Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Analytical Methods
2.3. Statistical Analyses
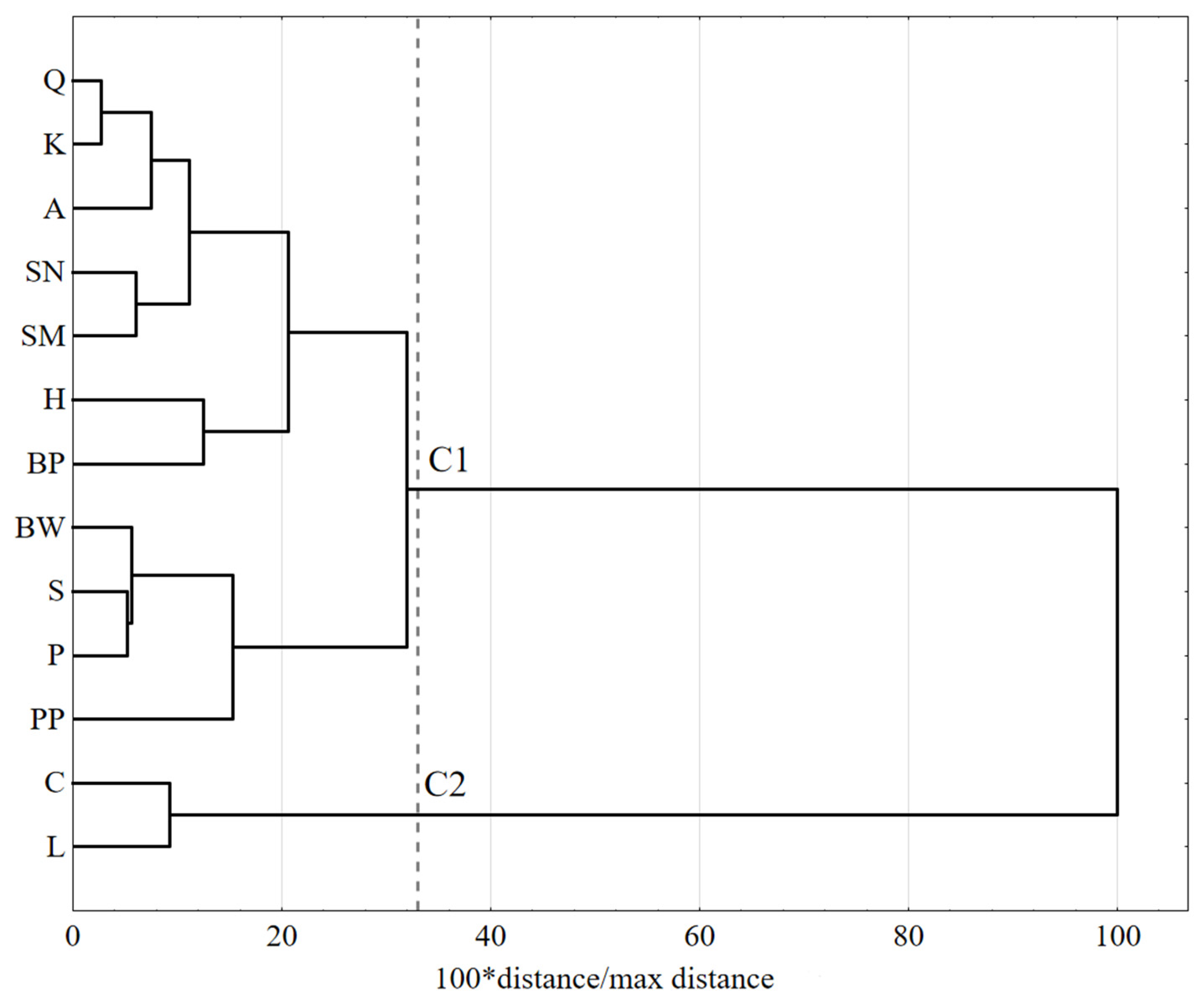
3. Results
4. Discussion
4.1. Seeds as a Source of Essential n-3 Polyunsaturated Fatty Acids
4.2. Pseudocereals as a Source of Fat and Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | amaranth |
| ALA | α-linolenic acid |
| BP | blue poppy |
| BW | buckwheat |
| C | chia |
| FA | fatty acids |
| FAME | fatty acid methyl esters |
| H | hemp |
| H/H | hypocholesterolemic/hypercholesterolemic ratio |
| IA | index of atherogenicity |
| IT | index of thrombogenicity |
| K | canihua |
| L | flax |
| LA | linoleic acid |
| MUFAs | monounsaturated fatty acids |
| P | pumpkin |
| PP | plantago |
| PUFAs | polyunsaturated fatty acids |
| Q | quinoa |
| S | sesame |
| SFAs | saturated fatty acids |
| SM | milk thistle |
| SN | sunflower |
References
- Bewley, J.D.; Black, M. Seeds. In Seeds: Physiology of Development and Germination; Bewley, J.D., Black, M., Eds.; Springer US: Boston, MA, USA, 1994; pp. 1–33. ISBN 978-1-4899-1002-8. [Google Scholar]
- Ros, E.; Hu, F.B. Consumption of Plant Seeds and Cardiovascular Health: Epidemiologic and Clinical Trial Evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty Seeds: Nutrients, Bioactives, Bioavailability, and Health Benefits: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.; Castro Pinho, O.; Ferreira, I.M.P.L.V.O. Food Industry By-Products Used as Functional Ingredients of Bakery