Abstract
Endoclita signifer is a prominent wood-boring insect species in eucalyptus plantations in Guangxi, China, causing significant ecological and economic damage. A novel approach to controlling the challenging wood-boring pest involves disrupting the olfactory communication between insects and the volatile compounds emitted by plants. To identify the olfactory proteins contributing to host selection based on 11 GC-EAD-active volatiles from eucalyptus leaves and to discover the highly expressed olfactory proteins, we conducted a study on the antennal transcriptomes of adult E. signifer and screened key olfactory proteins in the antennae. We identified a total of 69 olfactory proteins. When compared to the larval transcriptomes, the antennal transcriptome of adult E. signifer revealed the presence of 17 new odorant-binding proteins (OBPs), including 2 pheromone-binding proteins (PBPs), 7 previously unreported chemosensory proteins (CSPs), 17 new odorant receptors (ORs), 4 new gustatory receptors (GRs), 11 novel ionotropic receptors (IRs), and 2 sensory neuron membrane proteins (SNMPs). Through the phylogenetic tree of OBPs and ORs, we identified EsigPBP2 and EsigPBP3 as two of the three PBPs, designated EsigOR13 as EsigOrco, and recognized EsigOR10 and EsigOR22 as the newly discovered EsigPRs in E. signifer. In the adult antennae, the expression levels of EsigGOBP14, EsigGOBP13, EsigOBP14, EsigOBP17, EsigCSP14, and EsigOR16 were notably high, indicating that these proteins could be pivotal in binding to plant volatiles.
1. Introduction
Endoclita signifer Walker (ghost moth, Lepidoptera, Hepialidae) is the major wood-boring pest of eucalyptus that has spread extensively across Guangxi, China, causing substantial damage to eucalyptus trees and ecosystems [1]. As an omnivorous pest, the native host plants of E. signifer include 30 families, 40 genera, and 51 species [2]. Interestingly, with the widespread establishment of eucalyptus plantations in Guangxi, E. signifer has transitioned from its native host plants to eucalyptus, serving as a notable example of a native pest adapting to introduced hosts. Meanwhile, the third instar larvae of E. signifer display a unique behavior pattern: they move from the soil of eucalyptus plantations to standing trees, where they feed on bark and bore into the interior wood. And the phenomenon, along with the damage inflicted by E. signifer on eucalyptus trees within one to three years, indicates that both larval [3,4] and adult host selection contribute to its adaptation to eucalyptus. And because the sensitive olfactory system of the insect plays a key role in host selection, mating, and feeding, we hypothesized that the olfactory system of adult E. signifer significantly contributes to its host selection, with olfactory proteins serving as the functional components in this process.
The first odorant-binding protein (OBP) was discovered in Antheraea polyphemus. It was a small, 15 kD soluble protein uniquely found in the male antennae, capable of binding to labeled pheromones in native gels [5]. OBPs play a pivotal role in the initial stages of odor detection, involving the identification, screening, binding, and transportation of odor molecules. Studies examining their function have revealed their significance in neuronal activation. This was initially demonstrated through research on mutants lacking the OBP LUSH, which exhibited insensitivity to concentrations of the male-specific pheromone 11-cis vaccenyl acetate (cVA), which strongly activates neurons expressing the Or67d receptor subunit in Drosophila [6]. Chemosensory proteins (CSPs), unlike OBPs, exhibit a less rigid structure, allowing them to bind ligands with a broader ligand [7], and a study showed that CSPs in coleopteran insects have duplication and differentiation under the role of natural selection [8]. The mechanism of transporting odor molecules from binding proteins to receptors is similar in Ostrinia furnacalis. Specifically, the sex pheromones E-12-tetradecenyl acetate and Z-12-tetradecenyl acetate are recognized and bound by the pheromone-binding proteins (PBPs) OfurPBP3 and OfurPBP2. Subsequently, these bound pheromones are transported to the odorant receptors (ORs) OfurOR4 and OfurOR6, leading to their activation [9]. Factually, ORs function as the primary receptors, and they form heteromers with a shared, highly conserved subunit known as the OR coreceptor (Orco) in insects [10,11]. Orco has evolved to act as a coreceptor, enabling a unified mechanism for regulating odorant sensitivity that operates independently of the expression of individual tuning receptors [12]. The first identified pheromone receptor (PR) in Drosophila was Or67d. It was subsequently discovered that a mutation at amino acid 23 in Or67d caused the substitution of cysteine with tryptophan, and mutation Or67d still expressed but was entirely unresponsive to the cVA pheromone [13]. However, putative insect olfactory receptors are not highly conserved among insect species [14]. Indeed, in addition to odorant receptors (ORs), insects also have gustatory receptors (GRs), ionotropic receptors (IRs), and sensory neuron membrane proteins (SNMPs) that function as part of the olfactory receptor system. These various receptor types collectively contribute to the insects’ complex sensory perception, enabling them to detect and respond to a wide range of environmental cues.
