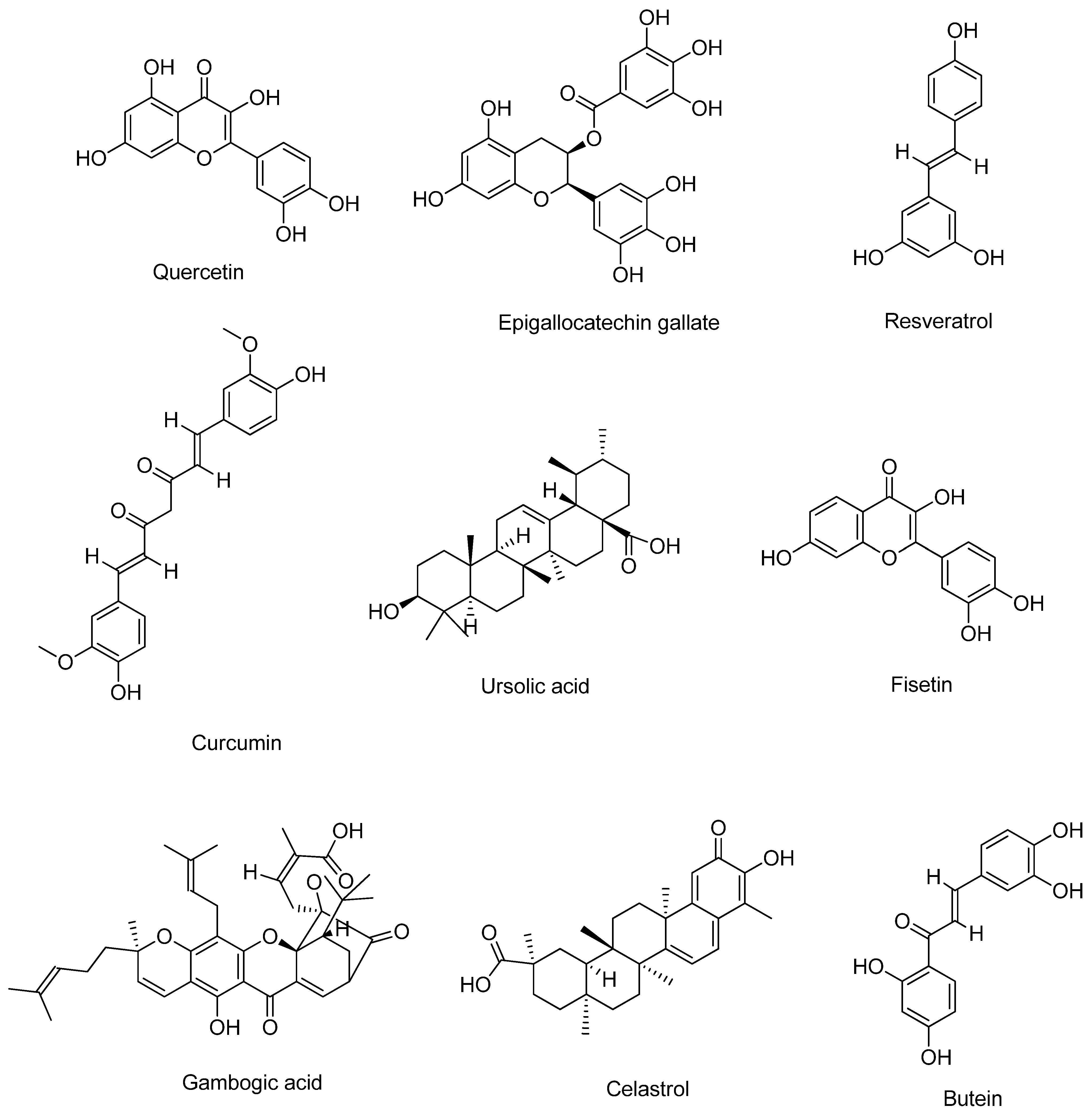
2. Bioactive Compounds
2.1. Quercetin
2.2. Epigallocatechin Gallate
2.3. Resveratrol
2.4. Curcumin
2.5. Ursolic Acid
2.6. Fisetin
2.7. Gambogic Acid
2.8. Celastrol
2.9. Butein
| Bioactive Compound | Experimental Models | Condition | Effective Dose | Mechanism | References |
|---|---|---|---|---|---|
| Quercetin | NB4, HL60, K562, Jurkat and Daudi | Leukaemia and lymphoma | 50 µmol/L | Apoptosis through activation of the Wnt signalling pathway | [40] |
| Quercetin | P39 | Leukaemia | 50, 100 µmol/L | Apoptosis through an upregulation of pro-apoptotic proteins and downregulation of anti-apoptotic proteins, autophagy, Akt-mTOR phosphorylation, tumour inhibition | [32] |
| Quercetin | Human232b4 | Chronic lymphocytic leukaemia | 24 µM | Decrease in the proliferative capacity of cells due to activation of caspase-3 and cell cycle arrest | [41] |
| Quercetin | MV4-11, HL-60 | Acute myeloid leukaemia | Dose-dependent | Apoptosis via downregulation of vascular endothelial growth factor signalling | [34] |
| Quercetin | HL-60, U937 | Leukaemia | 50 µmol/L | Demethylation of pro-apoptotic genes, BCL2L11 and DAPK1 | [47] |
| Quercetin | U937 | Leukaemia | 120 µM | Down regulation of Mcl-1, survivin and XIAP genes | [44] |
| Quercetin | Patients | Chronic lymphocytic leukaemia | 500 mg twice daily for 3 months | Reduction in lymphocyte number and a decline in lactate dehydrogenase | [54] |
| Quercetin and Curcumin | K562 | Chronic myeloid leukaemia | Dose-dependent | Apoptosis | [26] |
| Quercetin and TRAIL | KG-1 | Human myeloid leukaemia | 105.6 µM | Sensitise TRAIL to induce apoptosis | [33] |
| Quercetin and Adriamycin | HL-60 xenografts | Human leukaemia | 100 µM | Lower concentration of Adriamycin to inhibit proliferation of cells | [42] |
| Quercetin and Green Tea | HL-60 xenografts | Human leukaemia | 120:100 mg/kg | Tumour inhibition | [42] |
| Epigallocatechin Gallate | Leukemic mice | Acute promyelocytic leukaemia | 200 µM | Apoptosis, tumour inhibition via reduction in the number of promyelocytes and reduction in CD34+ haematopoietic progenitor cells | [63] |
| Epigallocatechin Gallate | Bcr/Abl+ | Chronic myeloid leukaemia | Dose-dependent | Caspase-independent apoptosis through upregulation of apoptosis-inducing factor | [67] |
| Epigallocatechin Gallate | NB4, NB4-R1, NB4-R2 | Promyelocytic leukaemia | 200 µM | Mitochondria damage, ROS activation, caspase activation | [62] |
| Epigallocatechin Gallate | Jurkat cells | T lymphoblastic leukaemia | 250 mg/kg | Apoptotic induction through increase in caspase-3 level and Fas expression | [59] |
| Epigallocatechin Gallate | NALM-6 | Acute myelocytic leukaemia | 45 µM | Downregulation of epigenetic modifiers such as DNMT1, HDAC1, HDAC2, G9a | [61] |
| Epigallocatechin Gallate + ponatinib | K562 | Chronic myeloid leukaemia | 87.13 nM and 50 µM | Cell cycle arrest | [70] |
| Resveratrol | Human multiple myeloma tissue-U266 and LP-1 | Multiple myeloma | Dose-dependent | Repression of NEAT1 and down regulation of c-Myc and MMP-7 genes | [30] |
| Resveratrol | Molt-4 and Jurkat | T-cell acute lymphoblastic leukaemia | 75 µM | Apoptosis and autophagy through regulation of pro-apoptotic and anti-apoptotic genes | [79] |
| Resveratrol + Carfilzomib | LP-1, U266 | Multiple myeloma | 200 µM | Modulation of metabolism, stress and apoptosis through ROS generation | [80] |
| Resveratrol + Bestatin | K562 | Chronic myeloid leukaemia | 10 µM | Downregulation of P-glycoprotein | [88] |
| Curcumin | A375 | Acute monocytic leukaemia | 80 µM | Reduction in tumour weight, disruption of mitochondria membrane potential | [95] |
| Curcumin + doxorubicin | REH and RSV | Acute lymphoblastic leukaemia | 100 µM | Enhanced sensitivity of drug to apoptosis induction | [97] |
| Curcumin+ cytarabine | Bone marrow samples | Acute myeloid leukaemia | 12.41;3.1 µM | Synergistic effect which enhanced the anti-proliferative capacity of cytarabine, Downregulation of MDR genes | [98] |
| Curcumin | Multiple myeloma patients | Multiple myeloma | 3–4 g daily for 3 months | Decrease in paraprotein load and plasmacytosis | [102] |
| Curcumin | SH-1 | Human monocytic leukaemia | 32.40 µM | Alteration of MAPK and MMP signalling | [109] |
| Curcumin | BL41-3, DG-75, THP1 | Burkitt Lymphoma | Dose-dependent | Downregulation of Glil-1, Notch 1 | [110] |
| Ursolic acid | HL-60, U937, Jurkat and THP-1 | Leukaemia | 25 µM | Suppression of cell proliferation | [115] |
| Ursolic acid | Jurkat | Leukaemia | 30 µM | Suppression of phytohemagglutinin induced IL-2 and TNF-α | [116] |
| Ursolic acid | K562 | Leukaemia | Dose-dependent | Downregulation of MCl-1 and p-Bad proteins | [117] |
| Ursolic acid | HL-60 | Leukaemia | 60 µmol/L | Monocytic differentiation through activation of the ERK signalling pathway | [118] |
| Ursolic acid | RPMI-8226 | Multiple myeloma | 40 µM | Downregulation of intracellular β-catenin levels and reduction in the expression of target β-catenin dependent genes such as c-Myc and cyclin D1 | [122] |
| Fisetin | K562 | Myeloid leukaemia | 163 µM | Apoptosis and mitochondrial membrane depolarisation through increase in caspase-3 activity and cell cycle arrest at S and G2 phases | [129] |
| Fisetin | U266 | Multiple myeloma | 60 µM | Downregulation of Bcl-2, Mcl-1 and upregulation of Bax and Bad | [131] |
| Gambogic acid | KBM5, K562 | Imatinib-resistant chronic myeloid leukaemia | 0.40 µmol/L | Sensitivity of cells to treatment | [137] |
| Gambogic acid + Bortezomib | MM.1S | Human myeloma | 0.9 µM GNA + 4.0 Nm BTZ | Enhancing the apoptosis-inducing effect of the drug through NFkB downregulation and caspase activation | [138] |
| Gambogic acid | DLBCL cell lines and mouse models | B-cell lymphoma | 0.30 µM | Proteasome inhibition resulting in NF-kB downregulation and caspase activation | [138] |
| Gambogic acid | U266 | Multiple myeloma | Dose-dependent | Suppression of HIF-1α | [143] |
