Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic–Ischemic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. ES-MSC Labeling with CellVue® NIR815 Fluorophore
2.3. Hypoxic–Ischemic Brain Injury and the Administration of ES-MSCs
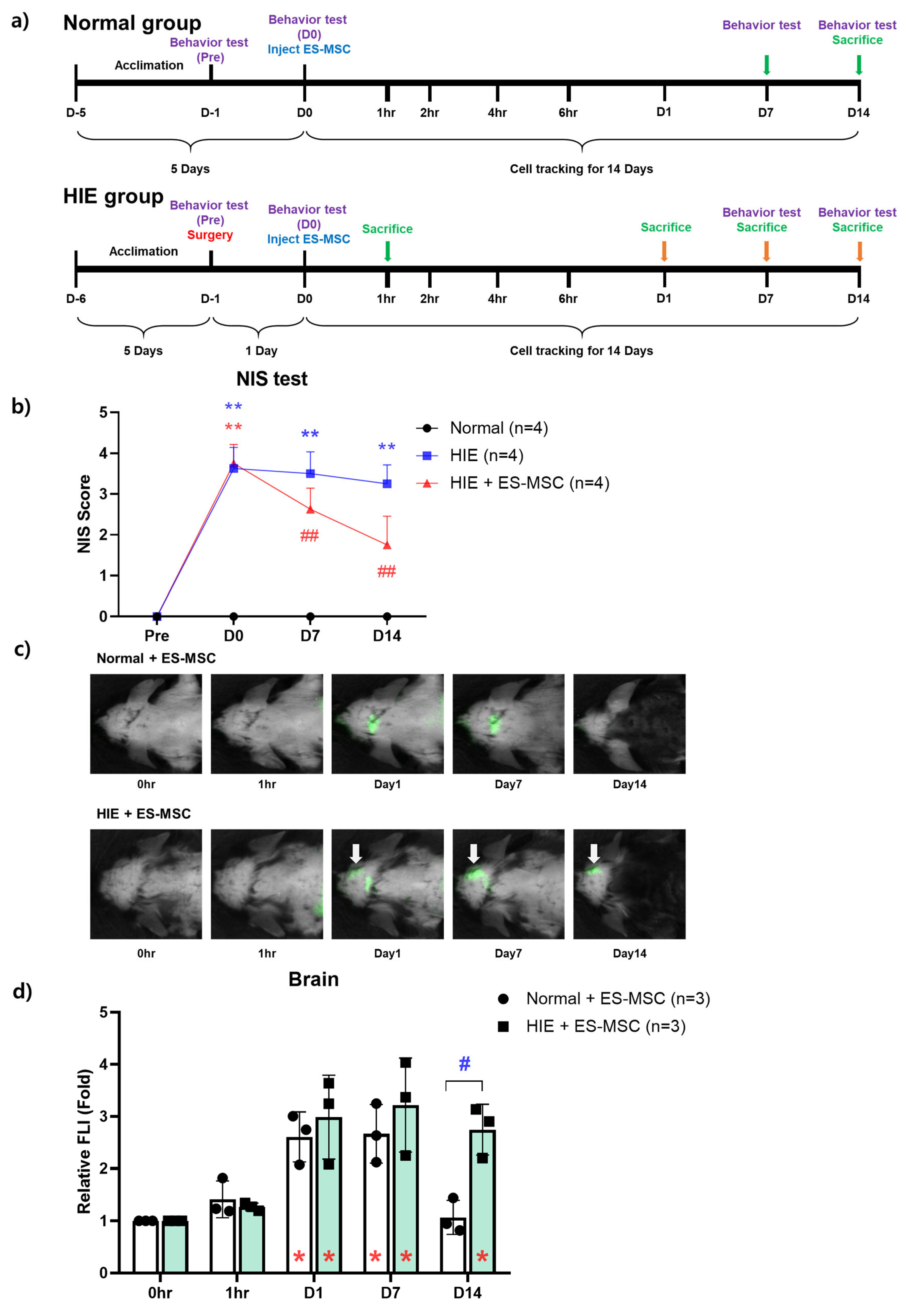
2.4. Experimental Design
2.5. Behavior Test (Neurologic Impairment Scoring)
2.6. In Vivo and Ex Vivo Imaging
2.7. Immunofluorescence Analysis
2.8. Statistical Analysis
3. Results
3.1. Improvement in Neurological Behavior in the ES-MSC–Administered HIE Group and Infiltration of the Cells in the Damaged Brain
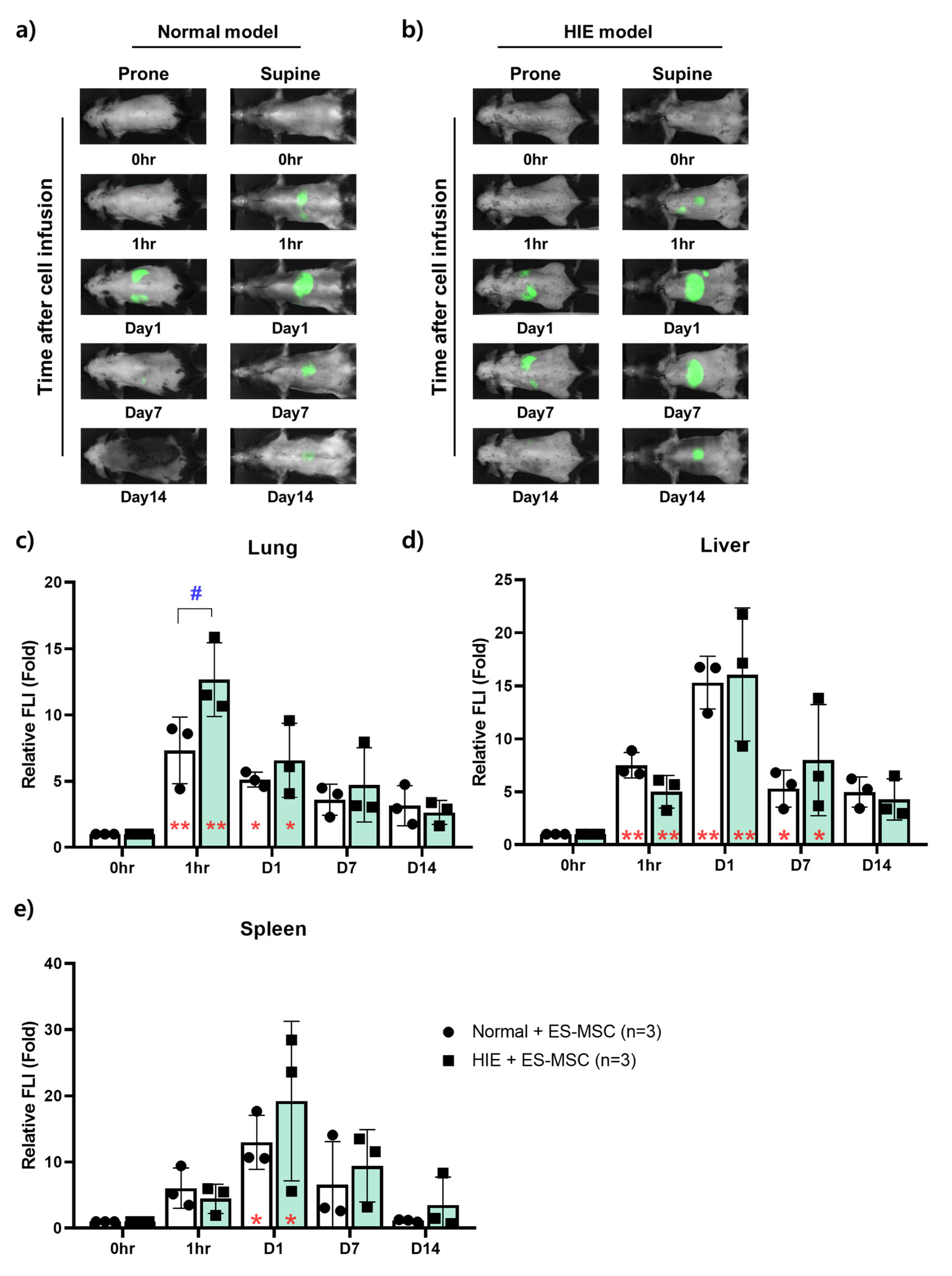
3.2. Changes in the Distribution of ES-MSCs in the Host Body by Lapse of Time
3.3. Ex Vivo NIR Fluorescent Imaging on the Infiltration Ability of Stem Cells for Each Organ over Time
3.4. Engraftment of ES-MSCs in Hypoxic–Ischemic Brain Lesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bang, O.Y.; Kim, E.H.; Cha, J.M.; Moon, G.J. Adult Stem Cell Therapy for Stroke: Challenges and Progress. J. Stroke 2016, 18, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Keep, R.F.; Hua, Y.; Xi, G. Critical Role of the Sphingolipid Pathway in Stroke: A Review of Current Utility and Potential Therapeutic Targets. Transl. Stroke Res. 2016, 7, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, R.J.; Lee, G.A. Two-vessel Occlusion Mouse Model of Cerebral Ischemia-reperfusion. J. Vis. Exp. 2019, 145, e59078. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.; Tatlisumak, T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007, 87, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Meyer, P.; Grandgirard, D.; Lehner, M.; Haenggi, M.; Leib, S.L. Grafted Neural Progenitor Cells Persist in the Injured Site and Differentiate Neuronally in a Rodent Model of Cardiac Arrest-Induced Global Brain Ischemia. Stem. Cells Dev. 2020, 29, 574–585. [Google Scholar] [CrossRef]
- Palma-Tortosa, S.; Tornero, D.; Gronning Hansen, M.; Monni, E.; Hajy, M.; Kartsivadze, S.; Aktay, S.; Tsupykov, O.; Parmar, M.; Deisseroth, K.; et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 9094–9100. [Google Scholar] [CrossRef]
- Tornero, D.; Tsupykov, O.; Granmo, M.; Rodriguez, C.; Gronning-Hansen, M.; Thelin, J.; Smozhanik, E.; Laterza, C.; Wattananit, S.; Ge, R.; et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain 2017, 140, 692–706. [Google Scholar] [CrossRef]
- Li, F.; Zhang, K.; Liu, H.; Yang, T.; Xiao, D.J.; Wang, Y.S. The neuroprotective effect of mesenchymal stem cells is mediated through inhibition of apoptosis in hypoxic ischemic injury. World J. Pediatr. 2020, 16, 193–200. [Google Scholar] [CrossRef]