Research in the field of insect olfaction has predominantly centered around behaviors linked to reproduction, including the detection of sex pheromones, mating, and the selection of host and predator volatiles [6,7,8]. In E. signifer larvae, our initial investigation focused on the volatiles emitted by eucalyptus trunks and the humus of the forest floor when the third instar larvae transitioned to eucalyptus as their host. We identified a total of 34 volatile compounds, and, among them, alpha-phellandrene stood out as one of the most prominent and volatile herbivore-induced plant volatiles (HIPVs) [4]. Secondly, we screened the function volatile by electrophysiology and behavior choice. We found that 11 volatiles were GC-EAD-active compounds, and o-cymene stimulated significant behavior attraction in third instar E. signifer larvae [4]. Finally, through the transcriptome of larvae heads (different instar) and tegument, we identified 62 olfactory proteins [15,16]; furthermore, we demonstrated that EsigGOBP1 was the key protein for binding alpha-phellandrene [17]. All the above results indicated that E. signifer larvae possess the ability to select their host, and olfactory proteins play a significant role in this selection process. In adult E. signifer, 40 volatile compounds were identified from one- and five-year-old eucalyptus leaves, in which 11 volatiles were GC-EAD-active compounds, and we observed an increasing trend in the electroantennogram (EAG) response as the concentration of the stimulus sample increased (unpublished data). However, we have yet to identify the key olfactory proteins of E. signifer adult and elucidate how they contribute to the host selection process.
This study examined the antennal transcriptomes of adult E. signifer and analyzed the expression profiles of these transcriptomes, with a particular focus on identifying key proteins associated with the recognition of 11 GC-EAD-active volatiles in adult E. signifer. By comparing these key proteins with previous findings, we aimed to uncover the olfactory proteins responsible for recognizing plant volatiles. This research has the potential to offer novel insights into pest control strategies.
2. Results
2.1. Transcriptome Sequencing and Sequence Assembly
We generated 48 million raw reads from each cDNA library of the E. signifer adult, averagely. Approximately 94.09% of these reads exhibited q30 quality scores on average. The number of 44,905 unigenes, with an N50 of 1488 bp, average length of 859 bp, were obtained (Figure 1 and Table 1). Additionally, BUSCO analysis indicated a completion rate of 89.30%.
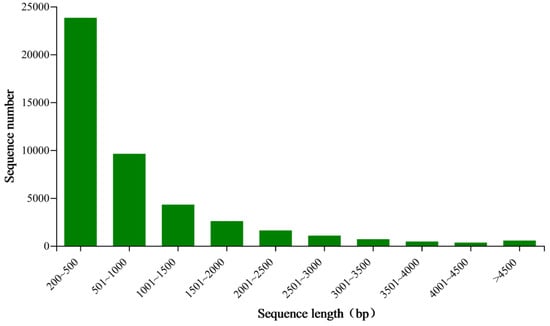
Figure 1.
Length distribution of unigenes in the antennal transcriptome of E. signifer.

Table 1.
Quality control, number, and length of unigenes in Endoclita signifer adult antennal transcriptome.
2.2. Homology Analysis and Gene Ontology Annotation
Approximately 34.82% showed matches to entries in the Nr protein database through BLASTx, with an E-value cutoff of 1e-5. The top sequence matches were found in Eumeta japonica (1228, 7.85%), followed by O. furnacalis (714, 4.57%), Chilo suppressalis (713, 4.56%), Spodoptera litura (706, 4.51%,) and so on (Figure 2).
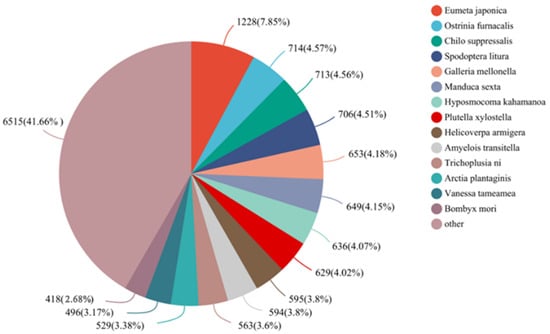
Figure 2.
Species distribution of antennal unigenes of E. signifer in the Nr database.
The gene ontology (GO) annotations classified the 11,147 transcripts into various functional groups using BLAST2GO with P value calculated by a hypergeometric distribution test and E-value less than 1 × 10–5. Binding accounted for most of the GO annotations in molecular function (52.24%), cellular process accounted for most in biology process (35.05%), and cell part (30.56%) accounted for most in cellular component, in antennal transcriptome of E. signifer (Figure 3).
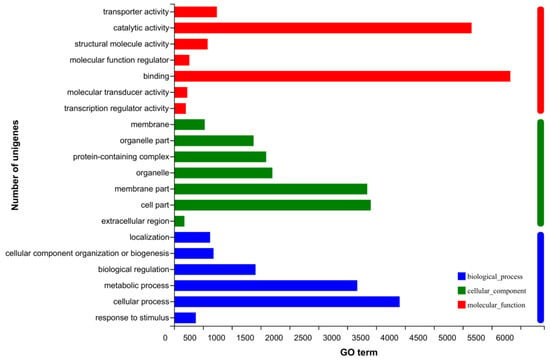
Figure 3.
Gene ontology (GO) classification of assembled E. signifer unigenes.
Through the integration of KEGG database analysis, we identified 8929 unigenes participating in 4 metabolic pathways. Among these pathways, the top three most annotated ones are as follows: signal transduction accounting for 11.98%, translation representing 8.65%; transport and catabolism contributing to 7.83%, respectively (Figure 4).
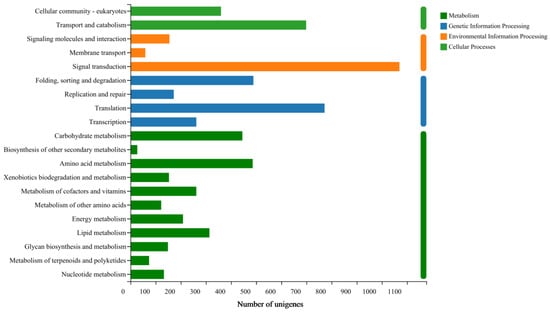
Figure 4.
Classification of E. signifer Walker transcriptome based on KEGG.