| Celastrol | MM.1S, MM.1R, U266 | Human myeloma | 500 nM | Induction of anti-proliferative activity, cell cycle arrest and proteasome inhibition | [155] |
| Celastrol | KBM5 | Chronic myeloid leukaemia | 525.4 nM | Downregulation of Bcl-Abl genes | [156] |
| Celastrol | U266, RPMI8226 | Multiple myeloma | Dose-dependent | Downregulation of the NF-kB and STAT3 pathway | [151] |
| Celastrol | HL-60 | Human acute promyelocytic leukaemia | 0.55 µM | Activation of the p-53 mitochondrial pathway with notable increase in uridine levels | [163] |
| Butein | RS4-11, MOLT-4 | Acute lymphoblastic leukaemia | 100 µM | Downregulation of cyclin E, CDK2 and the upregulation of caspase-3 expression through the FOXO3a signalling pathway | [166] |
| Butein | HTL-V1 infected T-cells | Adult T-cell leukaemia | 7.0 µM | Apoptosis induction through the regulation of other signalling pathways such as NF-kB, AP-1, P53 and Akt | [170] |
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | AKT serine/threonine kinase 1 |
| ALL | Acute lymphoblastic leukaemia |
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| AP-1 | Activator Protein 1 |
| Atg | Autophagy related |
| Bad | BCL2 Associated Agonist of Cell Death |
| Bax | Bcl-2 Associated X-protein |
| Bcl-2 | B-cell lymphoma 2 |
| BCL2L11 | Bcl-2-like protein 11 |
| Bcl-xL | B-cell lymphoma- extra large |
| Bcr-Abl | Breakpoint cluster region and Abelson murine leukaemia viral oncogene homologue |
| BCRP | Breast cancer resistance protein |
| Bim | BCL2-interacting mediator of cell death |
| CDK2 | Cyclin-dependent kinase 2 |
| CDKN1A | Cyclin-Dependent Kinase Inhibitor 1A] |
| c-Myc | Cellular-Master Regulator of Cell Cycle Entry |
| CXCR4 | C-X-C motif receptor 4 |
| DAPK1 | Death-Associated Protein Kinase 1 |
| DNMT1 | DNA methyltransferase |
| ERK | Extracellular signal-regulated kinase |
| Fas | Fas Cell Surface Death Receptor |
| FOXO3a | Forkhead box transcription factors |
| GADD45B | Growth Arrest and DNA Damage Inducible Beta |
| Gfi-1 protein | Growth Factor Independent 1 Transcriptional Repressor |
| Glil-1 | Glioma-associated oncogene homologue 1 |
| HDAC | Histone deacetylase |
| hTERT | human Telomerase reverse transcriptase |
| IL-2 | Interleukin-2 |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| JNK | c-Jun N-terminal kinases |
| LC3-I | Microtubule-associated protein 1A/1B-light chain 3-I |
| LC3-II | Microtubule-associated protein 1A/1B-light chain 3-II |
| LRP | Low-density lipoprotein receptor-related protein 1 |
| MAPK | Microtubule-Associated Protein Kinase |
| Mcl-1 | Myeloid cell leukaemia-1 |
| MDR1 | Multidrug resistance gene |
| miR-1290 | MicroRNA 1290 |
| miR-196b | MicroRNA 196b |
| miR-21 | MicroRNA 21 |
| MMP | Mitochondrial membrane potential |
| MMP-7 | Matrix metalloproteinase |
| MRp-1 | Multidrug resistance protein 1 |
| mTor | The mechanistic target of rapamycin |
| NEAT1 | Nuclear Paraspeckle Assembly Transcript 1 |
| NF-kB | Nuclear Factor-Kappa B |
| NFKBIA | NFKB Inhibitor Alpha |
| Notch-1 | Neurogenic locus notch homolog protein 1 |
| PARP-1 | Poly-Adenosine Diphosphate Ribose Polymerase-1 |
| p-ERK | Phospho-extracellular signal-related kinase |
| PI3K | Phosphoinositide 3-kinases |
| PI3K-AKT | Phosphatidylinositol-3-Kinase |
| p-JNK | Protein and c-jun NH2-terminal kinase |
| PKB | Protein kinase B |
| PMAIP1 | Phorbol-12-Myristate-13-Acetate-Induced Protein 1 |
| PTEN | Phosphatase and Tensin Homolog |
| ROS | Reactive oxygen species |
| SCID | Severe combined immunodeficiency |
| Stat 5 a/b | Signal transducer and activator of transcription 5 |
| TNF-α | Tumour Necrosis factor alpha |
| TRAIL | TNF-related apoptosis-induced ligand |
| VEGF | Vascular endothelial growth factor |
| Wnt/β | Wnt/beta-catenin |
| WST | Water soluble tetrazolium salt |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Anderson, K.C.; Alsina, M.; Atanackovic, D.; Biermann, J.S.; Chandler, J.C.; Costello, C.; Djulbegovic, B.; Fung, H.C.; Gasparetto, C.; Godby, K. NCCN guidelines insights: Multiple myeloma, version 3.2016. J. Nat. Compr. Cancer Netw. 2016, 14, 389–400. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancers by Body Location/System. Available online: https://www.cancer.gov/types/by-body-location#hematologicblood (accessed on 9 February 2023).
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood J. Am. Soc. Hematol. 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood J. Am. Soc. Hematol. 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Yanada, M.; Naoe, T. Acute myeloid leukemia in older adults. Int. J. Hematol. 2012, 96, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Brown, T.; Pilkington, G.; Bagust, A.; Boland, A.; Oyee, J.; Tudur-Smith, C.; Blundell, M.; Lai, M.; Saborido, M.; Greenhalgh, J. Clinical effectiveness and cost-effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, 1–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Tabbara, I.A.; Zimmerman, K.; Morgan, C.; Nahleh, Z. Allogeneic hematopoietic stem cell transplantation: Complications and results. Arch. Intern. Med. 2002, 162, 1558–1566. [Google Scholar] [CrossRef]
- Wildes, T.M.; Rosko, A.; Tuchman, S.A. Multiple myeloma in the older adult: Better prospects, more challenges. J. Clin. Oncol. 2014, 32, 2531. [Google Scholar] [CrossRef]
- Hainaut, P.; Plymoth, A. Targeting the hallmarks of cancer: Towards a rational approach to next-generation cancer therapy. Cur. Opin. Oncol. 2013, 25, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Wood, W.A.; Lee, S.J.; Shea, T.C.; Naresh, K.N.; Kazembe, P.N.; Casper, C.; Hesseling, P.B.; Mitsuyasu, R.T. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood Am. Soc. Hematol. 2012, 119, 5078–5087. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T.; Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators*. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A.; Gross, A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004, 350, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Kafuko, G.W.; Burkitt, D.P. Burkitt’s lymphoma and malaria. Int. J. Cancer. 1970, 6, 1–9. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. GLOBOCAN 2008 v1. 2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Canel, C.; Moraes, R.M.; Dayan, F.E.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cirla, A.; Mann, J. Combretastatins: From natural products to drug discovery. Nat. Prod. Rep. 2003, 20, 558–564. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Bankole, E.O.; Iheagwam, F.N.; Dania, O.E.; Ntite, U.F. Cytotoxic assessment of Xylopia aethiopica [Dun.] A. on human prostate and breast cancer cell lines. TJNPR 2020, 4, 1143–1146. [Google Scholar]