Products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Barsby, J.P.; Cowley, J.M.; Leemaqz, S.Y.; Grieger, J.A.; McKeating, D.R.; Perkins, A.V.; Bastian, S.E.P.; Burton, R.A.; Bianco-Miotto, T. Nutritional Properties of Selected Superfood Extracts and Their Potential Health Benefits. PeerJ 2021, 9, e12525. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Białek, M.; Jelinska, M.; Tokarz, A. Fatty Acid Composition and Oxidative Characteristics of Novel Edible Oils in Poland. CyTA-J. Food 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty Acid Composition and Oxidative Stability of Cold-pressed Edible Seed Oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid Components of Flax, Perilla, and Chia Seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- Cox, S.; Garcia, K.; Carlson, C. Superfood Seeds. In Superfoods: Cultural and Scientific Perspectives; Miller, J.P., Van Buiten, C., Eds.; Food and Health; Springer International Publishing: Cham, Switzerland, 2022; pp. 125–139. ISBN 978-3-030-93240-4. [Google Scholar]
- Schoenlechner, R.; Siebenhandl, S.; Berghofer, E. 7-Pseudocereals. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Dal Bello, F., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2008; pp. 149–VI. ISBN 978-0-12-373739-7. [Google Scholar]
- Sandoval-Oliveros, M.R.; Paredes-López, O. Isolation and Characterization of Proteins from Chia Seeds (Salvia hispanica L.). Available online: https://pubs.acs.org/doi/abs/10.1021/jf3034978 (accessed on 9 April 2019).
