The Impact of COVID-19 Pandemic on Surgical Treatment of Resectable Non-Small Cell Lung Cancer in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data
2.3. Statistical Analysis
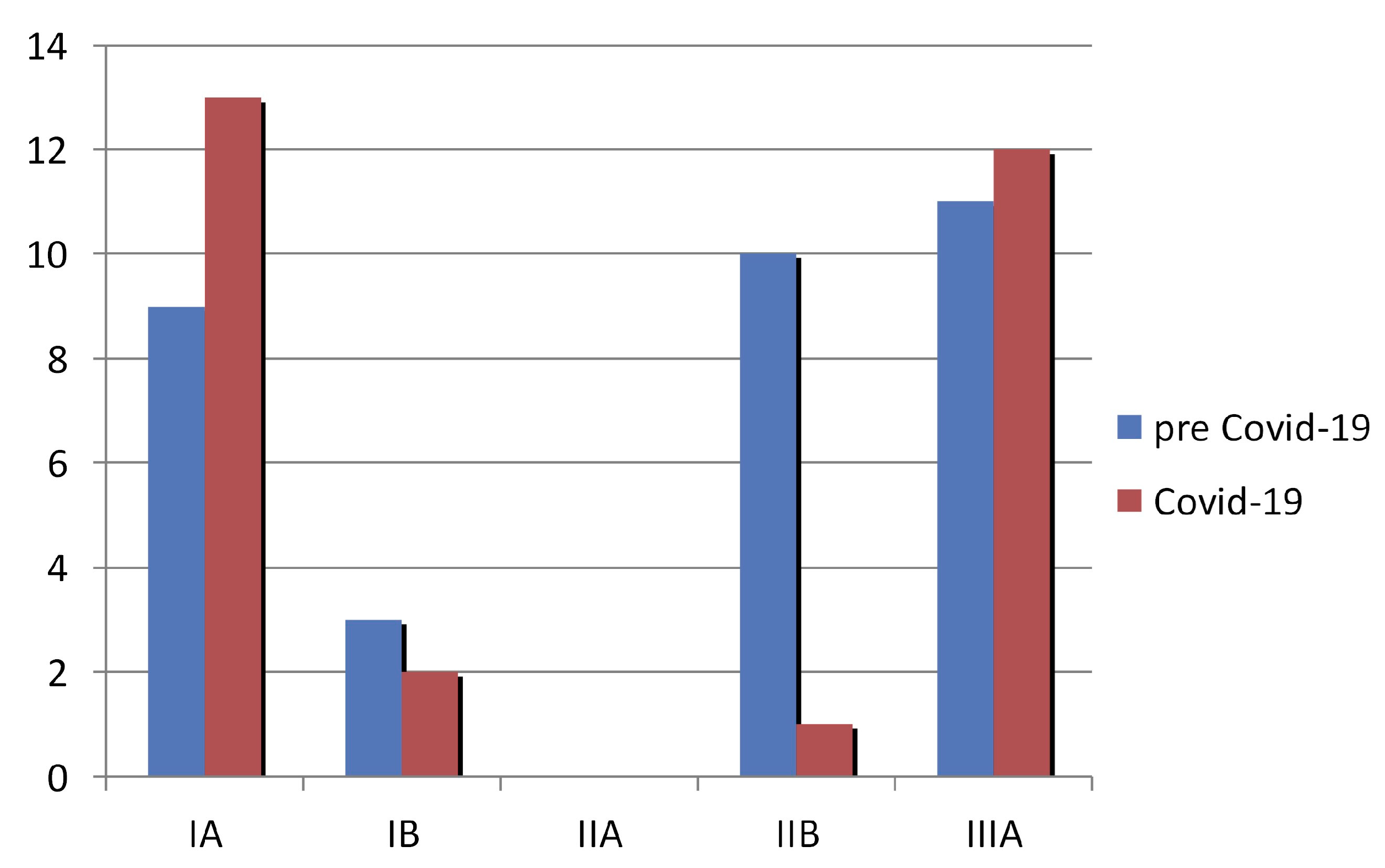
3. Results
3.1. Demographic and Clinical Characteristics of the Study Patients
3.2. Consequences regarding the Waiting Time to Surgical Treatment
3.3. Consequences regarding Disease Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kapetanakis, E.I.; Tomos, I.P.; Karakatsani, A.; Koumarianou, A.; Tomos, P.I. Management of surgical lung cancer patients during the COVID-19 pandemic in the financially and resource strained Greek health care system. J. Surg. Oncol. 2020, 122, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Chang, K.; Mi, J.; Liang, Z.; Hu, L.; Long, F.; Shi, H.; Lin, Z.; Wang, X.; Pei, X. The impact of the COVID-19 pandemic on lung cancer patients. Ann. Palliat. Med. 2020, 9, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Yang, Y.; Zhou, W.; Zhang, M.; Shen, Y.; Tao, D.; Wang, L.; Lei, Q.; Wang, Y.; Wu, Y. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer 2021, 157, 60–65. [Google Scholar] [CrossRef]
- Bakouny, Z.; Hawley, J.E.; Choueiri, T.K.; Peters, S.; Rini, B.I.; Warner, J.L.; Painter, C.A. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell 2020, 38, 629–646. [Google Scholar] [CrossRef]
- Tomos, I.; Kostikas, K.; Hillas, G.; Bakakos, P.; Loukides, S. Primary care and COVID-19: Cutting the Gordian knot-the Greek experience and algorithm. ERJ Open Res. 2020, 6, 00468–2020. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Akinosoglou, K.; Karampitsakos, T.; Panou, V.; Tomos, I.; Tsoukalas, G.; Stratiki, M.; Dimakou, K.; Chrysikos, S.; Papaioannou, O.; et al. Epidemiological characteristics and outcomes from 187 patients with COVID-19 admitted to 6 reference centers in Greece: An observational study during the first wave of the COVID-19 pandemic. Adv. Respir. Med. 2021, 89, 378–385. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.-A.; You, H.; Wu, M.; Zheng, Q.-C.; et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 28, 1054–1062. [Google Scholar] [CrossRef]