- Yakubu, O.F.; Adebayo, A.H.; Dokunmu, T.M.; Zhang, Y.J.; Iweala, E.E. Cytotoxic effects of compounds isolated from Ricinodendron heudelotii. Molecules 2019, 24, 145. [Google Scholar] [CrossRef]
- Kim, W.; Lee, W.-B.; Lee, J.-W.; Min, B.-I.; Baek, S.K.; Lee, H.S.; Cho, S.-H. Traditional herbal medicine as adjunctive therapy for breast cancer: A systematic review. Complement. Therap. Med. 2015, 23, 626–632. [Google Scholar] [CrossRef]
- Kuo, Y.-T.; Liao, H.-H.; Chiang, J.-H.; Wu, M.-Y.; Chen, B.-C.; Chang, C.-M.; Yeh, M.-H.; Chang, T.-T.; Sun, M.-F.; Yeh, C.-C. Complementary Chinese herbal medicine therapy improves survival of patients with pancreatic cancer in Taiwan: A nationwide population-based cohort study. Integr. Cancer Therap. 2018, 17, 411–422. [Google Scholar] [CrossRef]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.Y.; Lu, R.; Wang, J.H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Altundağ, E.; Yılmaz, A.M.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells. Nutr. Cancer 2018, 70, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambo, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights. Int. J. Funct. Nutri. 2020, 1, 9. [Google Scholar] [CrossRef]
- Pojero, F.; Poma, P.; Spano, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Geng, W.; Guo, X.; Zhang, L.; Ma, Y.; Wang, L.; Liu, Z.; Ji, H.; Xiong, Y. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2018, 107, 484–494. [Google Scholar] [CrossRef]
- Hsiao, P.-C.; Chang, J.-H.; Lee, W.-J.; Ku, C.-C.; Tsai, M.-Y.; Yang, S.-F.; Chien, M.-H. The curcumin analogue, EF-24, triggers p38 MAPK-mediated apoptotic cell death via inducing PP2A-modulated ERK deactivation in human acute myeloid leukemia cells. Cancers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Maso, V.; Calgarotto, A.K.; Franchi, G.C.; Nowill, A.E.; Vassallo, J.; Saad, S.T.O. Multitarget Effects of Quercetin in LeukemiaMultitarget Effects of Quercetin in Leukemia. Cancer Prev. Res. 2014, 7, 1240–1250. [Google Scholar] [CrossRef]
- Naimi, A.; Entezari, A.; Hagh, M.F.; Hassanzadeh, A.; Saraei, R.; Solali, S. Quercetin sensitizes human myeloid leukemia KG-1 cells against TRAIL-induced apoptosis. J. Cell. Physiol. 2019, 234, 13233–13241. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.-Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z.-X.; Chen, P.-H.; Dai, H.-Q.; Feng, J.; Chatterjee, S. Quercetin induces apoptosis via downregulation of vascular endothelial growth factor/Akt signaling pathway in acute myeloid leukemia cells. Front. Pharmacol. 2020, 11, 534171. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Anti-oxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef]
- Kawahara, T.; Kawaguchi-Ihara, N.; Okuhashi, Y.; Itoh, M.; Nara, N.; Tohda, S. Cyclopamine and quercetin suppress the growth of leukemia and lymphoma cells. Anticancer Res. 2009, 29, 4629–4632. [Google Scholar]
- Gokbulut, A.A.; Apohan, E.; Baran, Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology 2013, 18, 144–150. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Maso, V.; Junior, G.C.F.; Nowill, A.E.; Vassallo, J.; Saad, S.T.O. Anti-tumor activities of quercetin and green tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459. [Google Scholar] [CrossRef]
- Niu, G.; Yin, S.; Xie, S.; Li, Y.; Nie, D.; Ma, L.; Wang, X.; Wu, Y. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim. Biophys. Sin. 2011, 43, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Cerella, C.; Russo, M.; Chateauvieux, S.; Diederich, M.; Russo, G.L. Quercetin downregulates Mcl-1 by acting on mRNA stability and protein degradation. Br. J. Cancer 2011, 105, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Chow, J.M.; Chang, J.H.; Wen, Y.C.; Lin, Y.W.; Yang, S.F.; Lee, W.J.; Chien, M.H. Quercetin simultaneously induces G0/G1-phase arrest and caspase-mediated crosstalk between apoptosis and autophagy in human leukemia HL-60 cells. Environ. Toxicol. 2017, 32, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic compounds cannabidiol, curcumin and quercetin cause mitochondrial dysfunction and suppress acute lymphoblastic leukemia cells. Int. J. Mol. Sci. 2020, 22, 204. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.C.; Maso, V.; Torello, C.O.; Ferro, K.P.; Saad, S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenet. 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Hackanson, B.; Poetsch, A.R.; Zucknick, M.; Sonnet, M.; Blagitko-Dorfs, N.; Hiller, J.; Wilop, S.; Brümmendorf, T.H.; Galm, O. Quantitative analyses of DAPK1 methylation in AML and MDS. Int. J. Cancer 2012, 131, E138–E142. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Treppendahl, M.B.; Asmar, F.; Girkov, M.S.; Nielsen, H.M.; Kjeldsen, T.E.; Ralfkiaer, E.; Hansen, L.L.; Grønbæk, K. Investigation of MGMT and DAPK1 methylation patterns in diffuse large B-cell lymphoma using allelic MSP-pyrosequencing. Sci. Rep. 2013, 3, 2789. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Bio. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Ding, Y.; Xiu, B.; Li, P.; Dong, Y.; Zhu, Q.; Liang, A. Up-regulation of VEGF and its receptor in refractory leukemia cells. Int. J. Clin. Exper. Pathol. 2015, 8, 5282. [Google Scholar]
- Lee, W.-J.; Hsiao, M.; Chang, J.-L.; Yang, S.-F.; Tseng, T.-H.; Cheng, C.-W.; Chow, J.-M.; Lin, K.-H.; Lin, Y.-W.; Liu, C.-C. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117. [Google Scholar] [CrossRef]
