Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)?
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Olfactory Dysfunction
2.2. Analyses of Data and Statistical Tests
3. Results
3.1. Within-Group Comparison
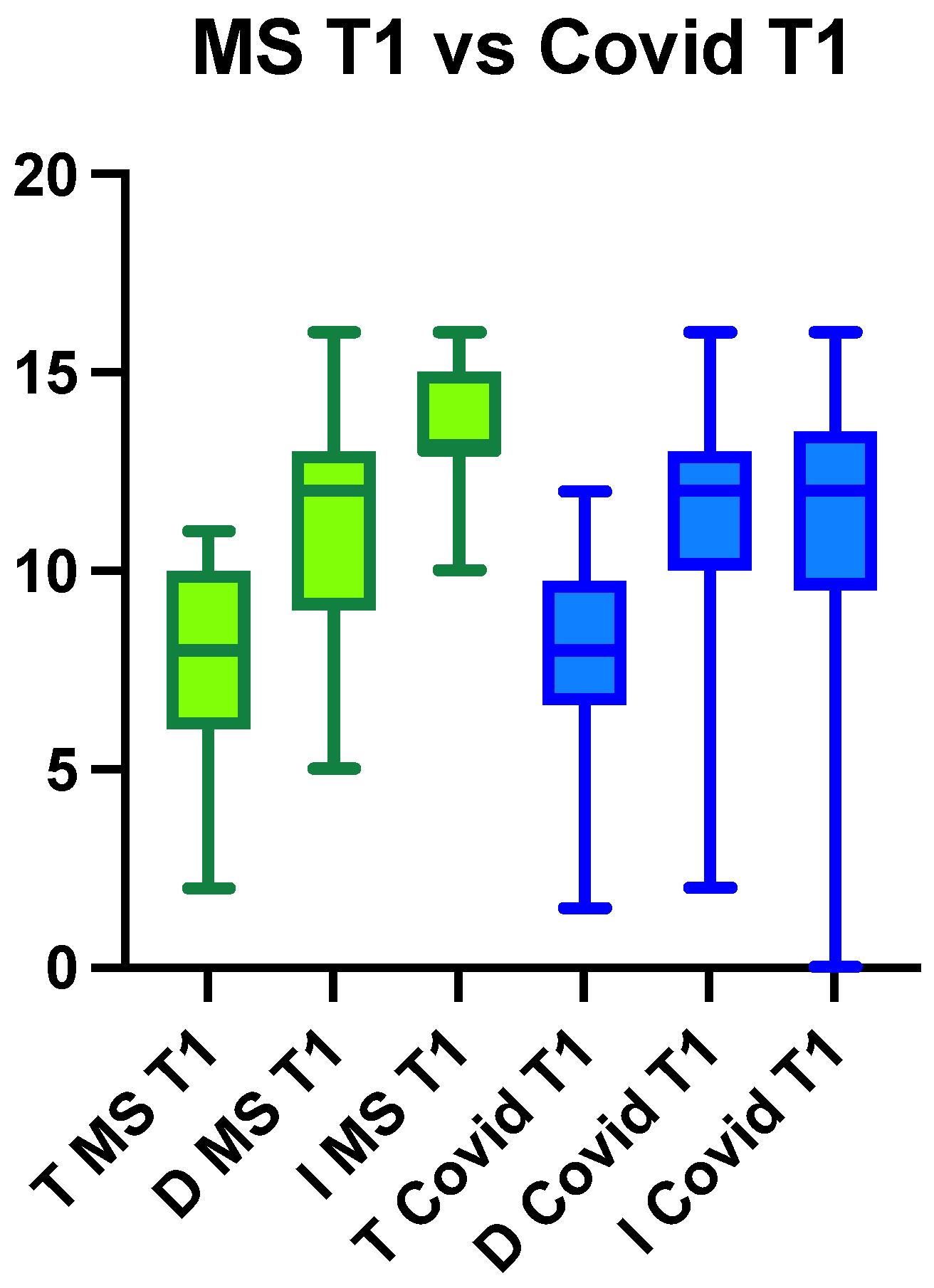
3.1.1. Multiple Sclerosis Group
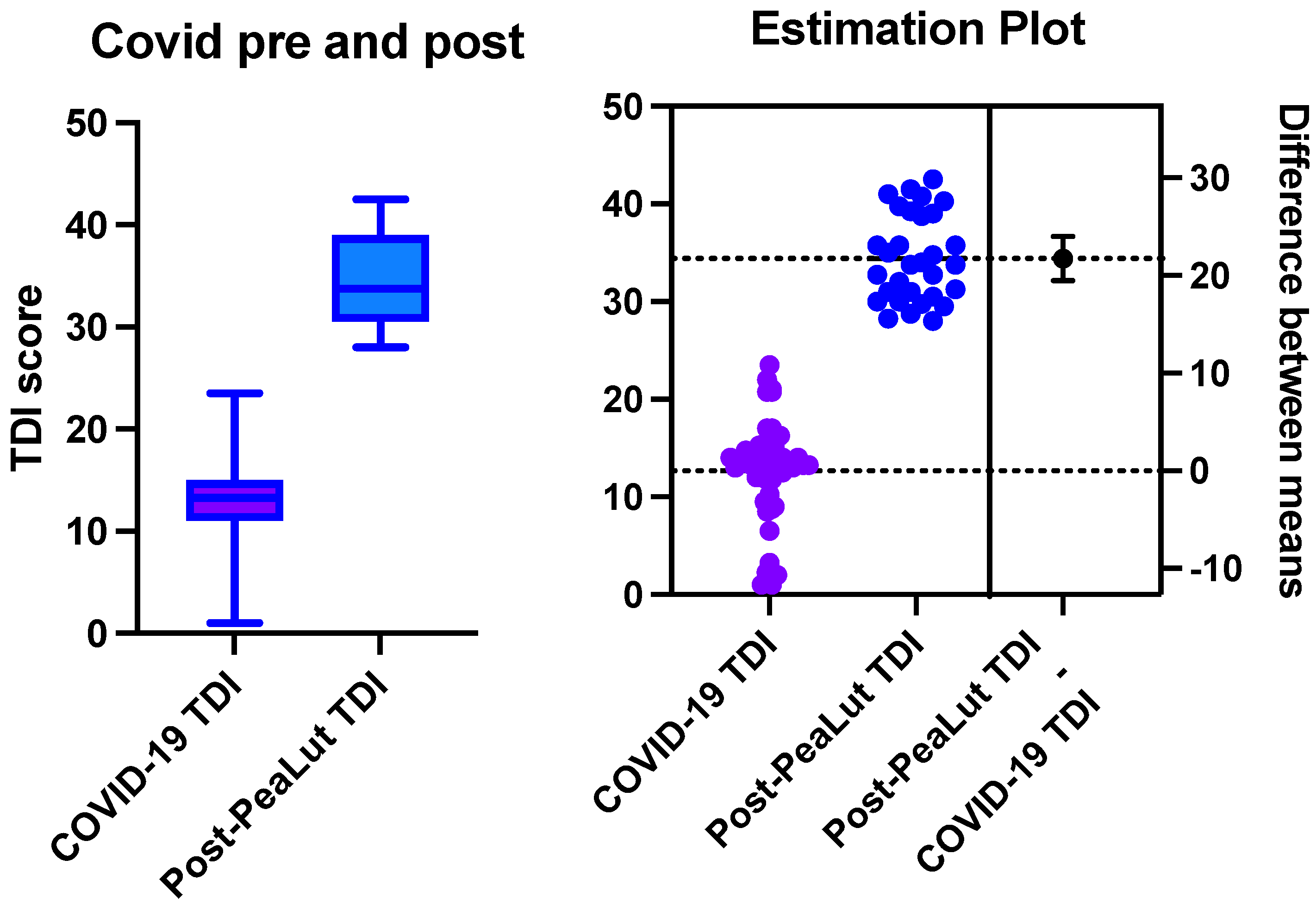
3.1.2. Long COVID Group
3.2. Between-Group Comparison
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hüttenbrink, K.B.; Hummel, T.; Berg, D.; Gasser, T.; Hähner, A. Olfactory dysfunction: Common in later life and early warning of neurodegenerative disease. Dtsch. Arztebl. Int. 2013, 110, 1–7.e1. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Klein, R.S. Neuroinflammation and COVID-19. Curr. Opin. Neurobiol. 2022, 76, 102608. [Google Scholar] [CrossRef]
- Khoy, K.; Mariotte, D.; Defer, G.; Petit, G.; Toutirais, O.; Le Mauff, B. Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring. Front. Immunol. 2020, 11, 549842. [Google Scholar] [CrossRef]
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple sclerosis--the plaque and its pathogenesis. N. Engl. J. Med. 2006, 354, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Raps, E.C.; Cohen, J.A.; West, S.E.; Doty, R.L. Olfactory disturbances as the initial or most prominent symptom of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1011–1012. [Google Scholar] [CrossRef]
- Doty, R.L.; Li, C.; Mannon, L.J.; Yousem, D.M. Olfactory dysfunction in multiple sclerosis. N. Engl. J. Med. 1997, 336, 1918–1919, Erratum in N. Engl. J. Med. 1997, 337, 507. [Google Scholar] [CrossRef]
- Doty, R.L.; Li, C.; Mannon, L.J.; Yousem, D.M. Olfactory dysfunction in multiple sclerosis: Relation to longitudinal changes in plaque numbers in central olfactory structures. Neurology 1999, 53, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Shephard, B.C.; Kobal, G. Assessment of olfaction in multiple sclerosis: Evidence of dysfunction by olfactory evoked response and identification tests. J. Neurol. Neurosurg. Psychiatry 1997, 63, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Good, K.P.; Tourbier, I.A.; Moberg, P.; Cuzzocreo, J.L.; Geckle, R.J.; Yousem, D.M.; Pham, D.L.; Doty, R.L. Unilateral olfactory sensitivity in multiple sclerosis. Physiol. Behav. 2017, 168, 24–30. [Google Scholar] [CrossRef]
- Zivadinov, R.; Zorzon, M.; Monti Bragadin, L.; Pagliaro, G.; Cazzato, G. Olfactory loss in multiple sclerosis. J. Neurol. Sci. 1999, 168, 127–130. [Google Scholar] [CrossRef]