2.3. Olfactory Proteins
A total of 69 olfactory genes were identified. There are 22 transcripts encoding putative OBPs in E. signifer adult antennae, of which 5 were identified in the before head, thorax, and abdomen cuticula transcriptomes (Table 2 labeled with underline) [15]. Furthermore, among the identified OBPs, the top three in terms of expression levels were as follows: EsigPBP2 exhibited the highest expression, with a fragments per kilobase million (FPKM) value of 62,318.74; EsigPBP1 (also known as EsigGOBP7) followed closely behind, with an FPKM value of 53,518.17; EsigGOBP13 ranked third in expression levels, with an FPKM value of 33,180 (Table 2). We identified 10 chemosensory proteins (CSPs), among which EsigCSP1, EsigCSP2, and EsigCSP7 were previously identified (labeled with underline) [15], and the FPKM value showed EsigCSP14, EsigCSP15, and EsigCSP10 were the top three genes in terms of expression levels (Table 2). A total of 19 ORs were identified in adult antennal transcriptome, in which only EsigOR1 was identified before. It is interesting to observe that all EsigORs had much lower expression levels than binding proteins (EsigOBPs and EsigCSPs). Among EsigORs, EsigOR4, EsigOR18, and EsigOR16 were most highly expressed (Table 2). We identified four transcripts encoding new gustatory receptor GRs. It is noteworthy that EsigGRs had much lower expression levels than most EsigORs, compared with EsigORs (Table 2). We identified 12 ionotropic receptors, IRs, among which known EsigIR75p-6 was most highly expressed in the adult antennae (labeled with underline). Additionally, we successfully identified two new sensory neuron membrane proteins (SNMPs) in the antenna of E. signifer adult. All sequences of olfactory proteins were listed in Supplementary Materials Additional File S1.

Table 2.
Best BLASTx hits for putative chemosensory proteins of Endoclita signifer.
2.4. Phylogenetic Analysis of OBPs and ORs
According to the phylogenetic tree of OBPs supporting EsigGOBP7 as the PBP of E. signifer [15], in this study we changed EsigGOBP7 to EsigPBP1. In this phylogenetic tree of OBPs (Figure 5), the PBPs clades included EsigPBP1 (EsigGOBP7, red), EsigPBP2 (red), EsigPBP3 (red), PxylGOBP1, and all Lepidoptera PBPs. The PBP clade had a 100% support rate (labeled with blue). In the NJ tree of ORs (Figure 6), we found there were three clades; the PRs clade (yellow) was found to be a sister clade to the clade that contained the novel lineage of PRs clade (blue), Orco clade (pink), and all other EsigORs. What is more, there is the close relationship between the clade of Orco and novel lineage of PRs. Additionally, EsigOR10 and EsigOR22 are in the clade of novel lineage of PRs, while EsigOR13 was positioned within the Orco clade. Interestingly, there were no EsigORs found within the PRs clade itself (yellow).
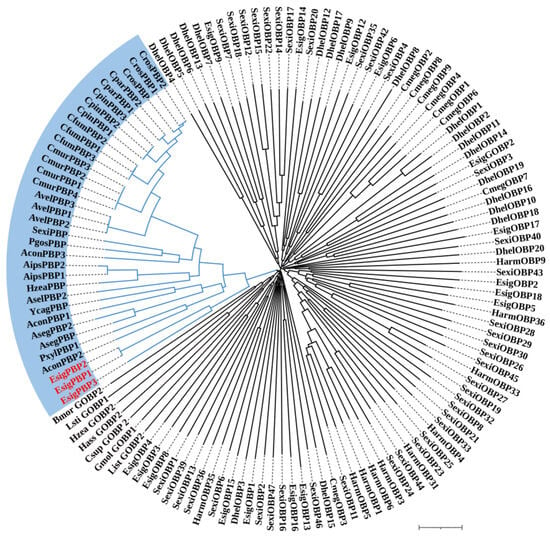
Figure 5.
Neighbor-joining phylogenetic tree of odorant-binding proteins (OBPs).
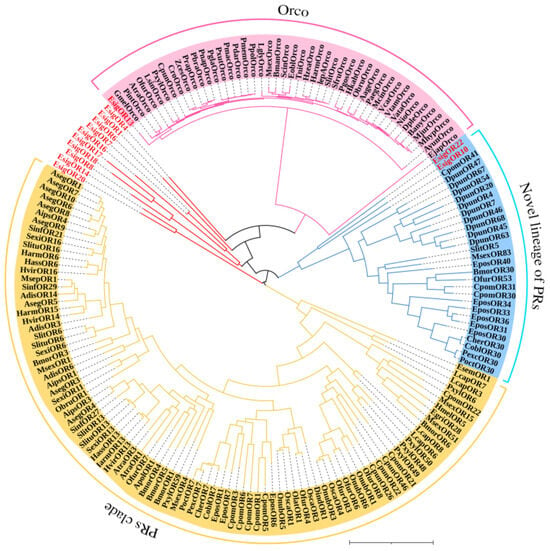
Figure 6.
Neighbor-joining phylogenetic tree of odorant receptors (ORs).
The NJ phylogenetic analysis of OBPs of E. signifer was performed with reference OBPs of Dastarcus helophoroides [18], Chrysomya megacephala [19], Plutella xylostella [20], S. exigua [21,22], H. armigera [23], and PBPs of Lepidoptera. Blue branch was PBPs clade. The stability of the nodes was assessed by bootstrap analysis with 1000 replications. The scale bar represents 0.1 substitutions per site.