- Rahbaran, M.; Razeghian, E. Anti-leukemic effects of the quercetin on human leukemia U937 cells mediated by down-regulation of Mcl-1, survivin, and XIAP. Ann. Cancer Res. Therap. 2021, 29, 55–61. [Google Scholar] [CrossRef]
- Baron, B.W.; Thirman, M.J.; Giurcanu, M.C.; Baron, J.M. Quercetin therapy for selected patients with PIM1 kinase-positive chronic lymphocytic leukemia/small lymphocytic lymphoma: A pilot study. Acta Haematol. 2018, 139, 132–139. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Cri. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Pirbaluti, M.; Pourgheysari, B.; Shirzad, H.; Sourani, Z.; Beshkar, P. The inhibitory effect of Epigallocatechin gallate on the viability of T lymphoblastic leukemia cells is associated with increase of caspase-3 level and Fas expression. Indian J. Hematol. Blood Transfus. 2018, 34, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.H.; Sedighi, S.; Moradzadeh, M. A comparative study of anti-leukemic effects of kaempferol and epigallocatechin-3-gallate (EGCG) on human leukemia HL-60 cells. Avicenna J. Phytomed. 2021, 11, 314. [Google Scholar] [PubMed]
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. Leuk. Lymphoma 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.-S.; Xu, P.-P.; Qian, Y.; Wang, A.-H.; Xiao, D.; Zhao, Y.; Sheng, Y.; Wen, X.-Q.; Zhao, W.-L. Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARα oncoprotein degradation. J. Hematol. Oncol. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Torello, C.O.; Shiraishi, R.N.; Della Via, F.I.; de Castro, T.C.L.; Longhini, A.L.; Santos, I.; Bombeiro, A.L.; Silva, C.L.A.; de Souza Queiroz, M.L.; Rego, E.M. Reactive oxygen species production triggers green tea-induced anti-leukaemic effects on acute promyelocytic leukaemia model. Cancer Lett. 2018, 414, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.; Ward, J.; Anver, M.; Berman, J.; Brayton, C.; Cardiff, R.; Carter, J.; de Coronado, S.; Downing, J.; Fredrickson, T. Hematopathology subcommittee of the Mouse Models of Human Cancers Consortium Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood 2002, 100, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Della Via, F.I.; Shiraishi, R.N.; Santos, I.; Ferro, K.P.; Salazar-Terreros, M.J.; Franchi Junior, G.C.; Rego, E.M.; Saad, S.T.O.; Torello, C.O. (–)-Epigallocatechin-3-gallate induces apoptosis and differentiation in leukaemia by targeting reactive oxygen species and PIN1. Sci. Rep. 2021, 11, 9103. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (−)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK 2/STAT 3/AKT signalling pathways. Clin. Exper. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef]
- Kroemer, G.; Martin, S.J. Caspase-independent cell death. Nat. Med. 2005, 11, 725–730. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Roustazadeh, A.; Tabarraei, A.; Erfanian, S.; Sahebkar, A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARα and HDAC1. Phytother. Res. 2018, 32, 471–479. [Google Scholar] [CrossRef]
- Goker, B.; Caliskan, C.; Caglar, H.O.; Kayabasi, C.; Balci, T.; Tepedelen, B.E.; Aygunes, D.; Susluer, S.Y.; Mutlu, Z.; Selvi, N. Synergistic effect of ponatinib and epigallocatechin-3-gallate induces apoptosis in chronic myeloid leukemia cells through altering expressions of cell cycle regulatory genes. J. BUON Off. J. Balk. Union Oncol. 2014, 19, 992–998. [Google Scholar]
- Wang, Y.-N.; Wang, J.; Yang, H.-N.; Zhang, B.-L.; Zhang, P.; Sun, P.-Y.; Zhang, N.; Wang, Y.; Sheng, J.; Wang, X.-J. The oxidation of (−)-epigallocatechin-3-gallate inhibits T-cell acute lymphoblastic leukemia cell line HPB-ALL via the regulation of Notch1 expression. RSC Adv. 2020, 10, 1679–1684. [Google Scholar] [CrossRef]
- Westphal, S.; McGeary, A.; Rudloff, S.; Wilke, A.; Penack, O. The green tea catechin epigallocatechin gallate ameliorates graft-versus-host disease. PLoS ONE 2017, 12, e0169630. [Google Scholar] [CrossRef]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and anti-oxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Cur. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A systematic review on natural anti-oxidant properties of resveratrol. Nat. Product Comm. 2018, 13, 1934578X1801300923. [Google Scholar]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Resveratrol: Biological and pharmaceutical properties as anti-cancer molecule. Biofactors 2010, 36, 360–369. [Google Scholar] [CrossRef]
- Jiao, G.E.; Yan, L.I.U.; Qiang, L.I.; Xia, G.U.O.; Ling, G.U.; Gui, Z.; Zhu, Y.P. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed. Environ. Sci. 2013, 26, 902–911. [Google Scholar]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol sensitizes carfilzomib-induced apoptosis via promoting oxidative stress in multiple myeloma cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Canturk, Z.; Dikmen, M.; Artagan, O.; Ozarda, M.G.; Ozturk, N. Cytotoxic Effects of Resveratrol, Rutin and Rosmarinic Acid on ARH–77 Human (Multiple Myeloma) Cell Line. Nat. Prod. Comm. 2016, 11, 1934578X1601101007. [Google Scholar] [CrossRef]
- Ma, R.; Yu, D.; Peng, Y.; Yi, H.; Wang, Y.; Cheng, T.; Shi, B.; Yang, G.; Lai, W.; Wu, X.; et al. Resveratrol induces AMPK and mTOR signaling inhibition-mediated autophagy and apoptosis in multiple myeloma cells. Acta Biochim. Biophys. Sin. 2021, 53, 775–783. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zheng, H.; Chan, M.T.; Wu, W.K.K. NEAT 1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017, 50, e12329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, S.; Ying, Y.; Zhou, R.; Mao, P. miR-196b/miR-1290 participate in the anti-tumor effect of resveratrol via regulation of IGFBP3 expression in acute lymphoblastic leukemia. Oncol. Rep. 2017, 37, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gao, Y.-Y.; Liu, B.-Q.; Niu, X.-F.; Zhuang, Y.; Wang, H.-Q. Resveratrol-induced cytotoxicity in human Burkitt’s lymphoma cells is coupled to the unfolded protein response. BMC Cancer 2010, 10, 445. [Google Scholar] [CrossRef]
