Antibacterial Effects of Phytocannabinoids
Abstract
:1. Introduction
2. Pharmacology of Antibiotics
2.1. Antibiotics Targeting Cell Wall Receptors
2.2. Inhibition of Protein Biosynthesis
2.3. Inhibition of DNA and RNA Biosynthesis
2.4. Inhibition of Metabolic Pathways
2.5. Inhibition of Membrane Function
2.6. Antibacterial Resistance
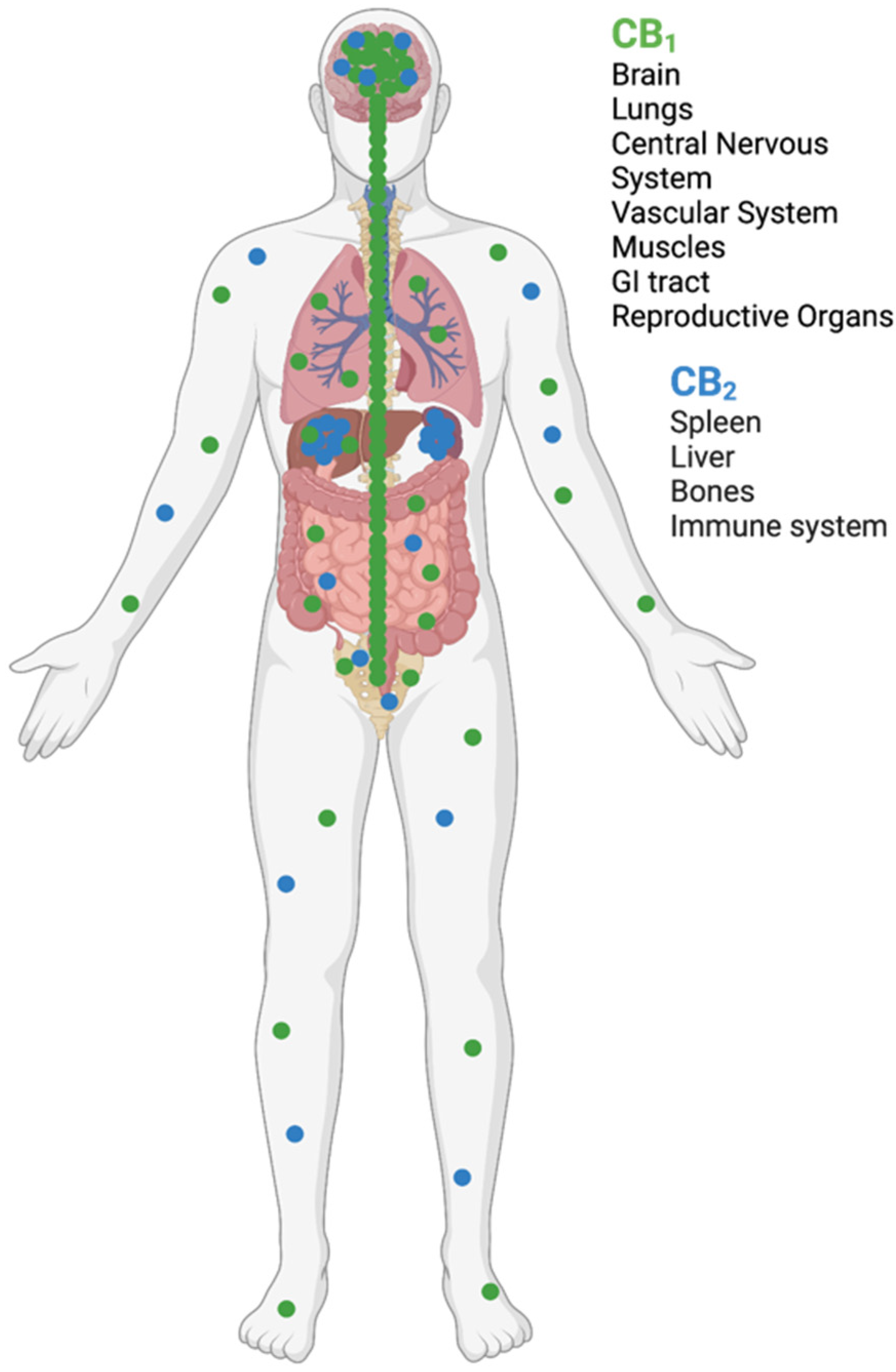
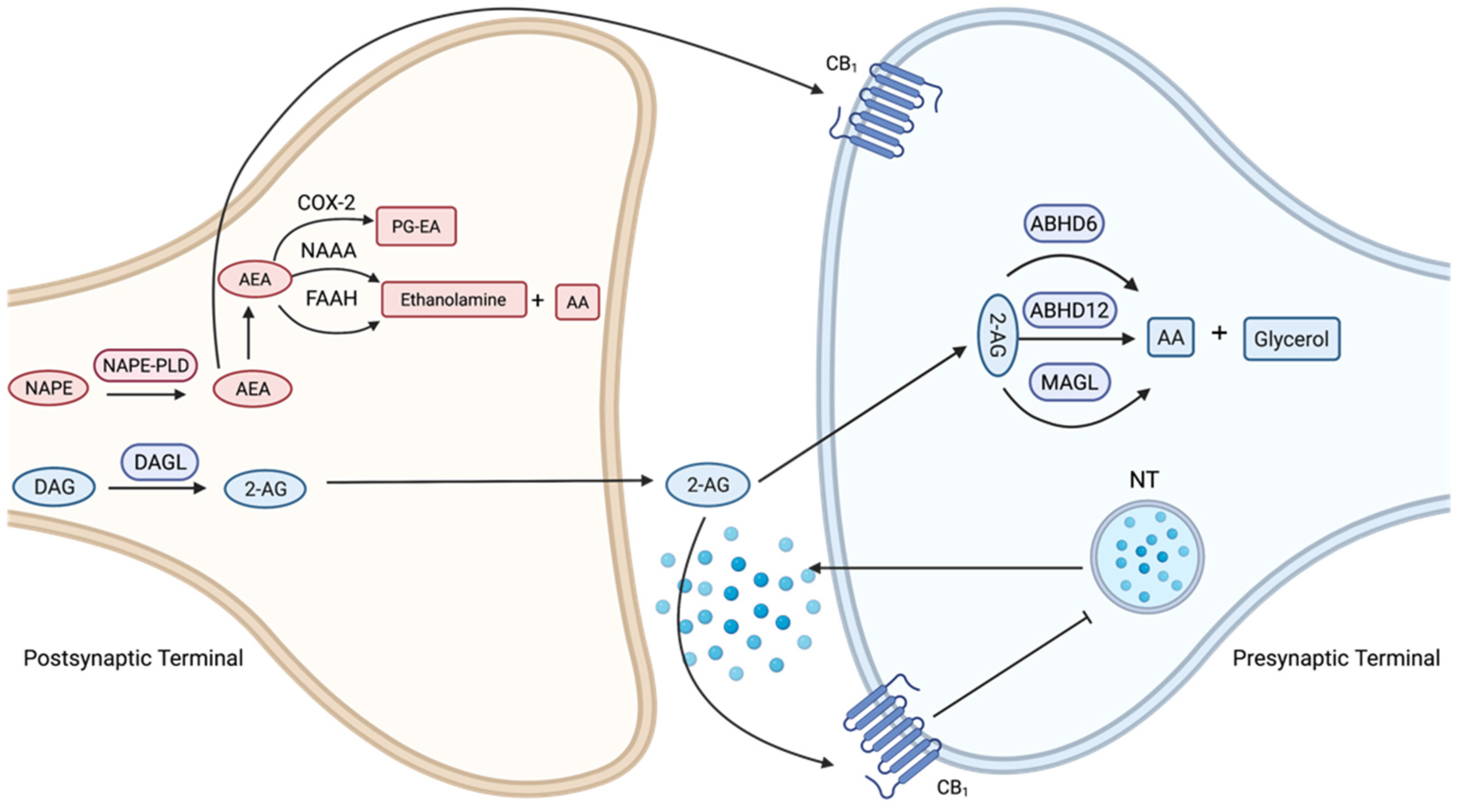
3. The Endocannabinoid System
3.1. Endocannabinoid Receptors
3.2. Cannabinoid Ligands and Enzyme Inhibitors
4. Antibacterial Effects of Cannabinoids
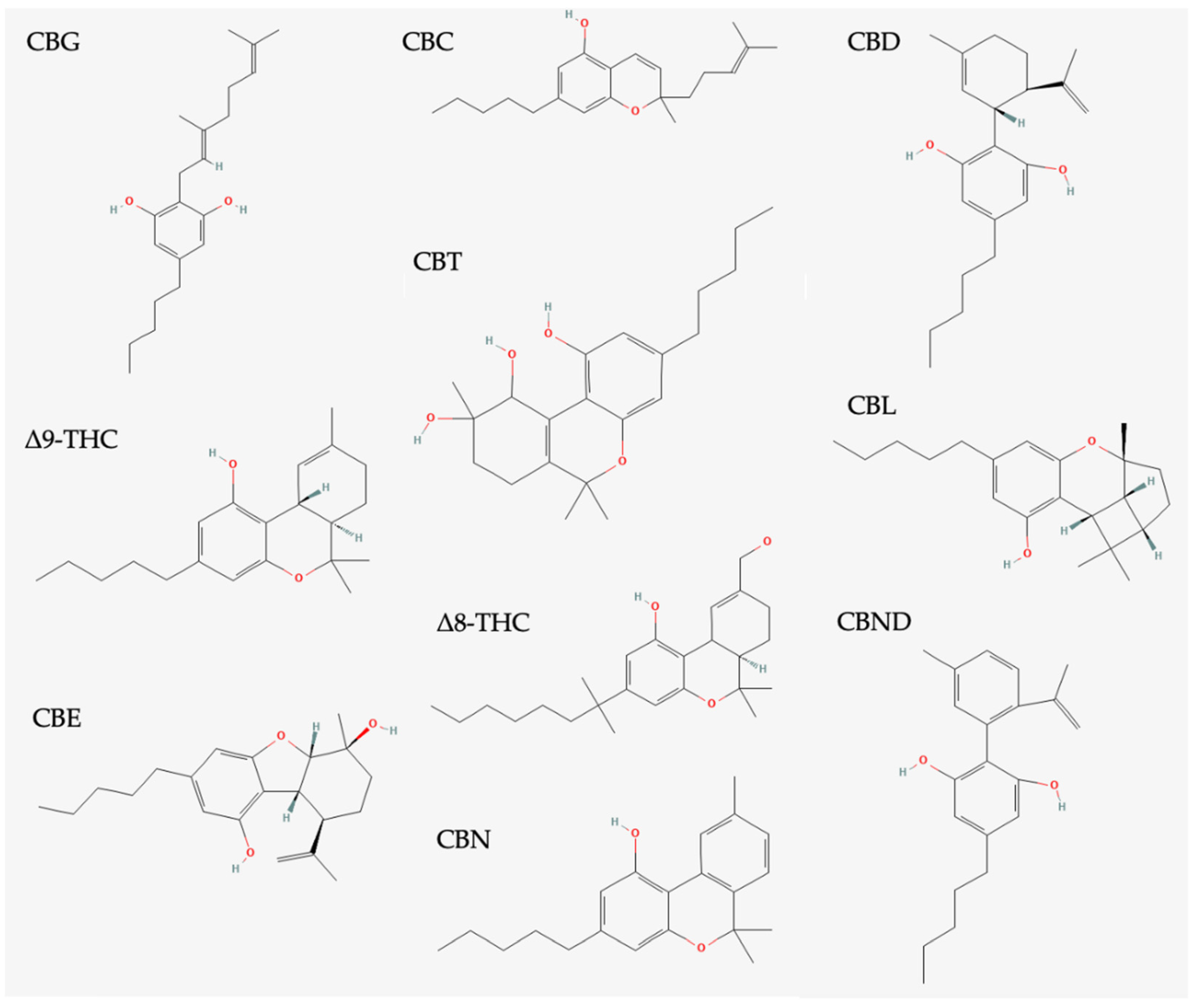
4.1. Classification of Phytocannabinoids
4.2. Antibacterial Effects of Cannabidiol (CBD)
4.3. Antibacterial Effects of Cannabichromene (CBC)
4.4. Antibacterial Effects of Cannabigerol (CBG)
4.5. Antibacterial Effects of Cannabidiolic Acid (CBDA)
4.6. Antibacterial Effects of Other CBR Agonists
| Strain | CBD | CBCA | CBG | THC | CBDA | BCP |
|---|---|---|---|---|---|---|
| Staphylococcus aureus (MRSA) | 4 2 | 3.9 | 2 | 2 | 4 | 16 |
| Enterococcus faecalis | 8 1 | 7.8 | 1 | |||
| Listeria monocytogenes | 4 1 | 2 | ||||
| Streptococcus pneumoniae | 1–2 | 6250 | ||||
| Clostridioides difficile | 2–4 | |||||
| Staphylococcus epidermidis | 2 | 4 | ||||
| Bacillus subtilis | 8 | 1 |
4.7. The Potential Role of Cannabinoid Structure on Antibacterial Activity
5. Therapeutic Applications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PBP | Penicillin Binding Proteins |
| MDR | Multidrug Resistance |
| HGT | Horizontal Gene Transfer |
| GPCR | G Protein Coupled Receptors |
| CB1 | Cannabinoid Receptor One |
| CB2 | Cannabinoid Receptor Two |
| CNS | Central Nervous System |
| PLC | Phospholipase C |