- Zorzon, M.; Ukmar, M.; Bragadin, L.M.; Zanier, F.; Antonello, R.M.; Cazzato, G.; Zivadinov, R. Olfactory dysfunction and extent of white matter abnormalities in multiple sclerosis: A clinical and MR study. Mult. Scler. 2000, 6, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, A.; Vedovello, M.; Reindl, M.; Ehling, R.; DiPauli, F.; Kuenz, B.; Gneiss, C.; Deisenhammer, F.; Berger, T. Olfactory threshold is impaired in early, active multiple sclerosis. Mult. Scler. 2011, 17, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Hegen, H.; Ladstätter, F.; Berek, K.; Amprosi, M.; Wurth, S.; Auer, M.; Di Pauli, F.; Deisenhammer, F.; Reindl, M.; et al. Change of olfactory function as a marker of inflammatory activity and disability progression in MS. Mult. Scler. 2019, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Berek, K.; Hegen, H.; Teuchner, B.; Auer, M.; Wurth, S.; Di Pauli, F.; Deisenhammer, F.; Berger, T. Smelling multiple sclerosis: Different qualities of olfactory function reflect either inflammatory activity or neurodegeneration. Mult. Scler. 2020, 26, 57–68. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Zelic, M.; Pontarelli, F.; Woodworth, L.; Zhu, C.; Mahan, A.; Ren, Y.; LaMorte, M.; Gruber, R.; Keane, A.; Loring, P.; et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 2021, 35, 109112. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- D’Ascanio, L.; Pandolfini, M.; Cingolani, C.; Latini, G.; Gradoni, P.; Capalbo, M.; Frausini, G.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol.–Head Neck Surg. 2021, 164, 82–86. [Google Scholar] [CrossRef]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 2022, 43, 1548–1560. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. TLC Study Group. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; D’Ascanio, L.; Vaira, L.A.; Cantone, E.; De Luca, P.; Cingolani, C.; Motta, G.; De Riu, G.; Vitelli, F.; Spriano, G.; et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo-Controlled Clinical Trial. Curr. Neuropharmacol. 2022, 20, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- D’Ascanio, L.; Vitelli, F.; Cingolani, C.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Randomized clinical trial “olfactory dysfunction after COVID-19: Olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4156–4162. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Camaioni, A.; Marra, P.; Salzano, G.; Carriere, G.; Ricciardi, L.; Pucci, R.; Montemurro, N.; Brenner, M.J.; Di Stadio, A. Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study. Cells 2022, 11, 2552. [Google Scholar] [CrossRef]