The NJ phylogenetic analysis of ORs of E. signifer (red) was performed with reference ORs of the novel lineage of candidate pheromone receptors and pheromone receptor clade sequence in Bastin-He’line et al. [24] and Orcos of moth. Blue branch was the novel lineage of PRs clade. Yellow branch was the PRs clade. Pink branch was the Orco clade. The stability of the nodes was assessed by bootstrap analysis with 1000 replications. The scale bar represents 1.0 substitutions per site.
2.5. Expression of Binding Proteins and Olfactory Receptors in Adult Antenna
Based on the designed qRT-PCR primers and reference genes (Ribosomal protein, RIB, and Elongation factors, EF) [25]. The expression of 11 OBPs, 6 CSPs, and 4 ORs were determined in the adult antennae (Figure 7). All tested genes were expressed in adult antennae (Figure 7). For binding protein, EsigCSP14, EsigPBP2, and EsigGOBP14 exhibited the highest levels of gene expression. Among OBPs, EsigPBP2 and EsigGOBP14 had significantly higher expression levels than other all OBPs (p < 0.05). For PBPs, EsigPBP2 had significantly higher expression than EsigPBP3 (p < 0.05). EsigGOBP14 displayed significantly higher levels of expression compared to the other OBPs (p < 0.05), while the expression of the remaining OBPs did not exhibit any significant differences (Figure 7). In CSPs, the expression of EsigCSP14 was significantly higher than that of the other five CSPs (p < 0.05) (Figure 7). In ORs, their expression levels were generally lower compared to those of the binding proteins. The expression levels of EsigOR13 and EsigOR16 were the highest among the odorant receptors, with no significant difference observed between them. Both were significantly different from EsigOR14 and EsigOR22 (p < 0.05) (Figure 7).
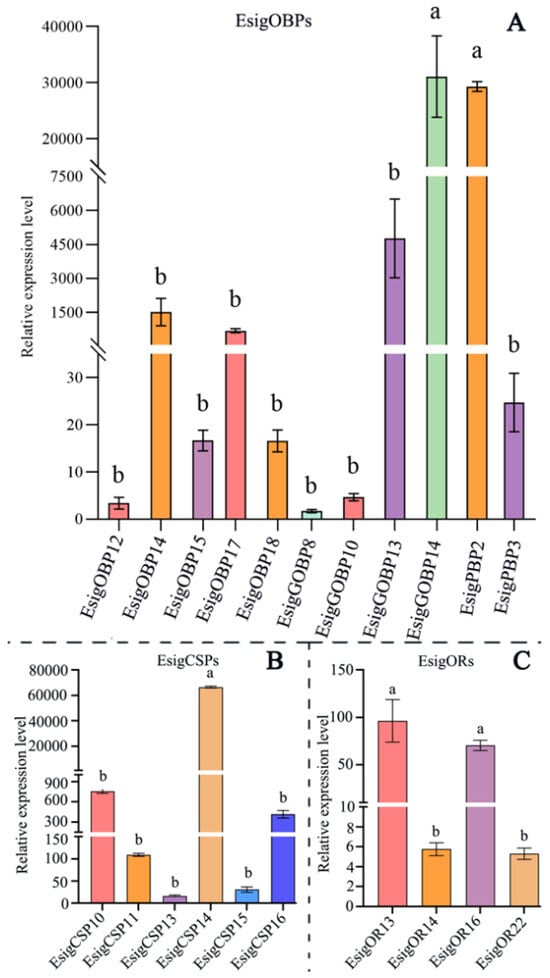
Figure 7.
Expression of olfactory-related proteins in the antennae of E. signifer Walker. Note: (A) EsigOBPs, (B) EsigCSPs, (C) EsigORs. The error bar represents the standard error. The different small letters a, b on the error bar indicates significant differences at p < 0.05.
3. Discussion
In the adult antennal transcriptome of E. signifer, we identified 69 olfactory-related genes, including 22 OBPs, 10 CSPs, 19 ORs, 4 GRs, 12 IRs, and 2 SNMPs. Interestingly, in comparison to the larvae, the adult antennal transcriptome unveiled a significant increase, with the identification 17 new OBPs (including 2 pheromone binding proteins), 7 new CSPs, 17 new ORs, 4 new GRs, 11 new IRs, and 2 new SNMPs [15,16]. This notable expansion in olfactory proteins in adults can be attributed to their heightened need for olfactory capabilities in order to recognize a more complex chemical environment. Another reason is the size difference between the tiny larvae antennae and the adult antennae. The adult antennae are more specialized for olfaction, and therefore, the focus on olfactory proteins and the annotation of a greater number of olfactory proteins is naturally more pronounced. Interestingly, the consistent expression of EsigGOBP2, EsigGOBP5, EsigCSP1, and EsigCSP2 across all E. signifer transcriptomes suggests that these proteins play a central role in olfaction throughout both the larval and adult stages. When comparing expression in olfactory tissues (larval head and adult antennae) from different development stages, EsigGOBP2, EsigGOBP5, EsigCSP1, EsigCSP2, EsigCSP7, EsigOR4, and EsigIR75p-6 were consistently expressed among the third and twelfth larvae and adults, showing their crucial role in olfaction and implying their main olfactory function. Odorant receptors were found to be less common across different tissues and developmental stages in E. signifer [15,16]. All were similar to Spodoptera littoralis, in that the caterpillars expressed a smaller set of olfactory genes than the adults, with the exception of SlitOBP21 and SlitGOBP1, which were adult-specific [26]. The identification of 69 olfactory-related genes in the antennal transcriptome of E. signifer adults places the species in the middle range when compared to other insect species. The number of olfactory-related genes is similar to that found in the antennal transcriptome of Glenea cantor [27], but fewer than in species like Mythimna loreyi with 138 olfactory-related genes [28], Monochamus saltuarius with 117 [29], and Podabrus annulatus with 101 [30]. On the other hand, E. signifer surpasses species like O. loti with 47 olfactory candidate genes in their antennae transcriptome [31] and Reticulitermes aculabialis with 16 olfactory genes [32].