| IP3 | Inositol 1,4,5-Triphosphate |
| THC | 9-tetrahydrocannabinol |
| 2-AG | 2-Arachidonooylglycerol |
| FAAH | Fatty Acid Amide Hydrolase |
| COX-2 | Cyclooxygenase-2 |
| NAAA | N-Acylethanolamine-Hydrolysing Acid Amidase |
| MAGL | Monoacylglycerol Lipase |
| ABHD6 | Alpha/Beta Domain Hydrolases 6 |
| ABHD12 | Alpha/Beta Domain Hydrolases 12 |
| CBD | Cannabidiol |
| BAC | Bacitracin |
| MIC | Minimum Inhibitory Concentration |
| LPS | Lipopolysaccharides |
| CBC | Cannabichromene |
| CBN | Cannabinol |
| CBGA | Cannabigerolic Acid |
| CBG | Cannabigerol |
| CBDA | Cannabidiolic Acid |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| CCA | Cannabiorcichromenic acid |
| MIC | Minimum Inhibitory Concentration |
| GRAS | Generally Recognised as Safe |
| FDA | Food and Drug Administration |
| IV | Intravenous |
References
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Russo, E.B.; Jiang, H.E.; Li, X.; Sutton, A.; Carboni, A.; Del Bianco, F.; Mandolino, G.; Potter, D.J.; Zhao, Y.X.; Bera, S.; et al. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J. Exp. Bot. 2008, 59, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Hawley, P.; Gobbo, M.; Afghari, N. The impact of legalization of access to recreational Cannabis on Canadian medical users with Cancer. BMC Health Serv. Res. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Sadhana, D.; Kathleen, M.; Jonathan, D.K. Marijuana and the Pediatric Population. Pediatrics 2020, 146, 279–289. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Yoneyama, H.; Katsumata, R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci. Biotechnol. Biochem. 2006, 70, 1060–1075. [Google Scholar] [CrossRef]