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOAC International. AOAC Fatty Acid in Oils and Fats Preparation of Methyl Ester Boron Trifluoride Method. In AOAC Official Method 969.33; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Białek, A.; Białek, M.; Jelinska, M.; Tokarz, A. Fatty Acid Profile of New Promising Unconventional Plant Oils for Cosmetic Use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical Properties of Cold Pressed Sunflower, Peanut, Rapeseed, Mustard and Olive Oils Grown in the Eastern Mediterranean Region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiri, H.A.; Ahmed, N.; Al-Sharrah, T. Fatty Acid Profiles, Cholesterol Composition, and Nutritional Quality Indices of 37 Commonly Consumed Local Foods in Kuwait in Relation to Cardiovascular Health. medRxiv 2020. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical Content, Oxidative Stability, and Nutritional Properties of Unconventional Cold-Pressed Edible Oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef]
- Sumara, A.; Stachniuk, A.; Montowska, M.; Kotecka-Majchrzak, K.; Grywalska, E.; Mitura, P.; Saftić Martinović, L.; Kraljević Pavelić, S.; Fornal, E. Comprehensive Review of Seven Plant Seed Oils: Chemical Composition, Nutritional Properties, and Biomedical Functions. Food Rev. Int. 2022, 1–21. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Camp, J.V.; Trawka, A.; Verhé, R. Phenolic Compounds and Some Quality Parameters of Pumpkin Seed Oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Pholphana, N.; Mahidol, C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad, J. Variation of Sesamin, Sesamolin and Tocopherols in Sesame (Sesamum indicum L.) Seeds and Oil Products in Thailand. Food Chem. 2010, 122, 724–730. [Google Scholar] [CrossRef]
- Çelik, H.T.; Gürü, M. Extraction of Oil and Silybin Compounds from Milk Thistle Seeds Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2015, 100, 105–109. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S. Milk Thistle Seed Oil Constituents from Different Varieties Grown in Iran. J. Am. Oil Chem. Soc. 2009, 86, 643–649. [Google Scholar] [CrossRef]
- Krist, S.; Stuebiger, G.; Bail, S.; Unterweger, H. Detection of Adulteration of Poppy Seed Oil with Sunflower Oil Based on Volatiles and Triacylglycerol Composition. J. Agric. Food Chem. 2006, 54, 6385–6389. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical Composition and Profile Characterisation of Pumpkin (Cucurbita maxima) Seed Oil. Ind. Crops Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Were, B.A.; Onkware, A.O.; Gudu, S.; Welander, M.; Carlsson, A.S. Seed Oil Content and Fatty Acid Composition in East African Sesame (Sesamum indicum L.) Accessions Evaluated over 3 Years. Field Crops Res. 2006, 97, 254–260. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines12. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Sayanova, O.; Napier, J.A. An Alternative to Fish Oils: Metabolic Engineering of Oil-Seed Crops to Produce Omega-3 Long Chain Polyunsaturated Fatty Acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Budoff, M.J.; Mason, R.P. A Revolution in Omega-3 Fatty Acid Research. J. Am. Coll. Cardiol. 