Colchicine versus Physical Therapy in Knee Osteoarthritis
Abstract
:1. Introduction
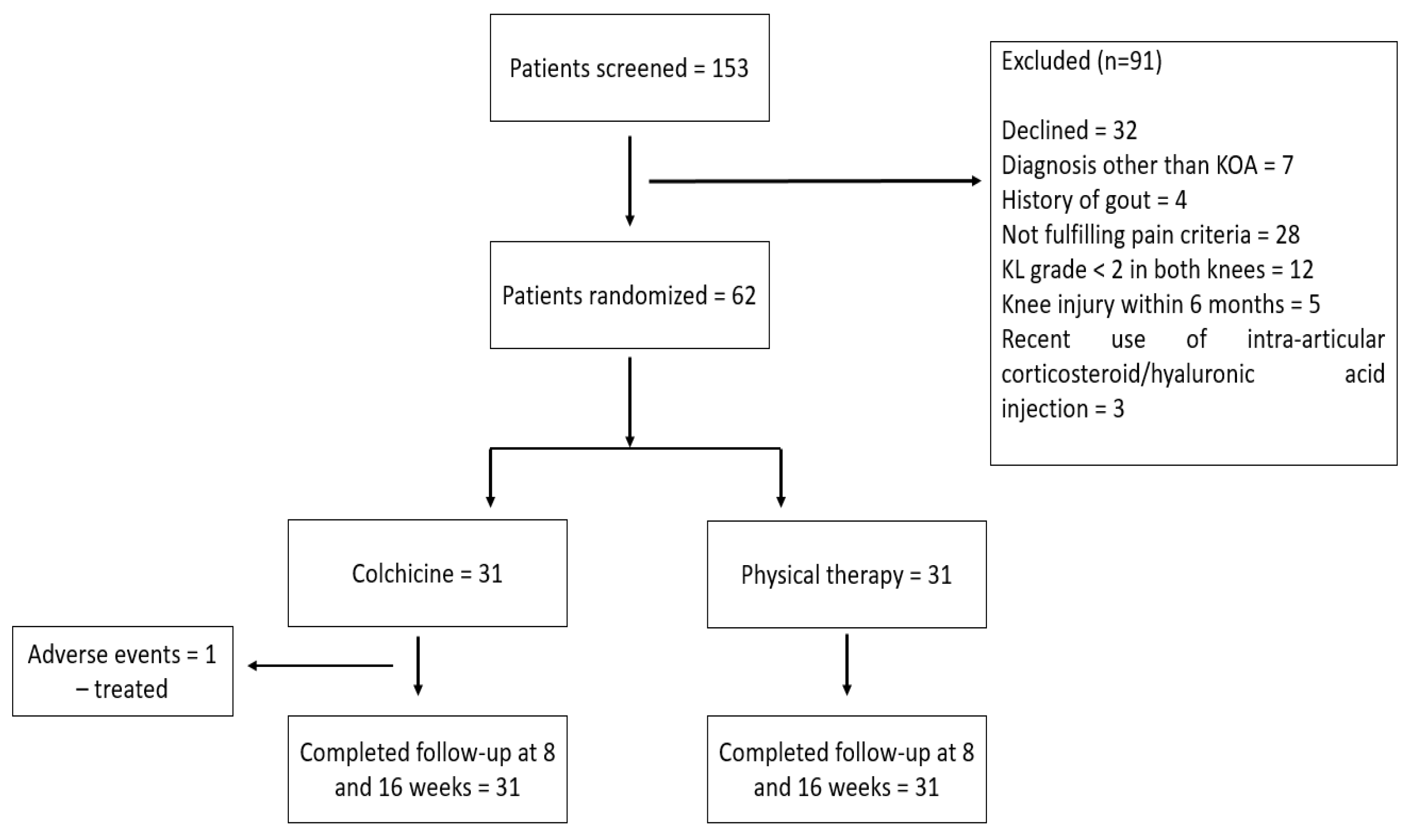
2. Materials and Methods
2.1. Patients
2.2. Demographic Characteristics and Assessment of Clinical, Laboratory and Imaging Data
2.3. Treatment and Outcome
2.4. Statistical Analysis
3. Results
3.1. Comparison between the Two Groups
3.2. Associations between Clinical and Paraclinical Data
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Taruc-Uy, R.L.; Lynch, S.A. Diagnosis and Treatment of Osteoarthritis. Prim. Care Clin. Off. Pract. 2013, 40, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Emmert, D.; Rasche, T.; Stieber, C.; Conrad, R.; Mücke, M. Knee Pain—Symptoms, Diagnosis and Therapy of Osteoarthritis. MMW Fortschr. Der Med. 2018, 160, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Cianni, L.; Bocchi, M.B.; Vitiello, R.; Greco, T.; de Marco, D.; Masci, G.; Maccauro, G.; Pitocco, D.; Perisano, C. Arthrodesis in the Charcot Foot: A Systematic Review. Orthop. Rev. 2020, 12, 64–68. [Google Scholar] [CrossRef]
- Lee, L.S.; Chan, P.K.; Fung, W.C.; Chan, V.W.K.; Yan, C.H.; Chiu, K.Y. Imaging of Knee Osteoarthritis: A Review of Current Evidence and Clinical Guidelines. Musculoskelet. Care 2021, 19, 363–374. [Google Scholar] [CrossRef]
- Möller, I.; Bong, D.; Naredo, E.; Filippucci, E.; Carrasco, I.; Moragues, C.; Iagnocco, A. Ultrasound in the Study and Monitoring of Osteoarthritis. Osteoarthr. Cartil. 2008, 16, S4–S7. [Google Scholar] [CrossRef]
- Oo, W.M.; Bo, M.T. Role of Ultrasonography in Knee Osteoarthritis. J. Clin. Rheumatol. 2016, 22, 324–329. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Engen, C.N.; Øiestad, B.E.; Engebretsen, L.; Risberg, M.A. Defining the Presence of Radiographic Knee Osteoarthritis: A Comparison between the Kellgren and Lawrence System and OARSI Atlas Criteria. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3532–3539. [Google Scholar] [CrossRef]