- Sammali, E.; Alia, C.; Vegliante, G.; Colombo, V.; Giordano, N.; Pischiutta, F.; Boncoraglio, G.B.; Barilani, M.; Lazzari, L.; Caleo, M.; et al. Intravenous infusion of human bone marrow mesenchymal stromal cells promotes functional recovery and neuroplasticity after ischemic stroke in mice. Sci. Rep. 2017, 7, 6962. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Corcelli, M.; Dowding, K.; Ranzoni, A.M.; Vlahova, F.; Hau, K.L.; Hunjan, A.; Peebles, D.; Gressens, P.; Hagberg, H.; et al. Embryonic Stem Cell-Derived Mesenchymal Stem Cells (MSCs) Have a Superior Neuroprotective Capacity Over Fetal MSCs in the Hypoxic-Ischemic Mouse Brain. Stem. Cells Transl. Med. 2018, 7, 439–449. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Nagai, A.; Sheikh, A.M.; Shiota, Y.; Narantuya, D.; Watanabe, T.; Masuda, J.; Kobayashi, S.; Kim, S.U.; Yamaguchi, S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J. Neurosci. Res. 2010, 88, 1017–1025. [Google Scholar] [CrossRef]
- Wang, X.; Kimbrel, E.A.; Ijichi, K.; Paul, D.; Lazorchak, A.S.; Chu, J.; Kouris, N.A.; Yavanian, G.J.; Lu, S.J.; Pachter, J.S.; et al. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem. Cell Rep. 2014, 3, 115–130. [Google Scholar] [CrossRef]
- Kim, G.H.; Subash, M.; Yoon, J.S.; Jo, D.; Han, J.; Hong, J.M.; Kim, S.S.; Suh-Kim, H. Neurogenin-1 Overexpression Increases the Therapeutic Effects of Mesenchymal Stem Cells through Enhanced Engraftment in an Ischemic Rat Brain. Int. J. Stem. Cells 2020, 13, 127–141. [Google Scholar] [CrossRef]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef]
- Bjorklund, A.; Lindvall, O. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 2000, 3, 537–544. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Kim, J.E.; Kalimuthu, S.; Ahn, B.C. In vivo cell tracking with bioluminescence imaging. Nucl. Med. Mol. Imaging 2015, 49, 3–10. [Google Scholar] [CrossRef]
- Cao, F.; Lin, S.; Xie, X.; Ray, P.; Patel, M.; Zhang, X.; Drukker, M.; Dylla, S.J.; Connolly, A.J.; Chen, X.; et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 2006, 113, 1005–1014. [Google Scholar] [CrossRef]
- Zeiser, R.; Nguyen, V.H.; Beilhack, A.; Buess, M.; Schulz, S.; Baker, J.; Contag, C.H.; Negrin, R.S. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006, 108, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Cobbs, C.; Jennings, M.; Bartholomew, A.; Hoffman, R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 2003, 101, 2999–3001. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jeong, C.H.; Woo, J.S.; Ryu, C.H.; Lee, J.H.; Jeun, S.S. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: Tropism for brain tumors and biodistribution. Int. J. Nanomed. 