- Muchtar, E.; Gertz, M.A.; Magen, H. A practical review on carfilzomib in multiple myeloma. Eur. J. Haematol. 2016, 96, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Jia, Y.; Liu, Z.; Shu, X.; Liu, K. Resveratrol increases anti-proliferative activity of bestatin through downregulating P-glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J. Cell. Biochem. 2016, 117, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.H.; Song, J.H.; Kim, T.S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Comm. 2010, 395, 104–110. [Google Scholar] [CrossRef]
- Wu, E.J.; Goussetis, D.J.; Beauchamp, E.; Kosciuczuk, E.M.; Altman, J.K.; Eklund, E.A.; Platanias, L.C. Resveratrol enhances the suppressive effects of arsenic trioxide on primitive leukemic progenitors. Cancer Biol. Therap. 2014, 15, 473–478. [Google Scholar] [CrossRef]
- Akram, M.; Shahab-Uddin, A.A.; Usmanghani, K.; Hannan, A.; Mohiuddin, E.; Asif, M. Curcuma longa and curcumin: A review article. Rom. J. Biol. Plant Biol. 2010, 55, 65–70. [Google Scholar]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. BioFactors 2013, 39, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Iweala, E.J.; Uche, M.E.; Dike, E.D.; Etumnu, L.R.; Dokunmu, T.M.; Oluwapelumi, A.E.; Okoro, B.C.; Dania, O.E.; Adebayo, A.H.; Ugbogu, E.A. Curcuma longa (Turmeric): Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicity profiles-A review. Pharmacol. Res.-Modern Chinese Med. 2023, 22, 100222. [Google Scholar] [CrossRef]
- Liao, W.; Xiang, W.; Wang, F.-F.; Wang, R.; Ding, Y. Curcumin inhibited growth of human melanoma A375 cells via inciting oxidative stress. Biomed. Pharmacother. 2017, 95, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J.-N. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Bio. Rep. 2015, 42, 267–275. [Google Scholar] [CrossRef]
- Mishra, D.; Singh, S.; Narayan, G. Curcumin induces apoptosis in Pre-B acute lymphoblastic leukemia cell lines via PARP-1 cleavage. Asian Pac. J. Cancer Prev. 2016, 17, 3865–3869. [Google Scholar]
- Shah, K.; Mirza, S.; Desai, U.; Jain, N.; Rawal, R. Synergism of curcumin and cytarabine in the down regulation of multi-drug resistance genes in acute myeloid leukemia. Anticancer Agents Med. Chem. 2016, 16, 128–135. [Google Scholar] [CrossRef]
- Pimentel-Gutiérrez, H.J.; Bobadilla-Morales, L.; Barba-Barba, C.C.; Ortega-De-La-Torre, C.; Sánchez-Zubieta, F.A.; Corona-Rivera, J.R.; González-Quezada, B.A.; Armendáriz-Borunda, J.S.; Silva-Cruz, R.; Corona-Rivera, A. Curcumin potentiates the effect of chemotherapy against acute lymphoblastic leukemia cells via downregulation of NF-κB. Oncol. Lett. 2016, 12, 4117–4124. [Google Scholar] [CrossRef]
- Pesakhov, S.; Nachliely, M.; Barvish, Z.; Aqaqe, N.; Schwartzman, B.; Voronov, E.; Sharoni, Y.; Studzinski, G.P.; Fishman, D.; Danilenko, M. Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 2016, 7, 31847. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W.; Szade, K.; Bukowska-Straková, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Develop. Therap. 2016, 10, 557. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Diamond, T.H.; Alexander, W.; Manoharan, A.; Golombick, T. Use of Curcumin in Multiple Myeloma patients intolerant of steroid therapy. Clin. Case Rep. 2020, 8, 739–744. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am. J. Hematol. 2012, 87, 455–460. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Long term use of curcumin in two smoldering multiple myeloma patients. J. Hematol. Malig. 2013, 3, 18–32. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. B-cell disorders and curcumin. Integr. Cancer Therap. 2017, 16, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shan, Q.; Gong, Y.; Lin, J.; Shi, F.; Shi, R.; Yang, X. Curcumin induces apoptosis via simultaneously targeting AKT/mTOR and RAF/MEK/ERK survival signaling pathways in human leukemia THP-1 cells. Die Pharm. Int. J. Pharm. Sci. 2014, 69, 229–233. [Google Scholar]
- Yang, C.-W.; Chang, C.-L.; Lee, H.-C.; Chi, C.-W.; Pan, J.-P.; Yang, W.-C. Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK Pathways. BMC Complement. Altern. Med. 2012, 12, 22. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Dai, H.-P.; Shen, Q.; Ji, O.; Zhang, Q.; Zhai, Y.-L. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharma. Biol. 2016, 54, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Shen, Q.; Jiang, H.; Ji, O.; Zhu, L.; Zhang, L. Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharma. Biol. 2020, 58, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Domina, A.; Lim, G.; Chang, T.; Zhang, T. Evaluation of curcumin, a natural product in turmeric, on Burkitt lymphoma and acute myeloid leukemia cancer stem cell markers. Fut. Oncol. 2018, 14, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Pironi, A.M.; de Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef]
- Mendes, V.I.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Wu, P.-P.; Zhang, B.-J.; Cui, X.-P.; Yang, Y.; Jiang, Z.-Y.; Zhou, Z.-H.; Zhong, Y.-Y.; Mai, Y.-Y.; Ouyang, Z.; Chen, H.-S. Synthesis and biological evaluation of novel ursolic acid analogues as potential α-glucosidase inhibitors. Sci. Rep. 2017, 7, 45578. [Google Scholar] [CrossRef]
- Uto, T.; Sakamoto, A.; Tung, N.H.; Fujiki, T.; Kishihara, K.; Oiso, S.; Kariyazono, H.; Morinaga, O.; Shoyama, Y. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines. Int. J. Mol. Sci. 2013, 14, 4106–4120. [Google Scholar] [CrossRef] [PubMed]
- Kaewthawee, N.; Brimson, S. The effects of ursolic acid on cytokine production via the MAPK pathways in leukemic T-cells. EXCLI J. 2013, 12, 102. [Google Scholar]