- Felson, D.T.; McAlindon, T.E.; Anderson, J.J.; Naimark, A.; Weissman, B.W.; Aliabadi, P.; Evans, S.; Levy, D.; Lavalley, M.P. Defining Radiographic Osteoarthritis for the Whole Knee. Osteoarthr. Cartil. 1997, 5, 241–250. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R.M.D. Knee Osteoarthritis: A Review of Management Options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J. Viscosupplementation for Osteoarthritis of the Knee. N. Engl. J. Med. 2015, 372, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Bejarano, T.; Novo, M. Current Interventions in the Management of Knee Osteoarthritis. J. Pharm. Bioallied Sci. 2013, 5, 30–38. [Google Scholar] [CrossRef]
- De l’Escalopier, N.; Anract, P.; Biau, D. Surgical Treatments for Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Haaland, B.; Huebner, J.L.; Wong, S.B.S.; Tjai, M.; Wang, C.; Chowbay, B.; Thumboo, J.; Chakraborty, B.; Tan, M.H.; et al. Colchicine Lack of Effectiveness in Symptom and Inflammation Modification in Knee Osteoarthritis (COLKOA): A Randomized Controlled Trial. Osteoarthr. Cartil. 2018, 26, 631–640. [Google Scholar] [CrossRef]
- Ma, C.A.; Leung, Y.Y. Exploring the Link between Uric Acid and Osteoarthritis. Front. Med. 2017, 4, 1. [Google Scholar] [CrossRef]
- El Said, T.O.; Elkosaier, M.A.; Elboghdady, I.A.; Elarman, M.M. Effect of Colchicine Treatment on Primary Knee Osteoarthritic Patients. Int. J. Clin. Rheumatol. 2018, 13, 370. [Google Scholar] [CrossRef]
- Salehi-Abari, I. 2016 ACR Revised Criteria for Early Diagnosis of Knee Osteoarthritis. Autoimmune Dis. Ther. Approaches Open Access 2016, 3, 1. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of Knee Function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63, S208–S228. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An Updated Algorithm Recommendation for the Management of Knee Osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Colchicine for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Available online: https://myorthoevidence.com/Blog/Show/138 (accessed on 9 July 2022).