Lepidoptera PBPs/GOBPs form a unique lineage of insect OBPs [33], and the number of PBPs varies among species [21]. The previous phylogenetic tree of OBPs supports EsigGOBP7 as the PBP of E. signifer [15]; in this study, the PBPs clades also included EsigPBP1, EsigPBP2, EsigPBP3, PxylGOBP1, and all Lepidoptera PBPs, further supporting the theory that EsigGOBP7 is a PBP. Gene expression analysis in PBPs revealed that EsigPBP2 had significantly higher expression than EsigPBP3 (p < 0.05), with FPKM values indicating a ten-thousand-fold higher expression level of EsigPBP2 compared to EsigPBP3. This pattern is similar to the expression of PBP1 and PBP2 in M. loreyi [28]. Additionally, EsigPBP1 also exhibited a ten-thousand-fold higher expression, suggesting that EsigPBP1 and EsigPBP3 have high expression tendencies and may serve as the main functional PBPs. Interestingly, in social insects, the expression of PBPs can exhibit role differentiation, with two PBPs showing significantly higher expression levels in alates compared to others in R. aculabialis [32]. For the newly identified OBPs, both qRT-PCR results and FPKM values supported EsigGOBP14, EsigGOBP13, EsigOBP14, and EsigOBP17 as the fourth highest expression OBPs in adult antennae, suggesting their potential roles as key proteins in binding plant volatiles. This pattern is similar to MusiOBP1, which was the highest expressed in primordial females in Megalurothrips usitatus [34]. In addition, OBPs often exhibited a male antennae-biased expression pattern, as observed in examples like OlotOBP1, OlotOBP4, and OlotOBP6 [31]. Moreover, OBPs are not restricted to expression in antennae; for instance, GcanOBP22 and GcanOBP25 were highly expressed in the wings and legs [27]. Furthermore, it is intriguing that the recognition between OBPs and host odors may be reduced by down-regulation in male M. saltuarius infested with Bursaphelenchus xylophilus, which could further affect the spread of B. xylophilus to new hosts [29]. Hence, it is crucial to explore the expression patterns of these key EsigOBPs in a more systematic manner to gain a better understanding of their roles in olfaction and host selection in E. signifer.
The results from both the FPKM values and qRT-PCR analysis confirm that EsigCSP14 exhibits higher expression compared to EsigCSP10, EsigCSP11, EsigCSP13, EsigCSP15, and EsigCSP16. The FPKM values indicate that EsigCSP14 is 50- to 10,000-fold more highly expressed than other EsigCSPs, suggesting that EsigCSP14 is the main and key CSP in adults and is not expressed in larvae. It is worth noting that the expression of CSPs can vary across different developmental stages; for example, GcanCSP4 showed the highest expression in G. cantor female antennae at 12 days [27], and MusiCSP1 was most highly expressed in M. usitatus primordial pupae [34]. Additionally, CSPs can be expressed in non-olfactory tissues and show division of labor biased in society insects. For example, five CSPs were more highly expressed in alates than in workers, soldiers, larvae, and nymphs, and the expression levels of RacuCSP6 were significantly higher in R. aculabialis nymphs [32]. However, the developmental stages and tissue-specific expression patterns of EsigCSP14 remain unknown, so further investigation is needed to reveal its detailed functions and roles in E. signifer.
It was found that ORs can be divided into two types: odorant receptorx (ORx) and Orco [35]. Functionally, Orco plays a crucial role in guiding the membrane targeting of canonical ORs [36]; moreover, it forms heteromerize with other ORs, through the conserved C-terminal and constituting ligand-gated ion channels, to become involved in the olfactory response of insect [37,38]. The homology of ORx among different insects was very low, and ORx was also varied among the same insects, which may be related to the recognition of odor substances in insect habitats [35]. However, Orco is a characteristic feature of olfactory sensory neurons (OSNs) expressing ORs and is highly conserved across various insect species and orders [35]. The phylogenetic evolution of insect ORs supported that the insect olfactory system has expanded its receptor repertoire with the occurrence of Orco proteins in Zygentoma, and the common origin of all insect Orco was Microcoryphia Orco [39]. In the case of E. signifer, all Lepidoptera Orco proteins formed a distinct cluster, and EsigOR13 was classified within the Orco clade, suggesting that it serves as the Orco receptor. Both the qRT-PCR results and FPKM values indicated that EsigOR16 exhibits the highest expression among the odorant receptors, making it the key receptor involved in odorant recognition. Likely, in O. furnacalis, OfurOR8, OfurOR7, and OfurOR5b primarily respond to the sex pheromone components of other Ostrinia species, while OfurOR27 strongly responds to plant odorants such as nonanal, octanal, and 1-octanol [9]. These findings highlight the specificity and diversity of odorant receptors in different insect species, reflecting the species’ adaptations to specific ecological niches and behaviors.