- Berger, J.R.; Brandstadter, R.; Bar-Or, A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e761. [Google Scholar] [CrossRef]
- O’Byrne, L.; Webster, K.E.; MacKeith, S.; Philpott, C.; Hopkins, C.; Burton, M.J. Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction. Cochrane Database Syst. Rev. 2022, 7, CD013876. [Google Scholar] [CrossRef]
- Di Stadio, A.; Severini, C.; Colizza, A.; De Vincentiis, M.; La Mantia, I. Investigational drugs for the treatment of olfactory dysfunction. Expert Opin. Investig. Drugs 2022, 31, 945–955. [Google Scholar] [CrossRef]
- Di Stadio, A.; D’Ascanio, L.; La Mantia, I.; Ralli, M.; Brenner, M.J. Parosmia after COVID-19: Olfactory training, neuroinflammation and distortions of smell. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, K.; Tariq, E.; Nzvere, F.P.; Miqdad, M.; Cancarevic, I. Role of Smoking in the Pathogenesis of Multiple Sclerosis: A Review Article. Cureus 2020, 12, e9564. [Google Scholar] [CrossRef]
- Figueroa-Vargas, A.; Cárcamo, C.; Henríquez-Ch, R.; Zamorano, F.; Ciampi, E.; Uribe-San-Martin, R.; Vásquez, M.; Aboitiz, F.; Billeke, P. Frontoparietal connectivity correlates with working memory performance in multiple sclerosis. Sci. Rep. 2020, 10, 9310. [Google Scholar] [CrossRef]
- Di Stadio, A.; Brenner, M.J.; De Luca, P.; Albanese, M.; D’Ascanio, L.; Ralli, M.; Roccamatisi, D.; Cingolani, C.; Vitelli, F.; Camaioni, A.; et al. Olfactory Dysfunction, Headache, and Mental Clouding in Adults with Long-COVID-19: What Is the Link between Cognition and Olfaction? A Cross-Sectional Study. Brain Sci. 2022, 12, 154. [Google Scholar] [CrossRef]
- Di Stadio, A.; D’Ascanio, L.; De Luca, P.; Roccamatisi, D.; La Mantia, I.; Brenner, M.J. Hyperosmia after COVID-19: Hedonic perception or hypersensitivity? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2196–2200. [Google Scholar] [CrossRef]
- Di Sabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Di Stadio, A.; Bernitsas, E.; Ralli, M.; Severini, C.; Brenner, M.J.; Angelini, C. OAS1 gene, Spike protein variants and persistent COVID-19-related anosmia: May the olfactory disfunction be a harbinger of future neurodegenerative disease? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 347–349. [Google Scholar] [CrossRef]