- Das, S.K.; Ramakrishnan, S.; Mishra, K.; Srivastava, R.; Agarwal, G.G.; Singh, R.; Sircar, A.R. A Randomized Controlled Trial to Evaluate the Slow-Acting Symptom-Modifying Effects of Colchicine in Osteoarthritis of the Knee: A Preliminary Report. Arthritis Care Res. 2002, 47, 280–284. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, K.; Ramakrishnan, S.; Srivastava, R.; Agarwal, G.G.; Singh, R.; Sircar, A.R. A Randomized Controlled Trial to Evaluate the Slow-Acting Symptom Modifying Effects of a Regimen Containing Colchicine in a Subset of Patients with Osteoarthritis of the Knee. Osteoarthr. Cartil. 2002, 10, 247–252. [Google Scholar] [CrossRef]
- Deyle, G.D.; Allen, C.S.; Allison, S.C.; Gill, N.W.; Hando, B.R.; Petersen, E.J.; Dusenberry, D.I.; Rhon, D.I. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N. Engl. J. Med. 2020, 382, 1420–1429. [Google Scholar] [CrossRef]
- Wang, C.; Schmid, C.H.; Iversen, M.D.; Harvey, W.F.; Fielding, R.A.; Driban, J.B.; Price, L.L.; Wong, J.B.; Reid, K.F.; Rones, R.; et al. Comparative Effectiveness of Tai Chi Versus Physical Therapy for Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2016, 165, 77–86. [Google Scholar] [CrossRef]
- Samuels, J.; Abramson, S.B. Hyaluronan Drugs versus Physical Therapy in Knee Osteoarthritis: Commentary. Nat. Clin. Pract. Rheumatol. 2006, 2, 528–529. [Google Scholar] [CrossRef]