2016, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Romanko, M.; Kramer, B.C.; Gosiewska, A.; Chopp, M.; Hong, K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012, 1489, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Kuroda, S.; Sugiyama, T.; Kawabori, M.; Ito, M.; Shichinohe, H.; Kuge, Y.; Houkin, K.; Tamaki, N.; Iwasaki, Y. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats--in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery 2012, 70, 435–444; discussion 444. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Yoon, J.A.; Park, M.; Shin, E.Y.; Jung, S.; Lee, J.E.; Eum, J.H.; Song, H.; Lee, D.R.; Lee, W.S.; et al. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem. Cell Res. Ther. 2020, 11, 255. [Google Scholar] [CrossRef]
- Gupta, V.R.; Root, A.; Fisher, T.; Norberg, R.; David, J.; Clark, T.; Cohen, J.; May, C.; Giddabasappa, A. Molecular imaging reveals biodistribution of P-cadherin LP-DART bispecific and trafficking of adoptively transferred T cells in mouse xenograft model. Oncotarget 2020, 11, 1344–1357. [Google Scholar] [CrossRef][Green Version]
- Violatto, M.B.; Santangelo, C.; Capelli, C.; Frapolli, R.; Ferrari, R.; Sitia, L.; Tortarolo, M.; Talamini, L.; Previdi, S.; Moscatelli, D.; et al. Longitudinal tracking of triple labeled umbilical cord derived mesenchymal stromal cells in a mouse model of Amyotrophic Lateral Sclerosis. Stem. Cell. Res. 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Tashiro, Y.; Hollandsworth, H.M.; Nishino, H.; Yamamoto, J.; Amirfakhri, S.; Filemoni, F.; Sugisawa, N.; Aoki, T.; Murakami, M.; Hoffman, R.M.; et al. Indocyanine Green Labels an Orthotopic Nude-Mouse Model of Very-Early Colon-Cancer Liver Metastases. In Vivo 2020, 34, 2277–2280. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Garcia-Fossa, F.; Radaic, A.; Duran, N.; Favaro, W.J.; de Jesus, M.B. Biogenic silver nanoparticles: In vitro and in vivo antitumor activity in bladder cancer. Eur. J. Pharm. Biopharm. 2020, 151, 162–170. [Google Scholar] [CrossRef]
- Nguyen, H.; Zarriello, S.; Coats, A.; Nelson, C.; Kingsbury, C.; Gorsky, A.; Rajani, M.; Neal, E.G.; Borlongan, C.V. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol. Dis. 2019, 126, 85–104. [Google Scholar] [CrossRef]
- El-Kadiry, A.E.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Moon, S.H.; Cha, R.; Lee, G.L.; Lim, J.K.; Soh, K.S. Primo vascular system in the subarachnoid space of a mouse brain. Evid. Based Complement. Alternat. Med. 2013, 2013, 280418. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal stem cell homing: The devil is in the details. Cell. Stem. Cell. 2009, 4, 206–216. [Google Scholar] [CrossRef]