- Doyle, G.R.; McCutcheon, J.A. Mechanisms of Antibacterial Drugs. In Clinical Procedures for Safer Patient Care; BCcampus: Victoria, BC, Canada, 2015. [Google Scholar]
- Eyler, R.F.; Shvets, K. Clinical pharmacology of antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Munita, J.; Arias, C. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Origins and evolution of antibiotic resistance. Microbiologia 1996, 12, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Abood, M.E. CB1 & CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Wilson, C.D.; Berquist, M.D. Pro-psychotic effects of synthetic cannabinoids: Interactions with central dopamine, serotonin, and glutamate systems. Drug Metab. Rev. 2018, 50, 65–73. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L.T. Emerging role of the cannabinoid receptor CB 2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, 1–31. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Zoratti, C.; Kipmen-Korgun, D.; Osibow, K.; Malli, R.; Graier, W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003, 140, 1351–1362. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharmacol. 2015, 10, 193–203. [Google Scholar] [CrossRef]
- Hermanson, D.J.; Gamble-George, J.C.; Marnett, L.J.; Patel, S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol. Sci. 2014, 35, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytocannabinoids biosynthesis in angiosperms, fungi, and liverworts and their versatile role. Plants 2021, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Wang, J.-X.; Wang, Q.; Li, H.