- Tuna, S.; Çelik, B.; Balcl, N. The Effect of Physical Therapy and Exercise on Pain and Functional Capacity According to the Radiological Grade of Knee Osteoarthritis. J. Back Musculoskelet. Rehabil. 2022, 35, 341–346. [Google Scholar] [CrossRef]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of Obesity Is Directly Associated with the Clinical and Functional Consequences of Knee Osteoarthritis. Sci. Rep. 2020, 10, 3601–3607. [Google Scholar] [CrossRef] [Green Version]
- Teichtahl, A.J.; Wang, Y.; Wluka, A.E.; Cicuttini, F.M. Obesity and Knee Osteoarthritis: New Insights Provided by Body Composition Studies. Obesity 2008, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Jadelis, K.; Miller, M.E.; Ettinger, W.H.; Messier, S.P. Strength, Balance, and the Modifying Effects of Obesity and Knee Pain: Results from the Observational Arthritis Study in Seniors (OASIS). J. Am. Geriatr. Soc. 2001, 49, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Nicklas, B.J.; Davis, C.; Loeser, R.F.; Lenchik, L.; Messier, S.P. Intensive Weight Loss Program Improves Physical Function in Older Obese Adults with Knee Osteoarthritis. Obesity 2006, 14, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Wei, J.; Zeng, C.; Yang, T.; Li, H.; Wang, Y.L.; Long, H.Z.; Wu, Z.Y.; Qian, Y.X.; Li, K.H.; et al. Association between Metabolic Syndrome and Knee