2020, 76, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomás, M.C. Characterization of Chia Seed Oils Obtained by Pressing and Solvent Extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F.; Tassone, S. Fatty Acid Profile and Nutritive Value of Quinoa (Chenopodium quinoa Willd.) Seeds and Plants at Different Growth Stages. Anim. Feed Sci. Technol. 2013, 183, 56–61. [Google Scholar] [CrossRef]
- Ayerza (h), R.; Coates, W. Protein Content, Oil Content and Fatty Acid Profiles as Potential Criteria to Determine the Origin of Commercially Grown Chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar] [CrossRef]
- Hall, C.; Tulbek, M.C.; Xu, Y. Flaxseed. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 1–97. [Google Scholar]
- Ursoniu, S.; Sahebkar, A.; Andrica, F.; Serban, C.; Banach, M. Effects of Flaxseed Supplements on Blood Pressure: A Systematic Review and Meta-Analysis of Controlled Clinical Trial. Clin. Nutr. 2016, 35, 615–625. [Google Scholar] [CrossRef]
- Prasad, K. Flaxseed and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Gogna, S.; Kaur, J.; Sharma, K.; Bhadariya, V.; Singh, J.; Kumar, V.; Rasane, P.; Vipasha, V. A Systematic Review on the Role of Alpha Linolenic Acid (ALA) in Combating Non-Communicable Diseases (NCDs). Nutr. Food Sci. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Bassett, C.M.; McCullough, R.; Pierce, G.N. The Cardiovascular Effects of Flaxseed and Its Omega-3 Fatty Acid, Alpha-Linolenic Acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and Therapeutic Perspectives of Chia (Salvia hispanica L.): A Review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef]
- The European Food Safety Authority (EFSA). Labelling Reference Intake Values for N-3 and n-6 Polyunsaturated Fatty Acids. EFSA J. 2009, 7, 1176. [Google Scholar] [CrossRef]
- Woods, V.B.; Fearon, A.M. Dietary Sources of Unsaturated Fatty Acids for Animals and Their Transfer into Meat, Milk and Eggs: A Review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching Laying Hens Eggs by Feeding Diets with Different Fatty Acid Composition and Antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef] [PubMed]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of Dietary Incorporation of N-3 Polyunsaturated Fatty Acids Rich Oil Sources on Fatty Acid Profile, Keeping Quality and Sensory Attributes of Broiler Chicken Meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty Acid Composition and Oxidation Stability of Hemp (Cannabis sativa L.) Seed Oil Extracted by Supercritical Carbon Dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Ali, M.; Hussain, Y. Fatty Acids Characterization, Oxidative Perspectives and Consumer Acceptability of Oil Extracted from Pre-Treated Chia (Salvia hispanica L.) Seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic Components of Buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Omary, M.B.; Fong, C.; Rothschild, J.; Finney, P. REVIEW: Effects of Germination on the Nutritional Profile of Gluten-Free Cereals and Pseudocereals: A Review. Cereal Chem. 2012, 89, 1–14. [Google Scholar] [CrossRef]