-L.; Tao, M.; Luo, Q.; Liu, H. New chromane and chromene meroterpenoids from flowers of Rhododendron rubiginosum Franch. var. rubiginosum. Fitoterapia 2018, 127, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, L.; Rousseau, M.; Plauth, A.; Schroeder, F.C.; Sauer, S. Amorfrutins are natural PPARγ agonists with potent anti-inflammatory properties. J. Nat. Prod. 2015, 78, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2017, 123, 13–17. [Google Scholar] [CrossRef]
- Toyota, M.; Kinugawa, T.; Asakawa, Y. Bibenzyl Cannabinoid and Bisbibenzyl Derivative From the. Phytochemistry 1994, 37, 859–862. [Google Scholar] [CrossRef]
- Quaghebeur, K.; Coosemans, J.; Toppet, S.; Compernolle, F. Cannabiorci- and 8-chlorocannabiorcichromenic acid as fungal antagonists from Cylindrocarpon olidum. Phytochemistry 1994, 37, 159–161. [Google Scholar] [CrossRef]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 1–18. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Galletta, M.; Reekie, T.A.; Nagalingam, G.; Bottomley, A.L.; Harry, E.J.; Kassiou, M.; Triccas, J.A. Rapid antibacterial activity of cannabichromenic acid against methicillin-resistant staphylococcus aureus. Antibiotics 2020, 9, 523. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; Macnair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus mutans. Front. Microbiol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of β-caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, β-caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Yang, J.Y.; Lee, M.H.; Kim, H.W.; Kwon, H.J.; Park, M.; Kim, S.K.; Park, S.Y.; Kim, S.H.; Kim, J.B. Inhibitory effects of β-caryophyllene on helicobacter pylori infection in vitro and in vivo. Int. J. Mol. Sci. 2020, 21, 1008. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Park, M.H.; Kim, C.J.; Lee, J.Y.; Keum, C.Y.; Kim, I.S.; Yun, C.H.; Kim, S.K.; Kim, W.H.; Lee, Y.C. Effect of β-caryophyllene from cloves extract on helicobacter pylori eradication in mouse model. Nutrients 2020, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.A.; Souza, M.C.d.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [Green Version]
- Mitscher, L.A.; Park, Y.H.; Alshamma, A.; Hudson, P.B.; Haas, T. Amorfrutin A and B, bibenzyl antimicrobial agents from Amorpha fruticosa. Phytochemistry 1981, 20, 781–785. [Google Scholar] [CrossRef]
- Hakeem Said, I.; Rezk, A.; Hussain, I.; Grimbs, A.; Shrestha, A.; Schepker, H.; Brix, K.; Ullrich, M.S.; Kuhnert, N. Metabolome Comparison of Bioactive and Inactive Rhododendron Extracts and Identification of an Antibacterial Cannabinoid(s) from Rhododendron collettianum. Phytochem. Anal. 2017, 28, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Xu, C.; Chang, T.; Du, Y.; Yu, C.; Tan, X.; Li, X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019, 70, 103202. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and δ9-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, C.; Neira Agonh, D.; Lehmann, C. Antibacterial Effects of Phytocannabinoids. Life 2022, 12, 1394. https://doi.org/10.3390/life12091394
Scott C, Neira Agonh D, Lehmann C. Antibacterial Effects of Phytocannabinoids. Life. 2022; 12(9):1394. https://doi.org/10.3390/life12091394
Chicago/Turabian StyleScott, Cassidy, Daniel Neira Agonh, and Christian Lehmann. 2022. "Antibacterial Effects of Phytocannabinoids" Life 12, no. 9: 1394. https://doi.org/10.3390/life12091394
APA StyleScott, C., Neira Agonh, D., & Lehmann, C. (2022). Antibacterial Effects of Phytocannabinoids. Life, 12(9), 1394. https://doi.org/10.3390/life12091394