Osteoarthritis: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2017, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Zhang, Y.; Hannan, M.T.; Naimark, A.; Weissman, B.; Aliabadi, P.; Levy, D. Risk Factors for Incident Radiographic Knee Osteoarthritis in the Elderly. Arthritis Rheum. 1997, 40, 728–733. [Google Scholar] [CrossRef]
- Wang, H.; Ma, B. Healthcare and Scientific Treatment of Knee Osteoarthritis. J. Healthc. Eng. 2022, 2022, 5919686. [Google Scholar] [CrossRef]
- Thorlund, J.B.; Simic, M.; Pihl, K.; Berthelsen, D.B.; Day, R.; Koes, B.; Juhl, C.B. Similar Effects of Exercise Therapy, Nonsteroidal Anti-Inflammatory Drugs, and Opioids for Knee Osteoarthritis Pain: A Systematic Review with Network Meta-Analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 207–216. [Google Scholar] [CrossRef]

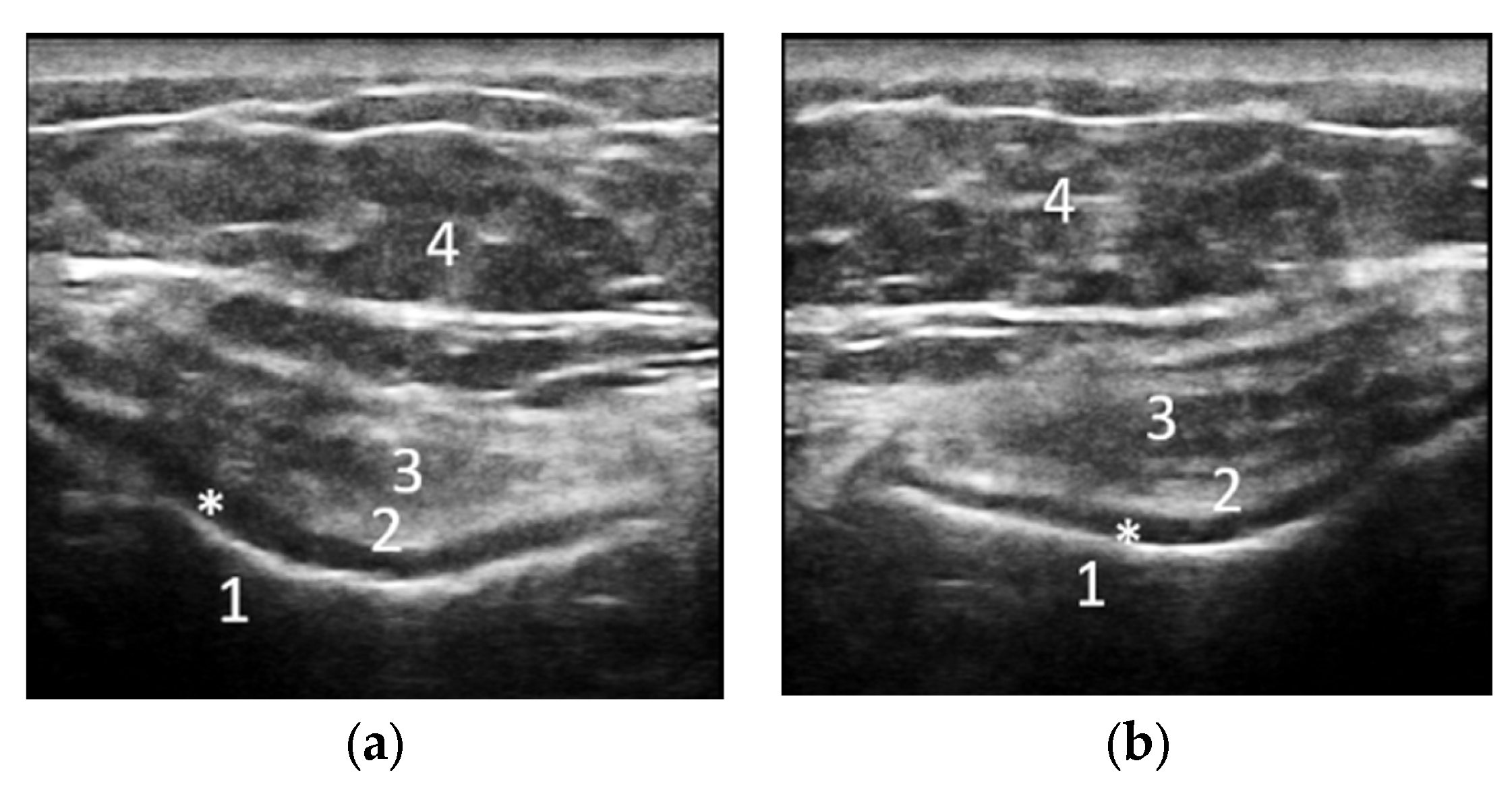
|
Demographic, Clinical and
Laboratory Features |
Patients n = 62 |
Group I (Colchicine) n = 31 |
Group II (Physical Therapy) n = 31 |
|---|---|---|---|
| Sex (patients, %) | 56 females (90.32%); 6 males (9.68%) | 28 females (90.32%); 3 males (9.68%) | 28 females (90.32%); 3 males (9.68%) |
| Age (years) (mean, SD) | 65.85 (7.42) | 65.25 (7.33) | 66.45 (7.46) |
| Disease duration (months) (mean, SD) | 29.83 (14.25) | 28.36 (13.98) | 27.68 (14.20) |
| Pain at mobilization (patients, %) | |||
| Right | 55 (88.70%) | 27 (87.09%) | 28 (90.32%) |