- Tiang, J.; Yuan, X.; Xiao, J.; Zhong, Q.; Yang, C.; Liu, B.; Cai, Y.; Lu, Z.; Wang, J.; Wang, Y.; et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Bestvina, C.; Velez Velez, M.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J. Immunother. Cancer 2021, 9, e002266. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Rosen, J.E.; Keshava, H.B.; Yao, X.; Kim, A.W.; Detterbeck, F.C.; Boffa, D.J. The Natural History of Operable Non-Small Cell Lung Cancer in the National Cancer Database. Ann. Thorac. Surg. 2016, 101, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Heiden, B.T.; Eaton, D.B.; Engelhardt, K.E.; Chang, S.-H.; Yan, Y.; Patel, M.R.; Kreisel, D.; Nava, R.G.; Meyers, B.F.; Kozower, B.D.; et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I Non-Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2111613. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Hu, K.; Wang, M. Clinical recommendations on lung cancer management during the COVID-19 pandemic. Thorac. Cancer 2020, 11, 2067–2074. [Google Scholar] [CrossRef]
- Chang, S.H.; Zervos, M.; Kent, A.; Chachoua, A.; Bizekis, C.; Pass, H.; Cerfolio, R.J. Safety of patients and providers in lung cancer surgery during the COVID-19 pandemic. Eur. J. Cardio-Thorac. Surg. 2020, 58, 1222–1227. [Google Scholar] [CrossRef]
- Yang, W.; Kandula, S.; Huynh, M.; Greene, S.K.; Van Wye, G.; Li, W.; Chan, H.T.; McGibbon, E.; Yeung, A.; Olson, D.; et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: A model-based analysis. Lancet Infect. Dis. 2021, 21, 203–212. [Google Scholar] [CrossRef]
- de Joode, K.; Dumoulin, D.W.; Engelen, V.; Bloemendal, H.J.; Verheij, M.; van Laarhoven, H.W.M.; Dingemans, I.H.; Dingemans, A.C.; van der Veldt, A.A.M. Impact of the coronavirus disease 2019 pandemic on cancer treatment: The patients’ perspective. Eur. J. Cancer 2020, 136, 132–139. [Google Scholar] [CrossRef]
- Cancino, R.S.; Su, Z.; Mesa, R.; Tomlinson, G.E.; Wang, J. The Impact of COVID-19 on Cancer Screening: Challenges and Opportunities. JMIR Cancer 2020, 6, e21697. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Murphy, M.; Price, S.; Lewis, R.; Denholm, R.; Horwood, J.; Palmer, T.; Salisbury, C. Changes in presentations with features potentially indicating cancer in primary care during the COVID-19 pandemic: A retrospective cohort study. BMJ Open 2021, 24, e050131. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.S.; Weller, D. Lung cancer and COVID-19: Lessons learnt from the pandemic and where do we go from here? NPJ Prim. Care Respir. Med. 2022, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Interim Guidance for Healthcare Facilities: Preparing for Community Transmission of COVID-19 in the United States. Available online: https://stacks.cdc.gov/view/cdc/85502 (accessed on 20 December 2022).