- Lin, Z.; Jiang, J.; Liu, X.-S. Ursolic acid-mediated apoptosis of K562 cells involves Stat5/Akt pathway inhibition through the induction of Gfi-1. Sci. Rep. 2016, 6, 33358. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.-M.; Wang, J.-S.; Shen, J.; Xing, Y.-Y.; Xi, T. Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPβ expression by ERK pathway activation. Anticancer Drugs 2011, 22, 158–165. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, S.; Budhraja, A.; Gao, Z.; Chen, J.; Liu, E.H.; Huang, C.; Chen, D.; Yang, Z.; Liu, Q. Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 2012, 165, 1813–1826. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, R.; Feng, T.; Chen, L.; Ying-Ying, X.; Tao, X. Ursolic acid induces U937 cells differentiation by PI3K/Akt pathway activation. Chin. J. Nat. Med. 2014, 12, 15–19. [Google Scholar]
- Wu, B.; Wang, X.; Chi, Z.-f.; Hu, R.; Zhang, R.; Yang, W.; Liu, Z.-G. Ursolic acid-induced apoptosis in K562 cells involving upregulation of PTEN gene expression and inactivation of the PI3K/Akt pathway. Arch. Pharmacal Res. 2012, 35, 543–548. [Google Scholar] [CrossRef]
- Song, G.R.; Park, Y.J.C.S.J.; Shin, S.; Lee, G.; Choi, H.J.; Lee, D.Y.; Song, G.-Y.; Oh, S. Root Bark of Morus alba L. and Its Bioactive Ingredient, Ursolic Acid, Suppress the Proliferation of Multiple Myeloma Cells by Inhibiting Wnt/β-Catenin Pathway. J. Microbiol. Biotechnol. 2021, 31, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, D.; Sennari, Y.; Zeng, Z.; Baba, R.; Morimoto, H.; Kitamura, N.; Nakanishi, T.; Tsukada, J.; Ueno, M. Pentacyclic triterpenoid ursolic acid induces apoptosis with mitochondrial dysfunction in adult T-cell leukemia MT-4 cells to promote surrounding cell growth. Med. Oncol. 2022, 39, 118. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.-Z.; Xuan, Y.-Y.; Ruan, S.-Q.; Sun, M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin. J. Integr. Med. 2011, 17, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.H.; Yun, J.-M. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid. Based Complement. Altern. Med. 2012, 2012, 639469. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, B.; Wang, X.; Zhu, Y.; Hu, L.; Li, P.; Zhang, A.; Chen, H.; Liu, M.; Tan, C. Fisetin lowers Streptococcus suis serotype 2 pathogenicity in mice by inhibiting the hemolytic activity of suilysin. Front. Microbiol. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Adan, A.; Baran, Y. Fisetin and hesperetin induced apoptosis and cell cycle arrest in chronic myeloid leukemia cells accompanied by modulation of cellular signaling. Tumor Biol. 2016, 37, 5781–5795. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Lin, J.-J.; Ma, Y.-S.; Peng, S.-F.; Huang, A.-C.; Huang, Y.-P.; Fan, M.-J.; Lien, J.-C.; Chung, J.-G. Fisetin inhibits cell proliferation through the induction of G0/G1 phase arrest and caspase-3-mediated apoptosis in mouse leukemia cells. Am. J. Chin. Med. 2019, 47, 841–863. [Google Scholar] [CrossRef]
- Jang, K.Y.; Jeong, S.-J.; Kim, S.-H.; Jung, J.H.; Kim, J.-H.; Koh, W.; Chen, C.-Y.; Kim, S.-H. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012, 319, 197–202. [Google Scholar] [CrossRef]
- Adan, A.; Baran, Y. The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumor Biol. 2015, 36, 8973–8984. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2010, 1805, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, X.; Su, L. Parthenolide induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wiśniewska, A.; Grzanka, D.; Czajkowska, P.; Hałas-Wiśniewska, M.; Durślewicz, J.; Antosik, P.; Grzanka, A.; Gagat, M. Cellular and molecular alterations induced by low-dose fisetin in human chronic myeloid leukemia cells. Int. J. Oncol. 2019, 55, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Mondal, R.; Tuli, H.S.; Kumar, G.; Sharma, A.K. Molecular targets of gambogic acid in cancer: Recent trends and advancements. Tumor Biol. 2016, 37, 12915–12925. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Liu, P.; Wu, X.; Chen, B. Gambogenic acid synergistically potentiates bortezomib-induced apoptosis in multiple myeloma. J. Cancer 2017, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, X.; Li, X.; Lan, X.; Zhao, C.; Liu, S.; Huang, H.; Liu, N.; Liao, S.; Song, W. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin. Cancer Res. 2014, 20, 151–163. [Google Scholar] [CrossRef]
- Shi, X.; Lan, X.; Chen, X.; Zhao, C.; Li, X.; Liu, S.; Huang, H.; Liu, N.; Zang, D.; Liao, Y. Gambogic acid induces apoptosis in diffuse large B-cell lymphoma cells via inducing proteasome inhibition. Sci. Rep. 2015, 5, 9694. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.; Zhang, Q.; Xu, J.; Ouyang, J. Anti-cancer effect and apoptosis induction of gambogic acid in human leukemia cell line K562 in vitro. Med. Sci. Monit. 2015, 21, 1604. [Google Scholar]
- Li, W.; Wang, S.; Feng, J.; Xiao, Y.; Xue, X.; Zhang, H.; Wang, Y.; Liang, X. Structure elucidation and NMR assignments for curcuminoids from the rhizomes of Curcuma longa. Magn. Reson. Chem. 2009, 47, 902–908. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Dai, L.; Ge, Z.; Gao, C.; Zhang, H.; Wang, F.; Zhang, X.; Chen, B. Gambogenic acid exerts anti-tumor activity in hypoxic multiple myeloma cells by regulation of miR-21. J. Cancer 2017, 8, 3278. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Guo, L.; Bao, W.; Jin, N.; Liu, R.; Liu, P.; Wang, Y.; Guo, Q.; Chen, B. Gambogic acid suppresses hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial growth factor expression via inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target protein of rapamycin pathway in multiple myeloma cells. Cancer Sci. 2014, 105, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Leone, E.; Morelli, E.; Di Martino, M.T.; Amodio, N.; Foresta, U.; Gullà, A.; Rossi, M.; Neri, A.; Giordano, A.; Munshi, N.C. Targeting miR-21 Inhibits In Vitro and In Vivo Multiple Myeloma Cell Growth Anti-tumor Activity of mir-21 Inhibitors in Multiple Myeloma. Clin. Cancer Res. 2013, 19, 2096–2106. [Google Scholar] [CrossRef]
- Wang, T.; Du, J.; Kong, D.; Yang, G.; Zhou, Q.; You, F.; Lin, Y.; Wang, Y. Gambogic acid inhibits proliferation and induces apoptosis of human acute T-cell leukemia cells by inducing autophagy and downregulating β-catenin signaling pathway: Mechanisms underlying the effect of Gambogic acid on T-ALL cells. Oncol. Rep. 2020, 44, 1747–1757. [Google Scholar] [CrossRef]