In the phylogenetic tree analysis, several interesting relationships among odorant receptors (ORs) in E. signifer were observed: (1) Relationship between the PRs clade and the novel lineage of PRs clade: The PRs clade was found to be the sister clade to the clade containing the novel lineage of PRs clade, the Orco clade, and all other EsigORs. This suggests a close relationship between the Orco clade and the novel lineage of PRs clade, both of which are distinct from the traditional PRs clade. (2) Absence of EsigORs in the traditional PRs clade: In Lepidoptera insects, there is a conserved clade of ORs that is specialized in sensing female sex pheromones and, thus, is called the traditional PRs clade [40], such as the five MlorPRs in this clad (PR1, PR2, PR3, OR1, and OR14), and each of the MlorPR is closely grouped with one or more PRs from other moths [28]. But, in our result, no EsigORs were found in this traditional PRs clade in E. signifer. (3) Novel PRs clade: Recent research has identified a new PR clade in Lepidoptera insects that is more closely related to general ORs [24]. This new clade was also observed in E. signifer, with EsigOR10 and EsigOR22 grouped within this novel PRs lineage. This suggests that these receptors may have functions related to sensing sex pheromones, similar to SlitOR5 in other moths [24]. (4) Evolutionary implications: The absence of traditional PRs in E. signifer and the closer relationship between the novel PRs lineage and Orco in the phylogenetic tree raise interesting evolutionary questions. This is combined with the view that the insect olfactory system has expanded its receptor repertoire with the occurrence of Orco proteins in Zygentoma [39] and ended up with the versatile OR complexes in flying insects [39,41] and the primitive moth E. signifer. It is possible that the novel PRs lineage represents a more primitive form of pheromone receptors in moths, and the evolution of specialized PRs may have occurred in a lineage-specific manner.
4. Materials and Methods
4.1. Collect Insect and Tissue
The E. signifer larvae were collected from a damaged eucalyptus plantation between December 2019 and April 2022 at the Gaofeng forest station (N 22.907°, E 108.266°), Guangxi, China. Subsequently, the larvae were artificial fed to adulthood, and their antennae were cut and collected for further analysis.
4.2. Construct cDNA Library and Sequence
The total adult antennal RNA was extracted by using a TRIzol reagent (Ambion, Naugatuck, UK) and the RNeasy Plus Mini Kit (No. 74134; Qiagen, Hilden, Germany); then, both density and quality were examined. Three cDNA library construction and Illumina sequencing (HiSeq2500 platform) of three separate RNA samples were performed at MajorBio Corporation (Shanghai, China), respectively. The entire cDNA library preparation process, including mRNA sample purification, fragmentation, first-strand cDNA synthesis, end repair, and PCR amplification, followed the methodology outlined by Zhang [15].
4.3. Assembly, Functional Annotation, and Olfactory Genes Identification
The raw reads acquisition, clean read assembly and evaluation were performed as per Zhang [15] and used BUSCO to evaluate the assembly integrity score—the higher the score, the better the integrity. NCBI BLASTx searches were used to annotate unigenes and the identification of olfactory proteins (OBP, CSP, OR, GR, IR, and SNMP) was checked by tBLASTn manually. The Blast2GO pipeline was used to perform GO annotation. The FPKM values (fragments per kilobase per million reads) were used to represent the genes’ expression levels [42], which were calculated by RSEM (RNA-Seq by Expectation-Maximization) (Version: 1.3.1) with default parameters [43].
4.4. Sequence and Phylogenetic Analysis
Muscle was used to align amino acid sequences, then the Mega v6.0 software package [44] was used to construct a neighbor-joining (NJ) tree [45] of OBPs with a P-distance model and a pairwise deletion of gaps. Color and arrangement of the NJ tree used FigTree (Version 1.4.2). The reliability of the tree structure and node support was evaluated by bootstrap analysis with 1000 replicates. Considering that E. signifer is a primitive Lepidoptera moth, the phylogenetic analyses of the OBPs were based on PBPs of Lepidoptera and OBPs of Dastarcus helophoroides (Coleoptera) [18], Chrysomya megacephala (Diptera) [19], Plutella xylostella [20], S. exigua [21,22], H. armigera [23] in Lepidoptera, and all OBPs of E. signifer [15,16], including those formerly and newly identified in larvae and adult antennal transcriptomes. The ORs tree used all Lepidoptera Orco, all ORs of E. signifer [15,16], and the novel lineage of PRs and PR clade sequence in Figure 4 of Bastin-He’line et al. [24]. The gene names and GenBank numbers of P. xylostella, H. armigera,Lepidoptera PBPs, and ORs in Bastin-He’line et al. [24] are listed in Supplementary Materials Additional File S2, and the other gene sequences are listed in the reference articles.
4.5. Expression Pattern of Olfactory Proteins in Adult Antennae
Expression patterns of 11 OBPs, 6 CSPs, and 4 ORs in adult antennae were constructed. The method used to extract the RNA of the adult antennae and test the quality was carried out as described before. cDNA was synthesized with the TransScript One-Step gDNA Removal and Synthesis Super Mix (No. O10306; Trans, Beijing, China). Primers were designed using Primer3 http://bioinfo.ut.ee/primer3-0.4.0/ (accessed on 15 December 2022) (Table 3), and the reference genes were determined as per Chen [25]. The PCR analysis was conducted using a Roche LIGHT CYCLE 480II (Colombia, SC, USA). Genious 2X SYBR Green Fast qPCR Mix (No ROX) (No. RK21205; ABclonal, Wuhan, China) was used for the PCR under a three-step amplification. Each PCR was conducted in a 20 µL reaction mixture containing 10 µL of Genious 2X SYBR Green Fast qPCR Mix (No ROX), 0.8 µL of each primer (10 mM), 2 µL of sample cDNA (2.5 ng of RNA), and 7.2 µL of dH2O (sterile distilled water). The qRT-PCR cycling parameters were as follows: 95 °C for 180 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 65 °C to 95 °C in increments of 0.5 °C for 5 s to generate the melting curves. The negative control was performed without either template. Each gene analysis was performed in three biological replicates and three technical replicates. Roche LIGHT CYCLE 480II was used to normalize the expression based on ΔΔCq values, using the reference genes EF and RIB, and using EsigGOBP8 and EsigOBP12 as control samples, and the 2−ΔΔCt method was used [46]. The normal distribution and equal variances test were performed and all the logarithm data that followed a normal distribution with equal variances were examined before comparative analyses. The comparative analyses for each gene were assessed by a one-way nested analysis of variance (ANOVA), followed by Tukey’s honestly significance difference (HSD) tests implemented in SPSS Statistics 18.0. The values are presented as the means ± SE.