| Left | 61 (98.38%) | 30 (96.77%) | 31 (100%) |
| Crackles (patients, %) | |||
| Right | 56 (90.32%) | 28 (90.32%) | 28 (90.32%) |
| Left | 56 (90.32%) | 28 (90.32%) | 28 (90.32%) |
| Patellar tap test (patients, %) | |||
| Right | 9 (14.51%) | 4 (12.90%) | 5 (16.12%) |
| Left | 13 (20.96%) | 8 (25.80%) | 5 (16.12%) |
| VAS (mm) (mean, SD) | |||
| Right | 61.19 (20.22) | 63.54 (18.05) | 60.32 (22.06) |
| Left | 66.45 (11.37) | 64.19 (12.89) | 68.70 (9.06) |
| WOMAC total | 48.19 (10.06) | 48.29 (9.11) | 48.09 (10.98) |
| ESR (mm/h) (mean, SD) | 29.56 (22.79) | 30.51 (22.37) | 28.61 (23.16) |
| CRP (mg/l) (mean, SD) | 22.60 (57.54) | 23.02 (53.54) | 22.17 (61.28) |
| Cholesterol (mean, SD) | 216.11 (41.63) | 208.87 (44.14) | 223.35 (37.59) |
| Triglycerides (mean, SD) | 116.67 (50.36) | 111.96 (49.17) | 121.38 (51.10) |
| MSUS Findings | Patients | Group I (Colchicine) | Group II (Physical Therapy) | |||
|---|---|---|---|---|---|---|
| Right n (%) n = 62 | Left n (%) n = 62 | Right n (%) n = 31 | Left n (%) n = 31 | Right n (%) n = 31 | Left n (%) n = 31 | |
| Inflammatory | ||||||
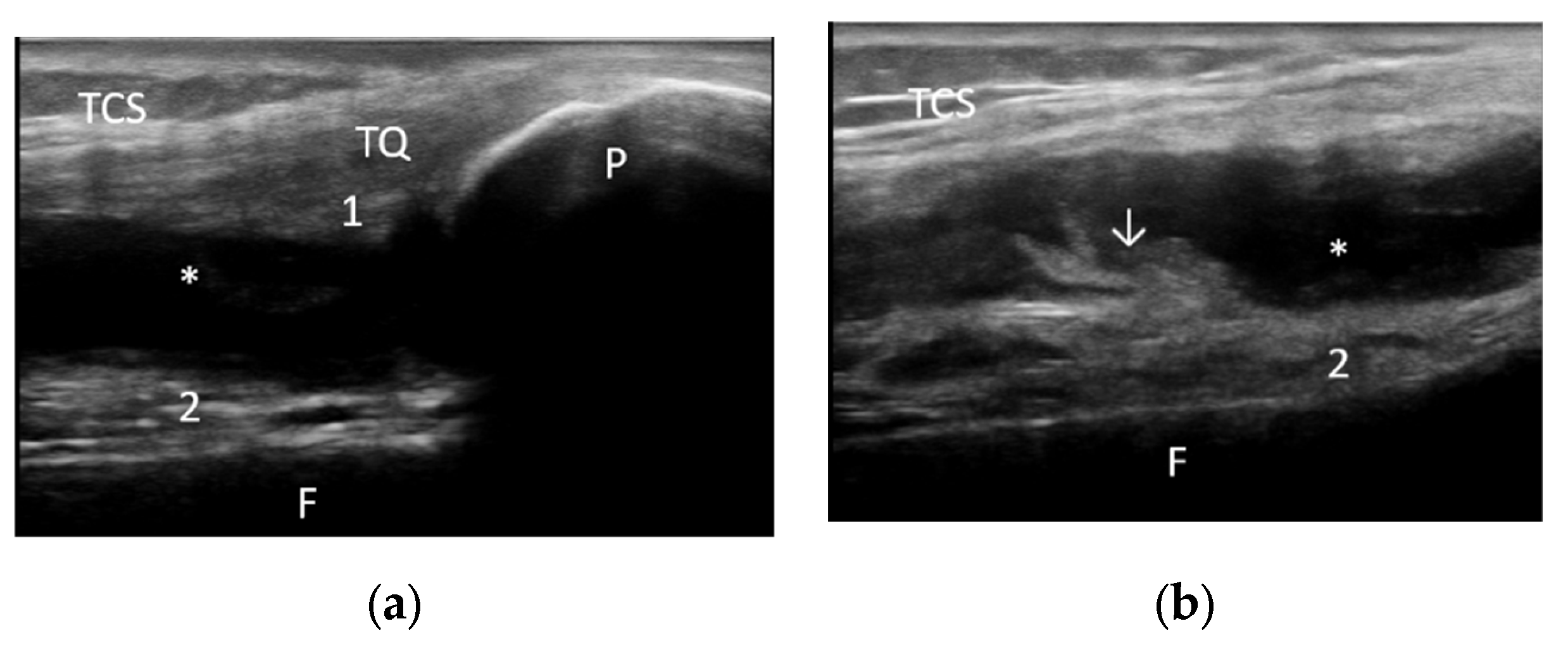
| Joint effusion | 33 (53.22%) | 36 (58.06%) | 16 (51.61%) | 15 (48.38%) | 17 (54.83%) | 21 (67.74%) |
| Synovial proliferation | 21 (33.87%) | 13 (20.96%) | 9 (29.03%) | 8 (25.80%) | 12 (38.70%) | 5 (16.12%) |