- Pandey, M.K.; Kale, V.P.; Song, C.; Sung, S.-s.; Sharma, A.K.; Talamo, G.; Dovat, S.; Amin, S.G. Gambogic acid inhibits multiple myeloma mediated osteoclastogenesis through suppression of chemokine receptor CXCR4 signaling pathways. Exp. Hematol. 2014, 42, 883–896. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Chen, Y.; Hui, H.; Li, Z.; Wang, H.-M.; You, Q.-D.; Lu, N. Gambogic acid induces growth inhibition and differentiation via upregulation of p21waf1/cip1 expression in acute myeloid leukemia cells. J. Asian Nat. Prod. Res. 2014, 16, 1000–1008. [Google Scholar] [CrossRef]
- Yang, L.-J.; Chen, Y.; He, J.; Yi, S.; Wen, L.; Zhao, S.; Cui, G.-H. Effects of gambogic acid on the activation of caspase-3 and downregulation of SIRT1 in RPMI-8226 multiple myeloma cells via the accumulation of ROS. Oncol. Lett. 2012, 3, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Hay, H.S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P. Celastrol inhibits proliferation and induces chemosensitization through down-regulation of NF-κB and STAT3 regulated gene products in multiple myeloma cells. Br. J. Pharmacol. 2011, 164, 1506–1521. [Google Scholar] [CrossRef]
- Ng, S.W.; Chan, Y.; Chellappan, D.K.; Madheswaran, T.; Zeeshan, F.; Chan, Y.L.; Collet, T.; Gupta, G.; Oliver, B.G.; Wark, P. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed. Pharmacother. 2019, 109, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Ocio, E.M.; San Miguel, J.F. Novel generation of agents with proven clinical activity in multiple myeloma. Semin. Oncol. 2013, 40, 618–633. [Google Scholar] [CrossRef] [PubMed]
- Vincenz, L.; Jäger, R.; O’Dwyer, M.; Samali, A. Endoplasmic Reticulum Stress and the Unfolded Protein Response: Targeting the Achilles Heel of Multiple MyelomaUPR in Multiple Myeloma. Mol. Cancer Ther. 2013, 12, 831–843. [Google Scholar] [CrossRef]
- Zhong, Y.-l.; Xu, G.-j.; Huang, S.; Zhao, L.; Zeng, Y.; Xiao, X.-f.; An, J.-l.; Liu, J.; Yang, T. Celastrol induce apoptosis of human multiple myeloma cells involving inhibition of proteasome activity. Eur. J. Pharmacol. 2019, 853, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jin, Y.; Qiu, L.; Lai, Y.; Pan, J. Celastrol, a novel HSP90 inhibitor, depletes Bcr–Abl and induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation. Cancer Lett. 2010, 290, 182–191. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, P.Y.; Mu, H.; Zhou, H.; Mak, D.H.; Schober, W.; Leverson, J.D.; Zhang, B.; Bhatia, R.; Huang, X. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl. Med. 2016, 8, ra117–ra355. [Google Scholar] [CrossRef]
- Yang, K.; Fu, L.-W. Mechanisms of resistance to BCR–ABL TKIs and the therapeutic strategies: A review. Crit. Rev. Oncol. Hematol. 2015, 93, 277–292. [Google Scholar] [CrossRef]
- Ni, H.; Zhao, W.; Kong, X.; Li, H.; Ouyang, J. NF-kappa B modulation is involved in celastrol induced human multiple myeloma cell apoptosis. PLoS ONE 2014, 9, e95846. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol attenuates the invasion and migration and augments the anti-cancer effects of bortezomib in a xenograft mouse model of multiple myeloma. Front. Pharmacol. 2018, 9, 365. [Google Scholar] [CrossRef]
- Tozawa, K.; Sagawa, M.; Kizaki, M. Quinone methide tripterine, celastrol, induces apoptosis in human myeloma cells via NF-κB pathway. Int. J. Oncol. 2011, 39, 1117–1122. [Google Scholar]
- Davenport, A.; Frezza, M.; Shen, M.; Ge, Y.; Huo, C.; Chan, T.H.; Dou, Q.P. Celastrol and an EGCG pro-drug exhibit potent chemosensitizing activity in human leukemia cells. Int. J. Mol. Med. 2010, 25, 465–470. [Google Scholar]
- Zhang, X.; Yang, J.; Chen, M.; Li, L.; Huan, F.; Li, A.; Liu, Y.; Xia, Y.; Duan, J.-a.; Ma, S. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 2016, 7, 46557. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Huang, L.-B.; Lin, W.-H.; Wang, L.-N.; Tian, Y.; Shi, D.; Wang, J.; Qin, G.; Li, A.; Liang, Y.-N. Butein inhibits cell proliferation and induces cell cycle arrest in acute lymphoblastic leukemia via FOXO3a/p27kip1 pathway. Oncotarget 2016, 7, 18651. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, L.; Shen, A.; Zhang, J.; Wang, Y.; Zhao, Y.; Zou, L.; Ke, Q.; He, F.; Wang, P. Clinical and biological significance of forkhead class box O 3a expression in glioma: Mediation of glioma malignancy by transcriptional regulation of p27kip1. J. Neuro-Oncol. 2010, 98, 57–69. [Google Scholar] [CrossRef]
- Yan, F.; Liao, R.; Lin, S.; Deng, X.; Little, P.J.; Zheng, W. Forkhead box protein O3 suppresses uveal melanoma development by increasing the expression of Bcl-2-like protein 11 and cyclin-dependent kinase inhibitor 1B. Mol. Med. Rep. 2018, 17, 3109–3114. [Google Scholar] [CrossRef]
- Moon, D.-O.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. Butein suppresses c-Myc-dependent transcription and Akt-dependent phosphorylation of hTERT in human leukemia cells. Cancer Lett. 2009, 286, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Senba, M.; Mori, N. Butein inhibits NF-κB, AP-1 and Akt activation in adult T-cell leukemia/lymphoma. Int. J. Oncol. 2017, 51, 633–643. [Google Scholar] [CrossRef]
- Woo, S.M.; Choi, Y.K.; Kim, A.J.; Cho, S.G.; Ko, S.G. p53 causes butein-mediated apoptosis of chronic myeloid leukemia cells. Mol. Med. Rep. 2016, 13, 1091–1096. [Google Scholar] [CrossRef]
- Kim, N. Butein sensitizes human leukemia cells to apoptosis induced by tumor necrosis factor-related apoptosis inducing ligand (TRAIL). Arch. Pharmacal Res. 2008, 31, 1179–1186. [Google Scholar] [CrossRef]
- Hong, X.Y.; Jm, W.; Luo, X.Q.; Liu, K.; Zhou, W.J.; Zhang, G.W. Research progress of inhibitory effects of polyphenolic compounds on xanthine oxidase. Food Mach. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and anti-oxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
 indicate downregulation and
indicate downregulation and  indicate upregulation.