- Walczak, P.; Zhang, J.; Gilad, A.A.; Kedziorek, D.A.; Ruiz-Cabello, J.; Young, R.G.; Pittenger, M.F.; van Zijl, P.C.; Huang, J.; Bulte, J.W. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008, 39, 1569–1574. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Gong, J.; Wang, J.; Fan, Y.; Cai, J.; Wang, Y.; Qiu, Y.; Wei, Y.; Xiong, C.; et al. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat. Commun. 2020, 11, 5196. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury—Disruption and repair. Brain Hemorrhages 2020, 1, 34–53. [Google Scholar] [CrossRef]
- Zhao, A.G.; Shah, K.; Cromer, B.; Sumer, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem. Cells Int. 2020, 2020, 8825771. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem. Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem. Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Hoover, J.; Kaneko, Y.; Borlongan, C.V. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke 2015, 46, 2616–2627. [Google Scholar] [CrossRef]
- Yang, B.; Hamilton, J.A.; Valenzuela, K.S.; Bogaerts, A.; Xi, X.; Aronowski, J.; Mays, R.W.; Savitz, S.I. Multipotent Adult Progenitor Cells Enhance Recovery After Stroke by Modulating the Immune Response from the Spleen. Stem. Cells 2017, 35, 1290–1302. [Google Scholar] [CrossRef]
- Pang, S.H.M.; D’Rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.L.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Xu, H.; Liu, Z. Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles. Biomaterials 2012, 33, 4872–4881. [Google Scholar] [CrossRef]
- Haley, M.J.; Lawrence, C.B. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow Metab. 2017, 37, 456–470. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Choung, J.S.; Kim, J.M.; Kim, H.; Kim, M. Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic–Ischemic Encephalopathy. Life 2023, 13, 227. https://doi.org/10.3390/life13010227
Lee SH, Choung JS, Kim JM, Kim H, Kim M. Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic–Ischemic Encephalopathy. Life. 2023; 13(1):227. https://doi.org/10.3390/life13010227
Chicago/Turabian StyleLee, Su Hyun, Jin Seung Choung, Jong Moon Kim, Hyunjin Kim, and MinYoung Kim. 2023. "Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic–Ischemic Encephalopathy" Life 13, no. 1: 227. https://doi.org/10.3390/life13010227
APA StyleLee, S. H., Choung, J. S., Kim, J. M., Kim, H., & Kim, M. (2023). Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic–Ischemic Encephalopathy. Life, 13(1), 227. https://doi.org/10.3390/life13010227