| Baker’s cyst | 8 (12.90%) | 9 (14.51%) | 5 (16.12%) | 3 (9.67%) | 3 (9.67%) | 6 (19.35%) |
| Structural | ||||||
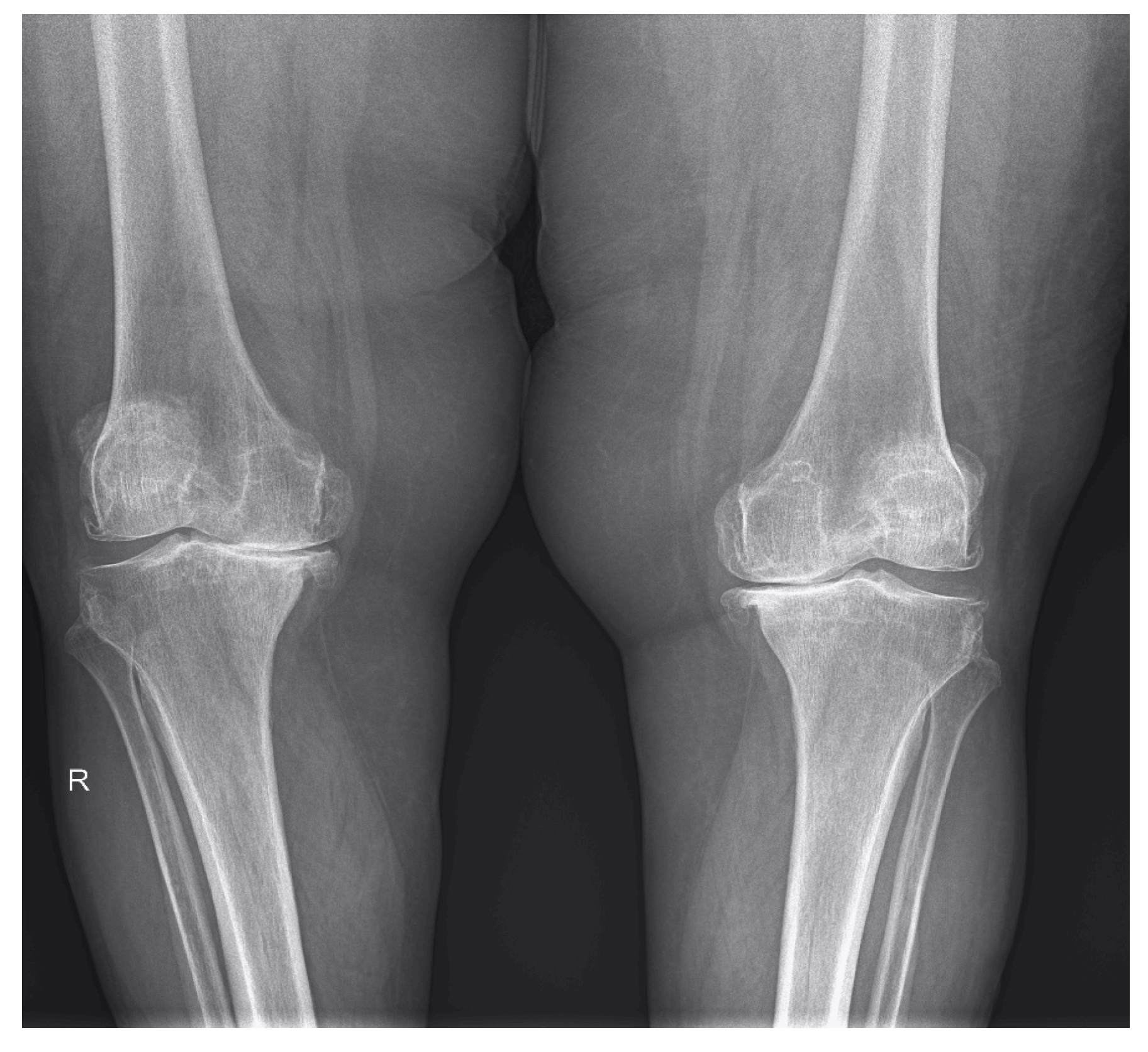
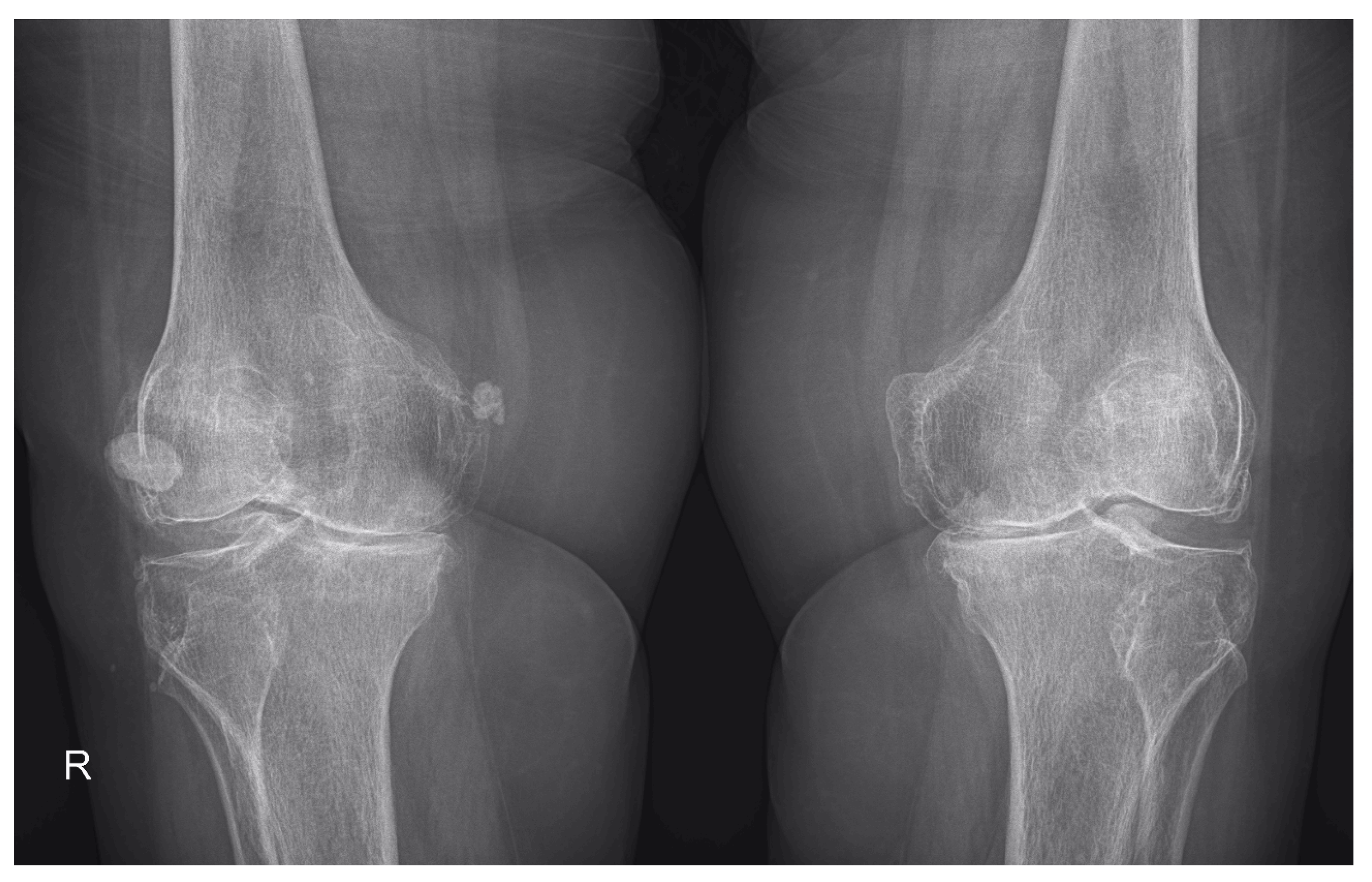
| Osteophytes | 52 (83.87%) | 57 (91.93%) | 22 (70.96%) | 30 (96.77%) | 30 (96.77%) | 27 (87.09%) |
| Cartilage | 59 (95.16%) | 62 (100%) | 29 (93.54%) | 31 (100%) | 30 (96.77%) | 31 (100%) |
| Medial meniscus | 37 (59.67%) | 38 (61.62%) | 18 (58.06%) | 20 (64.51%) | 19 (61.29%) | 18 (58.06%) |
| Lateral meniscus | 15 (24.19%) | 15 (24.19%) | 7 (22.58%) | 8 (25.80%) | 8 (25.80%) | 7 (22.58%) |
| Baseline | Week 8 | Week 16 | ||||
|---|---|---|---|---|---|---|
| Group I | Group II | Group I | Group II | Group I | Group II | |
| VAS left | 64.19 (12.89) | 68.70 (9.06) | 60.32 (10.92) p = 0.214 | 58.06 (7.79) p < 0.001 | 59.03 (11.73) p = 0.110 | 47.74 (8.31) p < 0.001 |
| VAS right | 63.54 (18.05) | 60.32 (22.06) | 58.70 (15.39) p = 0.268 | 52.58 (17.59) p = 0.138 | 55.16 (14.33) p = 0.051 | 45.16 (15.63) p = 0.003 |
| WOMAC | 48.29 (9.11) | 48.09 (10.93) | 47.48 (8.88) p = 0.729 | 45 (10.72) p = 0.272 | 46.90 (8.90) p = 0.533 | 42.22 (10.37) p = 0.037 |
| MSUS Findings | Group I Baseline | Group II Baseline | Group I 16 Weeks | Group II 16 Weeks | ||||
|---|---|---|---|---|---|---|---|---|
| Right n (%) n = 31 | Left n (%) n = 31 | Right n (%) n = 31 | Right n (%) n = 31 | Right n (%) n = 31 | Left n (%) n = 31 | Right n (%) n = 31 | Left n (%) n = 31 | |