- Gulpinar, A.R.; Erdogan Orhan, I.; Kan, A.; Senol, F.S.; Celik, S.A.; Kartal, M. Estimation of in Vitro Neuroprotective Properties and Quantification of Rutin and Fatty Acids in Buckwheat (Fagopyrum esculentum Moench) Cultivated in Turkey. Food Res. Int. 2012, 46, 536–543. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat Seed Milling Fractions: Description, Macronutrient Composition and Dietary Fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Zhou, M.-L.; Tang, Y.; Li, F.-L.; Tang, Y.-X.; Shao, J.-R.; Xue, W.-T.; Wu, Y.-M. Bioactive Compounds in Functional Buckwheat Food. Food Res. Int. 2012, 49, 389–395. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Zadernowski, R.; Czaplicki, S.; Derewiaka, D.; Wronowska, B. Amaranth Seeds and Products—The Source of Bioactive Compounds. Pol. J. Food Nutr. Sci. 2014, 64, 165–170. [Google Scholar] [CrossRef]
- Villa, D.; Russo, L.; Kerbab, K.; Landi, M.; Rastrelli, L. Chemical and Nutritional Characterization of Chenopodium pallidicaule (Cañ Ihua) and Chenopodium quinoa (Quinoa) Seeds. Emir. J. Food Agric. 2014, 26, 609. [Google Scholar] [CrossRef]
- Berganza, B.E.; Moran, A.W.; Rodríguez, G.M.; Coto, N.M.; Santamaría, M.; Bressani, R. Effect of Variety and Location on the Total Fat, Fatty Acids and Squalene Content of Amaranth. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional Constituents of Pseudo Cereals and Their Potential Use in Food Systems: A Review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
| Name | Family | Abbreviation |
|---|---|---|
| Pseudocereals: | ||
| amaranth (Amaranthus cruentus L.) | Amaranthaceae | A |
| buckwheat (Fagopyrum esculentum) | Polygonaceae | BW |
| canihua/kaniwa (Chenopodium pallidicaule) | Amaranthaceae | K |
| quinoa (Chenopodium quinoa) | Amaranthaceae | Q |
| Food functional ingredients: | ||
| chia (Salvia hispanica) | Lamiaceae/Labiatae | C |
| flax (Linum usitatissimum L.) | Linaceae | L |
| hemp (shelled seeds; Cannabis sativa L.) | Cannabaceae | H |
| milk thistle (Silybum marianum) | Asteraceae | SM |
| plantago (Plantago psyllium) | Plantaginaceae | PP |
| poppy (blue, Papaver somniferum) | Papaveraceae | BP |
| pumpkin (shelled seeds, Cucurbita L.) | Cucurbitaceae | P |
| sesame (shelled seeds, Sesamum indicum L.) | Pedaliaceae | S |
| sunflower (shelled seeds, Helianthus annuus) | Asteraceae | SN |
| PP | BW | Q | A | K | SM | C | L | P | BP | H | S | SN | p Value * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat [g 100 g −1] | ||||||||||||||
| 1.2 ± 1.2 a | 2.9 ± 0.2 a,b | 6.0 ± 1.1 b | 6.1 ± 0.6 b | 7.3 ± 0.7 b | 23.7 ± 3.2 | 29.4 ± 1.4 | 36.0 ± 2.0 c | 38.0 ± 5.9 c,d | 41.0 ± 3.9 d | 41.6 ± 3.1 d | 49.1 ± 3.1 e | 51.7 ± 3.1 e | <0.0001 | |
| SFAs [% of total FAs] | ||||||||||||||
| C12:0 | 0.17 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | - |
| C14:0 | 0.35 ± 0.08 | 0.13 ± 0.00 a | 0.17 ± 0.05 a,b | 0.23 ± 0.03 b | 0.15 ± 0.01 a | 0.11 ± 0.01 a | nd | nd | 0.12 ± 0.02 a | nd | nd | nd | nd | <0.0001 |