- Chabot, S.; Yong, V.W. Interferon beta-1b increases interleukin-10 in a model of T cell-microglia interaction: Relevance to MS. Neurology 2000, 55, 1497–1505. [Google Scholar] [CrossRef]
- Mudò, G.; Frinchi, M.; Nuzzo, D.; Scaduto, P.; Plescia, F.; Massenti, M.F.; Di Carlo, M.; Cannizzaro, C.; Cassata, G.; Cicero, L.; et al. Anti-inflammatory and cognitive effects of interferon-β1a (IFNβ1a) in a rat model of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 44. [Google Scholar] [CrossRef]
- Begum-Haque, S.; Christy, M.; Wang, Y.; Kasper, E.; Ochoa-Reparaz, J.; Smith, J.Y.; Haque, A.; Kasper, L.H. Glatiramer acetate biases dendritic cells towards an anti-inflammatory phenotype by modulating OPN, IL-17, and RORγt responses and by increasing IL-10 production in experimental allergic encephalomyelitis. J. Neuroimmunol. 2013, 254, 117–124. [Google Scholar] [CrossRef]
- Starossom, S.C.; Veremeyko, T.; Dukhinova, M.; Yung, A.W.; Ponomarev, E.D. Glatiramer acetate (copaxone) modulates platelet activation and inhibits thrombin-induced calcium influx: Possible role of copaxone in targeting platelets during autoimmune neuroinflammation. PLoS ONE 2014, 9, e96256. [Google Scholar] [CrossRef]
- De Luca, P.; Scarpa, A.; Ralli, M.; Tassone, D.; Simone, M.; De Campora, L.; Cassandro, C.; Di Stadio, A. Auditory Disturbances and SARS-CoV-2 Infection: Brain Inflammation or Cochlear Affection? Systematic Review and Discussion of Potential Pathogenesis. Front. Neurol. 2021, 12, 707207. [Google Scholar] [CrossRef]
- Desai, R.A.; Davies, A.L.; Del Rossi, N.; Tachrount, M.; Dyson, A.; Gustavson, B.; Kaynezhad, P.; Mackenzie, L.; van der Putten, M.A.; McElroy, D.; et al. Nimodipine Reduces Dysfunction and Demyelination in Models of Multiple Sclerosis. Ann. Neurol. 2020, 88, 123–136. [Google Scholar] [CrossRef]
- Orefice, N.S.; Alhouayek, M.; Carotenuto, A.; Montella, S.; Barbato, F.; Comelli, A.; Calignano, A.; Muccioli, G.G.; Orefice, G. Oral Palmitoylethanolamide Treatment Is Associated with Reduced Cutaneous Adverse Effects of Interferon-β1a and Circulating Proinflammatory Cytokines in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics 2016, 13, 428–438. [Google Scholar] [CrossRef]
- Nau, R.; Ribes, S.; Djukic, M.; Eiffert, H. Strategies to increase the activity of microglia as efficient protectors of the brain against infections. Front. Cell. Neurosci. 2014, 8, 138. [Google Scholar] [CrossRef]