Table 3.
Primers for real-time fluorescent quantitative PCR.
5. Conclusions
The analysis of the antennal transcriptome in adult E. signifer revealed a total of 69 olfactory-related genes, which included 22 OBPs, 10 CSPs, 19 ORs, 4 GRs, 12 IRs, and 2 SNMPs. When compared to the larval transcriptomes, this adult antennal transcriptome uncovered 17 new OBPs, comprising 2 PBPs, 7 CSPs, 17 ORs, 4 GRs, 11 IRs, and 2 SNMPs. Through the construction of phylogenetic trees for OBPs and ORs, three PBPs, one Orco, and two new PRs of E. signifer were identified. An in-depth analysis of the relationship between the novel PRs clade and Orco, the evolution of Orco and ORs, and the characteristics of the primitive moth E. signifer led to the hypothesis that the novel lineage of PRs clade may represent primitive PRs in moths.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13122264/s1, Additional File S1 Nucleic acid sequences of all candidates’ olfactory proteins identified in Endoclita signifer antennal transcriptome. Additional File S2 The protein names and gene accession numbers were used in phylogenetic trees.
Author Contributions
G.X. conducted insect collection, constructed antennal transcriptome, analyzed data, and wrote the manuscript. J.L. and H.F. constructed antennal transcriptome, conducted the qRT-PCR experiments, and analyzed data. P.H. conceived and designed the research and changed the manuscript. Z.Y. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 32001321 and NO. 32371884), and the Fund for Central Government Guide Development of Local Science and Technology (NO. Guike ZY21195019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, X.; Yang, X.; Xue, D.; Han, H. The complete mitochondrial genome of Endoclita signifer (Lepidoptera, Hepialidae). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 4620–4621. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, Y.; Wu, Y.; Zou, D.; Hu, P.; Wang, J. Distribution and Damage of Endoclita signifer Walker, as an important wood borer pest insect on forest. For. Pest Dis. 2021, 40, 34–40. [Google Scholar]
- Hu, P.; Qiu, Z.; Zhang, Y.; Xu, Y.; Yang, Z. Quick shift in volatile attraction between the third and fifth instar larvae of Endoclita signifer. Pest Manag. Sci. 2022, 79, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiu, Z.; Zhang, Y.; Zheng, X.; Lu, W.; Hu, P. Volatiles from eucalyptus trunks and forest floor humus influence the habitat transfer, host selection, and aggregation of Endoclita signifer larvae. Forests 2022, 13, 2058. [Google Scholar] [CrossRef]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161. [Google Scholar] [CrossRef]
- Xu, P.; Atkinson, R.; Jones, D.N.; Smith, D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 2005, 45, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Liu, G.; Ma, H.; Xie, H.; Xuan, N.; Guo, X.; Fan, Z.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.-F. Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: Role of CSP in insect defense. PLoS ONE 2016, 11, e0154706. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L. Current understandings of olfactory molecular events in the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Arch. Insect Biochem. Physiol. 2023, 112, e21996. [Google Scholar] [CrossRef]
- Jones, P.L.; Pask, G.M.; Rinker, D.C.; Zwiebel, L.J. Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. USA 2011, 108, 8821–8825. [Google Scholar] [CrossRef]
- Cao, L.-H.; Jing, B.-Y.; Yang, D.; Zeng, X.; Shen, Y.; Tu, Y.; Luo, D.-G. Distinct signaling of Drosophila chemoreceptors in olfactory sensory neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E902–E911. [Google Scholar] [CrossRef] [PubMed]
- Seeta, P.; Hao, G. Pkc98e regulates odorant responses in Drosophila melanogaster. J. Neurosci. 2021, 41, 3948–3957. [Google Scholar]
- Rinehart, J.P.; Hayward, S.A.; Elnitsky, M.A.; Sandro, L.H.; Lee, R.E., Jr.; Denlinger, D.L. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc. Natl. Acad. Sci. USA 2006, 103, 14223–14227. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Z.; Yang, X.; Ma, H.; Liu, X.; Hu, P. Olfactory Proteins and Their Expression Profiles in the Eucalyptus Pest Endoclita signifer Larvae. Front. Physiol. 2021, 12, 682537. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Qiu, Z.; Chen, X.; Xu, Y.; Su, X.; Yang, Z. Olfactory proteins of Endoclita signifer larvae and their roles in host recognition. Chem. Biol. Technol. Agric. 2022, 9, 54. [Google Scholar] [CrossRef]
- Hu, P.; Hao, E.; Yang, Z.; Qiu, Z.; Fu, H.; Lu, J.; He, Z.; Huang, Y. EsigGOBP1: The Key Protein Binding Alpha-Phellandrene in Endoclita signifer Larvae. Int. J. Mol. Sci. 2022, 23, 9269. [Google Scholar] [CrossRef]