- Passaro, A.; Addeo, A.; Von Garnier, C.; Blackhall, F.; Planchard, D.; Felip, E.; Dziadziuszko, R.; de Marinis, F.; Reck, M.; Bouchaab, H.; et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Lung cancer. ESMO Open 2020, 5, e000820. [Google Scholar] [CrossRef] [PubMed]
- Raskin, J.; Lebeer, M.; De Bondt, C.; Wener, R.; Janssens, A.; Van Meerbeeck, J.P. Cancer in the time of COVID: Expert opinion on how to adapt current practice. Eur. Respir. J. 2020; in press. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.C.; Soo, R.A.; Jazieh, A.R.; Rice, S.J.; Kim, Y.T.; Teo, L.L.S.; Warren, G.W.; Xiao, S.Y.; Smit, E.F.; Aerts, J.G.; et al. Treatment Guidance for Patients with Lung Cancer during the Coronavirus 2019 Pandemic. J. Thorac. Oncol. 2020, 15, 1119–1136. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e278S–e313S. [Google Scholar] [CrossRef]
- Yang, C.J.; Wang, H.; Kumar, A.; Wang, X.; Hartwig, M.G.; D’Amico, T.A.; Berry, M.F. Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma. Chest 2017, 152, 1239–1250. [Google Scholar] [CrossRef]
- Samson, P.; Patel, A.; Garrett, T.; Crabtree, T.; Kreisel, D.; Krupnick, A.S.; Patterson, G.A.; Broderick, S.; Meyers, B.F.; Puri, V. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann. Thorac. Surg. 2015, 99, 1906–1912. [Google Scholar] [CrossRef]
- Huang, C.S.; Hsu, P.-K.; Chen, C.-K.; Yeh, Y.-C.; Shih, C.-C.; Huang, B.-S. Delayed surgery after histologic or radiologic-diagnosed clinical stage I lung adenocarcinoma. J. Thorac. Dis. 2020, 12, 615–625. [Google Scholar] [CrossRef] [PubMed]
| Patients with Resectable NSCLC | |||
|---|---|---|---|
| Demographic Characteristics | Total (n = 61) | pre COVID-19 (Control Group) (n = 33) | COVID-19 (Study Group) (n = 28) |
| Age (mean, SD: years) | 67.0 (7.3) | 67.1 (7.5) | 67.0 (7.1) |
| Sex (n, %: males) | 50.0 (82.0) | 28.0 (84.8) | 22.0 (78.6) |
| Clinical Characteristics | |||
| Days waiting treatment (median, IQR) | 23.0 (14.0–37.0) | 18.0 (11.0–23.0) | 47.0 (23.0–100.0) |
| Increase of the size (n, %: yes) | 10.0 (16.4) | 2.0 (6.1) | 8.0 (28.6) |
| Increase of the stage (n, %: yes) | 7.0 (11.5) | 2.0 (6.1) | 5.0 (17.9) |