- Ye, S.; Liu, H.; Chen, Y.; Qiu, F.; Liang, C.L.; Zhang, Q.; Huang, H.; Wang, S.; Zhang, Z.D.; Lu, W.; et al. A Novel Immunosuppressant, Luteolin, Modulates Alloimmunity and Suppresses Murine Allograft Rejection. J. Immunol. 2019, 203, 3436–3446. [Google Scholar] [CrossRef]
- Noce, A.; Albanese, M.; Marrone, G.; Di Lauro, M.; Zaitseva, A.P.; Palazzetti, D.; Guerriero, C.; Paolino, A.; Pizzenti, G.; Di Daniele, F.; et al. Ultramicronized Palmitoylethanolamide (um-PEA): A New Possible Adjuvant Treatment in CO VID-19 patients. Pharmaceuticals 2021, 14, 336. [Google Scholar] [CrossRef]


| Age | Women, Men | Smokers | EDSS | Comorbidities | |
|---|---|---|---|---|---|
| MS | 48.5 ± 13.7 | 28, 12 | 20 | 1.9 ± 2.2 | n/a |
| COVID-19 | 39.5 ± 12.8 | 31, 14 | 12 | n/a | 9 (3 thyroid, 4 cardiovascular, 2 tumor) |
| Treatment | Fingolimod (Gilenya) | Teriflunomide (Aubagio) | Ocrelizumab | Cladribine (Mavenclad) | Dimethylfumarate (Tecfidera) | IFN-beta 1a (Avonex) | Natalizumab (Tysabri) | IFN-beta 1a (Plegridy) | Glatiramer Acetate (Copaxone) |
| Number of patients | 8 | 7 | 4 | 3 | 6 | 1 | 4 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stadio, A.; Bernitsas, E.; La Mantia, I.; Brenner, M.J.; Ralli, M.; Vaira, L.A.; Colizza, A.; Cavaliere, C.; Laudani, M.; Frohman, T.C.; et al. Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)? Life 2023, 13, 226. https://doi.org/10.3390/life13010226
Di Stadio A, Bernitsas E, La Mantia I, Brenner MJ, Ralli M, Vaira LA, Colizza A, Cavaliere C, Laudani M, Frohman TC, et al. Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)? Life. 2023; 13(1):226. https://doi.org/10.3390/life13010226
Chicago/Turabian StyleDi Stadio, Arianna, Evanthia Bernitsas, Ignazio La Mantia, Michael J. Brenner, Massimo Ralli, Luigi Angelo Vaira, Andrea Colizza, Carlo Cavaliere, Matteo Laudani, Teresa C. Frohman, and et al. 2023. "Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)?" Life 13, no. 1: 226. https://doi.org/10.3390/life13010226
APA StyleDi Stadio, A., Bernitsas, E., La Mantia, I., Brenner, M. J., Ralli, M., Vaira, L. A., Colizza, A., Cavaliere, C., Laudani, M., Frohman, T. C., De Vincentiis, M., Frohman, E. M., & Altieri, M. (2023). Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)? Life, 13(1), 226. https://doi.org/10.3390/life13010226