| C16:0 | 15.1 ± 2.4 e | 15.3 ± 1.0 e | 10.4 ± 0.7 c | 19.9 ± 2.2 | 14.2 ± 0.3 d,e | 8.62 ± 0.36 b,c | 7.40 ± 0.21 a,b | 6.17 ± 0.39 a | 12.5 ± 1.1 d | 9.65 ± 0.61 c | 6.33 ± 0.60 a | 9.73 ± 0.31 c | 6.26 ± 0.40 a | <0.0001 |
| C18:0 | 2.71 ± 0.28 d | 1.66 ± 0.20 b | 0.42 ± 0.08 a | 3.03 ± 0.52 d | 0.86 ± 0.19 a | 4.96 ± 0.44 e | 2.95 ± 0.56 d | 3.99 ± 0.55 | 6.10 ± 0.38 f | 1.93 ± 0.19 b,c | 2.68 ± 0.28 c,d | 5.57 ± 1.03 e,f | 2.44 ± 0.29 c,d | <0.0001 |
| C20:0 | 0.55 ± 0.08 d,e,f | 1.33 ± 0.19 | 0.43 ± 0.08 c,d,e | 0.72 ± 0.06 f | 0.60 ± 0.02 e,f | 2.70 ± 0.38 | 0.25 ± 0.03 a,b,c | 0.16 ± 0.02 a,b | 0.38 ± 0.03 b,c,d | 0.11 ± 0.01 a | 0.70 ± 0.10 f | 0.58 ± 0.05 d,e,f | 0.21 ± 0.02 a,b | <0.0001 |
| C21:0 | nd | 0.17 ± 0.01 a | nd | nd | 0.12 ± 0.01 a | nd | nd | nd | nd | nd | nd | nd | nd | <0.0001 |
| C22:0 | 0.67 ± 0.14 c | 1.56 ± 0.27 | 0.61 ± 0.08 c | 0.32 ± 0.04 a,b | 0.45 ± 0.02 b,c | 1.82 ± 0.28 | nd | 0.12 ± 0.01 a | 0.12 ± 0.01 a | nd | nd | 0.11 ± 0.01 a | 0.66 ± 0.08 c | <0.0001 |
| C23:0 | 0.27 ± 0.09 a,b | 0.19 ± 0.03 a | 0.36 ± 0.20 b | 0.44 ± 0.06 a | 0.19 ± 0.05 a | 0.18 ± 0.00 a | nd | nd | 0.13 ± 0.01 a | nd | nd | nd | 0.12 ± 0.02 a | <0.0001 |
| MUFAs [% of total FAs] | ||||||||||||||
| C14:1 | 0.24 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | - |
| C16:1 | 0.47 ± 0.17 | 0.22 ± 0.01 b,c | 0.14 ± 0.02 a,b | 0.31 ± 0.03 c | 0.10 ± 0.00 a | nd | 0.17 ± 0.01 a,b | nd | 0.15 ± 0.06 a,b | 0.14 ± 0.03 a,b | 0.11 ± 0.01 a,b | 0.12 ± 0.01 a,b | 0.12 ± 0.02 a,b | <0.0001 |
| C17:1 | 0.19 ± 0.04 a | nd | 0.21 ± 0.03 a,b | 0.71 ± 0.05 | 0.26 ± 0.04 b | nd | nd | nd | nd | nd | nd | nd | nd | <0.0001 |
| C18:1 | 28.7 ± 3.3 f,g | 33.7 ± 0.9 g,h | 24.1 ± 3.9 d,e,f | 21.1 ± 0.5 c,d,e | 24.0 ± 0.7 d,e,f | 24.4 ± 3.3 e,f | 5.64 ± 0.76 a | 17.3 ± 1.1 b,c,d | 33.2 ± 8.0 g | 16.6 ± 2.9 b,c | 11.3 ± 3.0 a,b | 40.0 ± 1.7 h | 33.7 ± 9.3 g,h | |
| C20:1 | nd | nd | 0.09 ± 0.00 | nd | nd | nd | nd | nd | nd | nd | 0.76 ± 0.61 | nd | nd | 0.347 |
| C22:1 n9 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.26 ± 0.06 | nd | nd | - |
| PUFAs [% of total FAs] | ||||||||||||||
| C18:2 n6 | 28.4 ± 3.9 | 37.5 ± 1.2 b | 50.4 ± 3.7 d,e | 45.8 ± 1.0 c,d | 48.9 ± 0.4 c,d,e | 55.1 ± 4.0 e | 19.6 ± 0.5 a | 19.1 ± 6.7 a | 46.6 ± 7.2 c,d | 69.2 ± 3.5 | 56.2 ± 1.4 e | 41.6 ± 2.1 b,c | 54.4 ± 8.8 e | <0.0001 |
| C18:3 n6 | nd | nd | 0.10 ± 0.00 a | 0.14 ± 0.04 a | nd | nd | 0.21 ± 0.01 b | 0.18 ± 0.02 b | nd | nd | 0.50 ± 0.00 | nd | nd | <0.0001 |
| C18:3 n3 | 14.5 ± 1.4 c | 2.12 ± 0.13 a,b | 5.78 ± 1.82 b | 0.83 ± 0.02 a | 5.06 ± 0.15 b | 0.22 ± 0.05 a | 62.0 ± 1.3 | 51.4 ± 6.7 | 0.24 ± 0.07 a | 0.71 ± 0.12 a | 17.3 ± 2.4 c | 0.33 ± 0.03 a | 0.25 ± 0.13 a | <0.0001 |
| C20:2 n6 | 0.18 ± 0.12 | nd | 0.14 ± 0.02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.416 |
| C20:3 n6 | nd | nd | nd | nd | 0.20 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd | - |
| C20:4 n6 | nd | nd | 0.22 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | - |
| C20:3 n3 | 0.27 ± 0.08 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | - |
| C20:5 n3 | 0.13 ± 0.00 a | 0.13 ± 0.00 a | 1.34 ± 0.09 | nd | 0.74 ± 0.03 | nd | nd | nd | nd | nd | 0.26 ± 0.06 | nd | nd | <0.0001 |
| C22:2 | nd | nd | 0.65 ± 0.15 a | 2.85 ± 1.26 | 0.78 ± 0.24 a | nd | nd | nd | 0.12 ± 0.01 a | nd | nd | nd | 0.18 ± 0.00 a | <0.0001 |
| PP | BW | Q | A | K | SM | C | L | P | BP | H | S | SN | p Value * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFAs | 21.3 ± 2.6 a,b | 21.6 ± 1.7 a | 13.0 ± 1.0 e | 25.5 ± 2.4 | 17.2 ± 0.3 c,d | 18.6 ± 1.2 c | 10.8 ± 0.7 e,f | 10.6 ± 0.9 f | 19.3 ± 1.1 b,c | 11.9 ± 0.8 f | 10.2 ± 0.8 f | 16.3 ± 1.0 d | 9.82 ± 0.52 f | <0.0001 |
| MUFAs | 32.0 ± 3.5 b,c | 36.1 ± 0.8 a,b | 25.7 ± 3.9 c,d | 23.0 ± 0.7 d,e | 25.2 ± 0.6 c,d | 24.9 ± 3.3 d | 5.92 ± 0.78 g | 17.6 ± 1.2 e,f | 33.5 ± 8.1 b | 17.1 ± 2.9 e,f | 12.8 ± 2.6 f,g | 40.9 ± 1.5 a | 34.5 ± 9.4 a,b | <0.0001 |
| PUFAs | 46.7 ± 5.4 e,f | 42.3 ± 1.6 f | 61.3 ± 3.3 c | 51.5 ± 1.9 d,e | 57.6 ± 0.7 c,d | 56.6 ± 4.2 c,d | 83.3 ± 1.5 a | 71.8 ± 1.9 b | 47.2 ± 7.3 e,f | 71.1 ± 3.5 b | 77.1 ± 3.1 a,b | 42.8 ± 2.3 f | 55.7 ± 9.1 c,d | <0.0001 |
| n3 PUFAs | 15.95 ± 1.6 a | 2.33 ± 0.19 c,d | 7.51 ± 1.90 b | 0.87 ± 0.03 d | 6.00 ± 0.17 b,c | 0.22 ± 0.06 d | 63.2 ± 1.3 | 52.3 ± 6.7 | 0.24 ± 0.07 d | 0.72 ± 0.12 d | 18.2 ± 2.9 a | 0.33 ± 0.03 d | 0.09 ± 0.15 d | <0.0001 |
| n6 PUFAs | 30.8 ± 4.5 | 40.0 ± 1.4 d | 53.8 ± 4.1 a–c | 50.6 ± 1.9 b,c | 51.6 ± 0.6 a–c | 56.3 ± 4.2 a,b | 20.1 ± 0.6 e | 19.6 ± 6.8 e | 47.0 ± 7.3 c,d | 70.4 ± 3.6 | 58.9 ± 0.3 a | 42.4 ± 2.3 d | 55.6 ± 9.1 a,b | <0.0001 |
| n3/n6 PUFAs | 0.52 ± 0.08 b | 0.06 ± 0.00 b,c | 0.14 ± 0.04 b,c | 0.02 ± 0.00 c | 0.12 ± 0.00 b,c | 0.00 ± 0.00 c | 3.14 ± 0.11 a | 2.97 ± 1.01 a | 0.01 ± 0.00 c | 0.01 ± 0.00 c | 0.31 ± 0.05 b,c | 0.01 ± 0.00 c | 0,00 ± 0.00 c | <0.0001 |
| IA | 0.37 ± 0.05 c | 0.44 ± 0.03 b,c | 0.36 ± 0.05 c | 0.90 ± 0.12 | 0.49 ± 0.01 a,b | 0.36 ± 0.05 c | 0.11 ± 0.00 e,f | 0.09 ± 0.01 f | 0.42 ± 0.16 c | 0.56 ± 0.07 a | 0.21 ± 0.02 d,e | 0.24 ± 0.01 d | 0.20 ± 0.05 d–f | <0.0001 |
| IT | 0.25 ± 0.04 d,e | 0.40 ± 0.04 a,b | 0.19 ± 0.02 f | 0.61 ± 0.08 | 0.28 ± 0.01 d | 0.34 ± 0.02 c | 0.05 ± 0.00 g | 0.06 ± 0.01 g | 0.46 ± 0.03 a | 0.26 ± 0.02 d | 0.11 ± 0.02 g | 0.37 ± 0.03 b,c | 0.20 ± 0.01 e,f | <0.0001 |
| H/H | 4.75 ± 0.93 e,f | 4.77 ± 0.39 e,f | 7.87 ± 0.67 c | 3.57 ± 0.49 f | 5.56 ± 0.13 d,e | 9.23 ± 0.49 b | 11.8 ± 0.41 | 14.3 ± 1.1 a | 6.41 ± 0.61 d | 9.00 ± 0.61 b,c | 13.5 ± 1.3 a | 8.44 ± 0.30 b,c | 14.2 ± 1.1 a | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwonka, M.; Białek, A. Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients. Life 2023, 13, 217. https://doi.org/10.3390/life13010217
Czerwonka M, Białek A. Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients. Life. 2023; 13(1):217. https://doi.org/10.3390/life13010217
Chicago/Turabian StyleCzerwonka, Małgorzata, and Agnieszka Białek. 2023. "Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients" Life 13, no. 1: 217. https://doi.org/10.3390/life13010217
APA StyleCzerwonka, M., & Białek, A. (2023). Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients. Life, 13(1), 217. https://doi.org/10.3390/life13010217