| Death (n, %: yes) | 1.0 (1.6) | 0.0 (0.0) | 1.0 (3.6) |
| Type of cancer (n, %) | |||
| Squamous | 28.0 (45.9) | 13.0 (39.4) | 15.0 (53.6) |
| Adenocarcinoma | 29.0 (47.5) | 17.0 (51.5) | 12.0 (42.9) |
| Other | 4.0 (6.6) | 3.0 (9.1) | 1.0 (3.6) |
| Lung Cancer Patients | Aσθενείς | ||
|---|---|---|---|
| Clinical Characteristics | pre COVID-19 (n = 33) | COVID-19 (n = 28) | p-Value |
| Days waiting treatment (median, IQR) | 18 (11–23) | 47 (23–100) | <0.001 1 * |
| Increase of the size (n, %: yes) | 2 (8.7) | 8 (44.4) | 0.012 2 * |
| Increase of the stage (n, %: yes) | 2 (8.7) | 5 (27.8) | 0.209 2 |
| Patient Group | b-coef | 95% C.I. for b-coef | p-Value | |
|---|---|---|---|---|
| Model 1 | Pre COVID-19 | Reference category | ||
| COVID-19 | 40.8 | (21.9 to 59.6) | <0.001 1 * | |
| OR | 95% C.I. for OR | p-Value | ||
| Model 2 | Pre COVID-19 | Reference category | ||
| COVID-19 | 10.7 | (1.7 to 69.0) | 0.012 2 * | |
| Model 3 | Pre COVID-19 | Reference category | ||
| COVID-19 | 5.9 | (0.7 to 46.2) | 0.093 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomos, I.; Kapetanakis, E.I.; Dimakopoulou, K.; Raptakis, T.; Kampoli, K.; Karakatsani, A.; Koumarianou, A.; Papiris, S.; Tomos, P. The Impact of COVID-19 Pandemic on Surgical Treatment of Resectable Non-Small Cell Lung Cancer in Greece. Life 2023, 13, 218. https://doi.org/10.3390/life13010218
Tomos I, Kapetanakis EI, Dimakopoulou K, Raptakis T, Kampoli K, Karakatsani A, Koumarianou A, Papiris S, Tomos P. The Impact of COVID-19 Pandemic on Surgical Treatment of Resectable Non-Small Cell Lung Cancer in Greece. Life. 2023; 13(1):218. https://doi.org/10.3390/life13010218
Chicago/Turabian StyleTomos, Ioannis, Emmanouil I. Kapetanakis, Konstantina Dimakopoulou, Thomas Raptakis, Katerina Kampoli, Anna Karakatsani, Anna Koumarianou, Spyros Papiris, and Periklis Tomos. 2023. "The Impact of COVID-19 Pandemic on Surgical Treatment of Resectable Non-Small Cell Lung Cancer in Greece" Life 13, no. 1: 218. https://doi.org/10.3390/life13010218
APA StyleTomos, I., Kapetanakis, E. I., Dimakopoulou, K., Raptakis, T., Kampoli, K., Karakatsani, A., Koumarianou, A., Papiris, S., & Tomos, P. (2023). The Impact of COVID-19 Pandemic on Surgical Treatment of Resectable Non-Small Cell Lung Cancer in Greece. Life, 13(1), 218. https://doi.org/10.3390/life13010218