indicate upregulation.
 indicate downregulation and
indicate downregulation and  indicate upregulation.
indicate upregulation.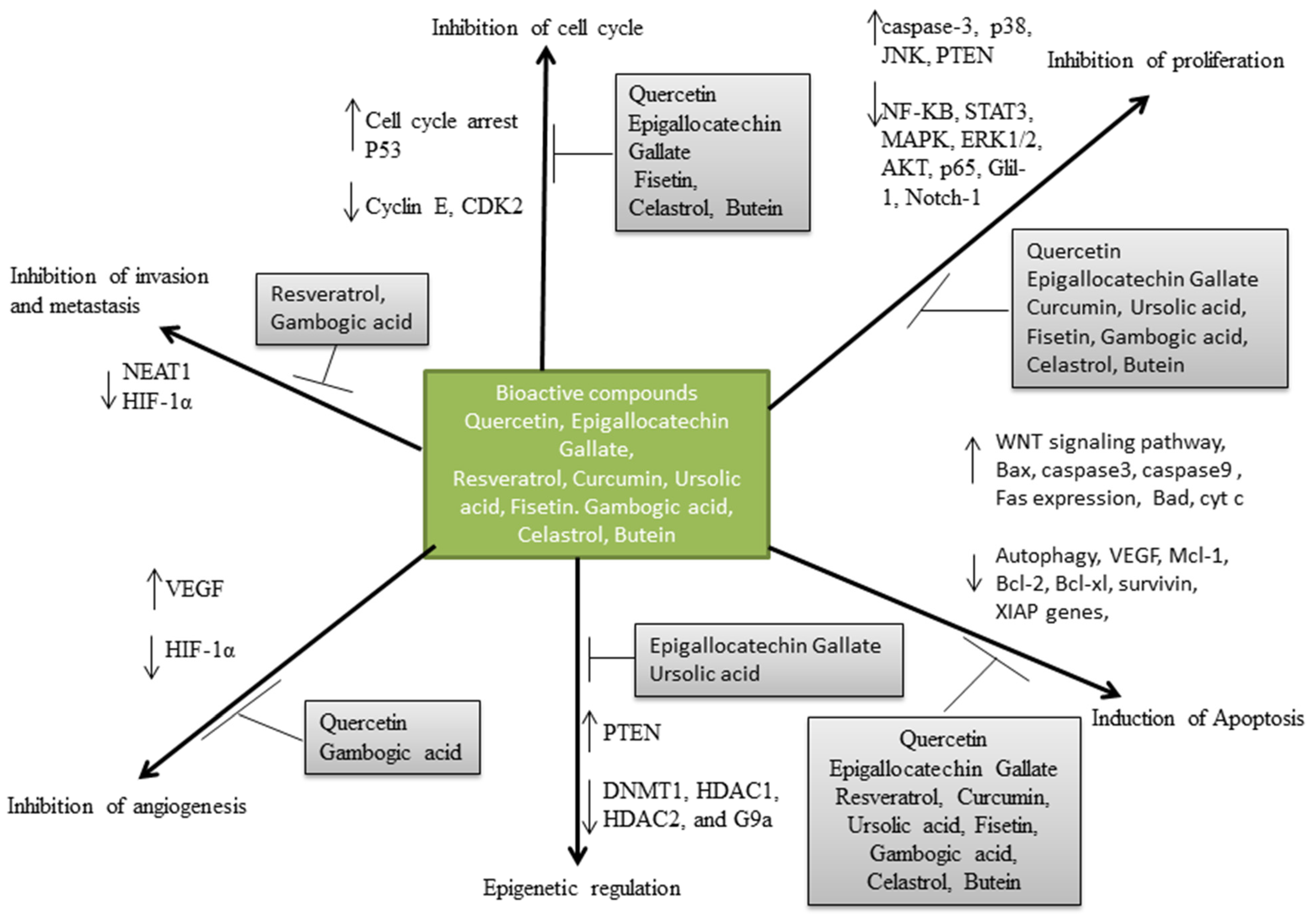
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iweala, E.J.; Oluwapelumi, A.E.; Dania, O.E.; Ugbogu, E.A. Bioactive Phytoconstituents and Their Therapeutic Potentials in the Treatment of Haematological Cancers: A Review. Life 2023, 13, 1422. https://doi.org/10.3390/life13071422
Iweala EJ, Oluwapelumi AE, Dania OE, Ugbogu EA. Bioactive Phytoconstituents and Their Therapeutic Potentials in the Treatment of Haematological Cancers: A Review. Life. 2023; 13(7):1422. https://doi.org/10.3390/life13071422
Chicago/Turabian StyleIweala, Emeka J., Adurosakin E. Oluwapelumi, Omoremime E. Dania, and Eziuche Amadike Ugbogu. 2023. "Bioactive Phytoconstituents and Their Therapeutic Potentials in the Treatment of Haematological Cancers: A Review" Life 13, no. 7: 1422. https://doi.org/10.3390/life13071422
APA StyleIweala, E. J., Oluwapelumi, A. E., Dania, O. E., & Ugbogu, E. A. (2023). Bioactive Phytoconstituents and Their Therapeutic Potentials in the Treatment of Haematological Cancers: A Review. Life, 13(7), 1422. https://doi.org/10.3390/life13071422