- Li, X.; Dong, G.; Fang, J.; Liu, H.; Guo, W.; Yin, H. Identification of putative olfactory genes in newly hatched larvae of a Coleopteran ectoparasitoid Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) by transcriptome analysis. Entomol. Res. 2020, 50, 329–342. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, M.; Lei, C.; Zhu, F. The developmental transcriptome of the synanthropic fly Chrysomya megacephala and insights into olfactory proteins. BMC Genom. 2015, 16, 20. [Google Scholar] [CrossRef]
- Zhu, J.; Ban, L.; Song, L.M.; Liu, Y.; Pelosi, P.; Wang, G. General odorant-binding proteins and sex pheromone guide larvae of Plutella xylostella to better food. Insect Biochem. Mol. Biol. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Liu, N.Y.; Zhang, T.; Ye, Z.F.; Li, F.; Dong, S.L. Identification and Characterization of Candidate Chemosensory Gene Families from Spodoptera exigua Developmental Transcriptomes. Int. J. Biol. Sci. 2015, 11, 1036–1048. [Google Scholar] [CrossRef]
- Llopis-Gimenez, A.; Carrasco-Oltra, T.; Jacquin-Joly, E.; Herrero, S.; Crava, C.M. Coupling Transcriptomics and Behaviour to Unveil the Olfactory System of Spodoptera exigua Larvae. J. Chem. Ecol. 2020, 46, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ai, D.; Zhang, J.; Dong, S.; Liu, Y.; Wang, G. Candidate odorant binding proteins and chemosensory proteins in the larval chemosensory tissues of two closely related noctuidae moths, Helicoverpa armigera and H. assulta. PLoS ONE 2017, 12, e0179243. [Google Scholar] [CrossRef] [PubMed]
- Bastin-Héline, L.; De Fouchier, A.; Cao, S.; Koutroumpa, F.; Caballero-Vidal, G.; Robakiewicz, S.; Monsempes, C.; François, M.-C.; Ribeyre, T.; Maria, A. A novel lineage of candidate pheromone receptors for sex communication in moths. Elife 2019, 8, e49826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, Z.-S.; Su, X.-Y.; Xu, Y.; Yang, Z.-D.; Hu, P. Screening of reference genes for RT-qPCR analysis in Endoclita signifer Walker larvae. J. Environ. Entomol. 2023, 45, 1016–1026. [Google Scholar]
- Poivet, E.; Gallot, A.; Montagne, N.; Glaser, N.; Legeai, F.; Jacquin-Joly, E. A comparison of the olfactory gene repertoires of adults and larvae in the noctuid moth Spodoptera littoralis. PLoS ONE 2013, 8, e60263. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Su, R.; Ouyang, H.; Zheng, X.; Lu, W.; Wang, X. Antennal Transcriptome Analysis and Identification of Olfactory Genes in Glenea cantor Fabricius (Cerambycidae: Lamiinae). Insects 2022, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Guo, J.-M.; Wei, Z.-Q.; Zhang, X.-T.; Liu, S.-R.; Guo, H.-F.; Dong, S.-L. Identification and sex expression profiles of olfactory-related genes in Mythimna loreyi based on antennal transcriptome analysis. J. Asia-Pac. Entomol. 2022, 25, 101934. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Zhang, Y.; Zheng, Y.; Fan, Z.; Zhang, R. Identification of olfactory genes in Monochamus saltuarius and effects of Bursaphelenchus xylophilus infestation on their expression. Forests 2022, 13, 258. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xie, G.; Wang, W.; Yang, Y. Identification and expression analyses of the olfactory-related genes in different tissues’ transcriptome of a predacious soldier beetle, Podabrus annulatus (Coleoptera, Cantharidae). Arch. Insect Biochem. Physiol. 2023, 112, e21997. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Du, L.; Ban, L. Antennal Transcriptome Analysis of Olfactory Genes and Characterization of Odorant Binding Proteins in Odontothrips loti (Thysanoptera: Thripidae). Int. J. Mol. Sci. 2023, 24, 5284. [Google Scholar] [CrossRef]
- Saba, N.u.; Ye, C.; Zhang, W.; Wu, T.; Wang, Y.; Zhang, X.; Song, Z.; Xing, L.; Su, X. The Antennal Sensilla and Expression Patterns of Olfactory Genes in the Lower Termite Reticulitermes aculabialis (Isoptera: Rhinotermitidae). J. Insect Sci. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, W.; Wang, X.; Sang, W.; Pan, H.; Ali, S.; Tang, L.; Wu, J. Transcriptome analysis of Megalurothrips usitatus (Bagnall) identifies olfactory genes with ligands binding characteristics of MusiOBP1 and MusiCSP1. Front. Physiol. 2022, 13, 978534. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004, 43, 703–714. [Google Scholar] [CrossRef]
- Pask, G.M.; Jones, P.L.; Rützler, M.; Rinker, D.C.; Zwiebel, L.J. Heteromeric anopheline odorant receptors exhibit distinct channel properties. PLoS ONE 2011, 6, e28774. [Google Scholar] [CrossRef]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 2008, 452, 1007. [Google Scholar] [CrossRef]
- Brand, P.; Robertson, H.M.; Lin, W.; Pothula, R.; Klingeman, W.E.; Jurat-Fuentes, J.L.; Johnson, B.R. The origin of the odorant receptor gene family in insects. Elife 2018, 7, e38340. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef]
- Thoma, M.; Missbach, C.; Jordan, M.D.; Grosse-Wilde, E.; Newcomb, R.D.; Hansson, B.S. Transcriptome surveys in silverfish suggest a multistep origin of the insect odorant receptor gene family. Front. Ecol. Evol. 2019, 7, 281. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).