| Inflammatory | ||||||||
| Joint effusion | 16 (51.61%) | 15 (48.38%) | 17 (54.83%) | 21 (67.74%) | 10 (32.25%) | 11 (35.48%) | 9 (29.03%) | 11 (35.48%) |
| Synovial proliferation | 9 (29.03%) | 8 (25.80%) | 12 (38.70%) | 15 (16.12%) | 4 (12.90%) | 3 (9.67%) | 6 (19.35%) | 3 (9.67%) |
| Baker’s cyst | 5 (16.12%) | 3 (9.67%) | 3 (9.67%) | 6 (19.35%) | 2 (6.67%) | 2 (6.67%) | 1 (3.22%) | 3 (9.67%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioroianu, G.O.; Florescu, A.; Mușetescu, A.E.; Sas, T.N.; Rogoveanu, O.C. Colchicine versus Physical Therapy in Knee Osteoarthritis. Life 2022, 12, 1297. https://doi.org/10.3390/life12091297
Cioroianu GO, Florescu A, Mușetescu AE, Sas TN, Rogoveanu OC. Colchicine versus Physical Therapy in Knee Osteoarthritis. Life. 2022; 12(9):1297. https://doi.org/10.3390/life12091297
Chicago/Turabian StyleCioroianu, George Ovidiu, Alesandra Florescu, Anca Emanuela Mușetescu, Teodor Nicușor Sas, and Otilia Constantina Rogoveanu. 2022. "Colchicine versus Physical Therapy in Knee Osteoarthritis" Life 12, no. 9: 1297. https://doi.org/10.3390/life12091297
APA StyleCioroianu, G. O., Florescu, A., Mușetescu, A. E., Sas, T. N., & Rogoveanu, O. C. (2022). Colchicine versus Physical Therapy in Knee Osteoarthritis. Life, 12(9), 1297. https://doi.org/10.3390/life12091297





