Folic Acid Reinforces Maize Tolerance to Sodic-Alkaline Stress through Modulation of Growth, Biochemical and Molecular Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions and Treatments
2.2. Foliar Applications of Folic Acid and the Experimental Layout
2.3. Growth Parameters and Life Pigments
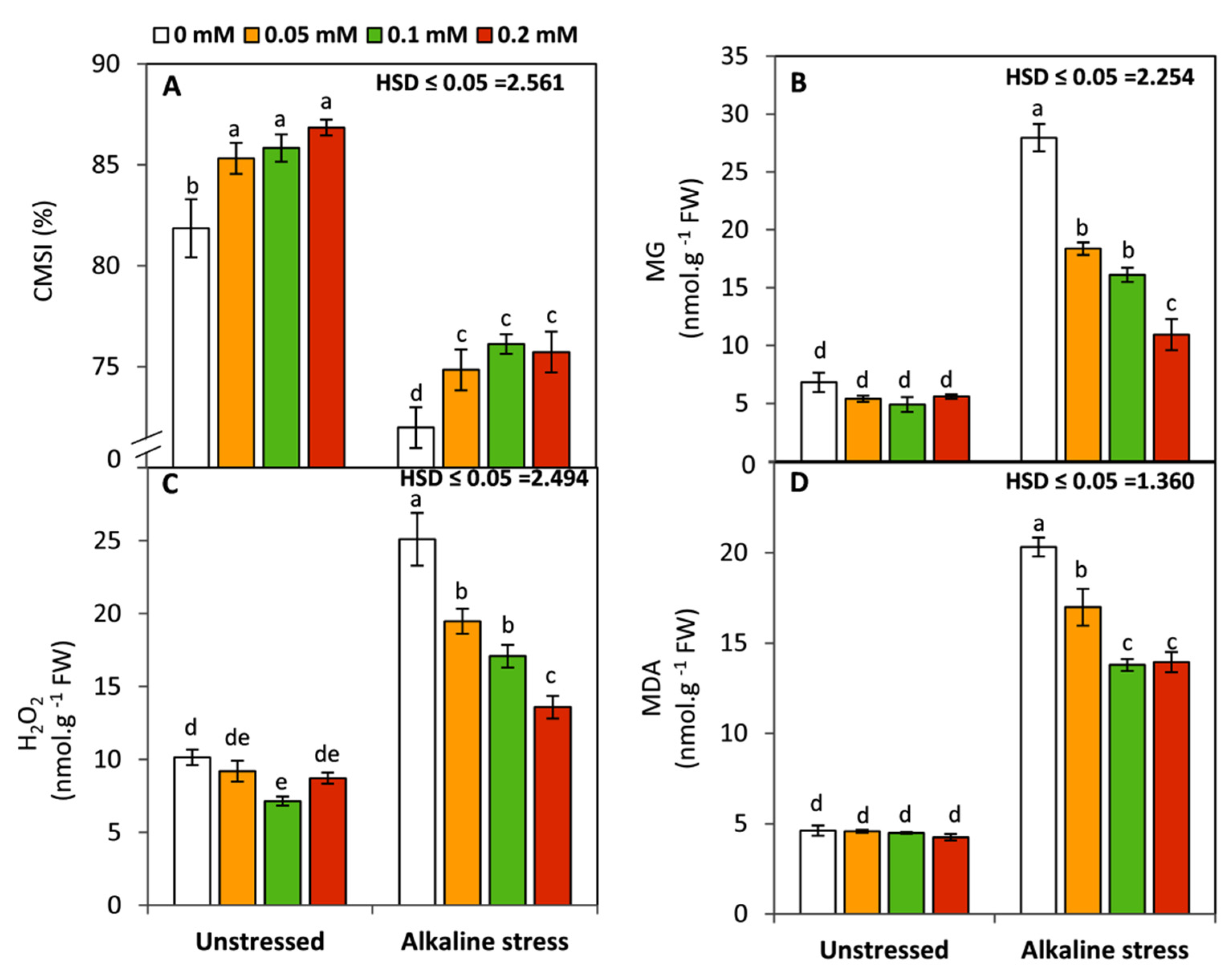
2.4. Cell Membrane Integrity and Oxidative Damage
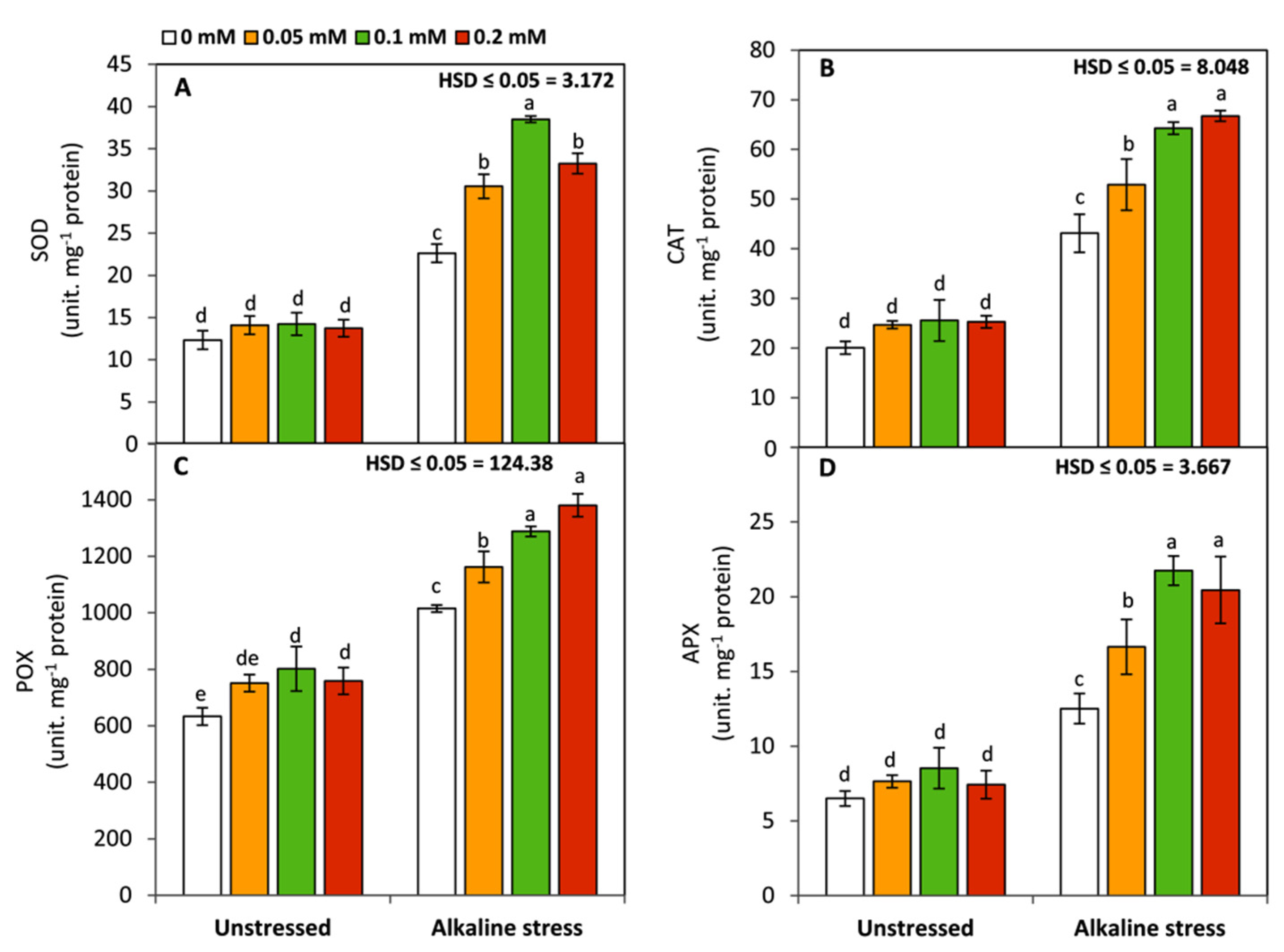
2.5. Determination of Total Soluble Protein and Activates of Antioxidant Enzymes
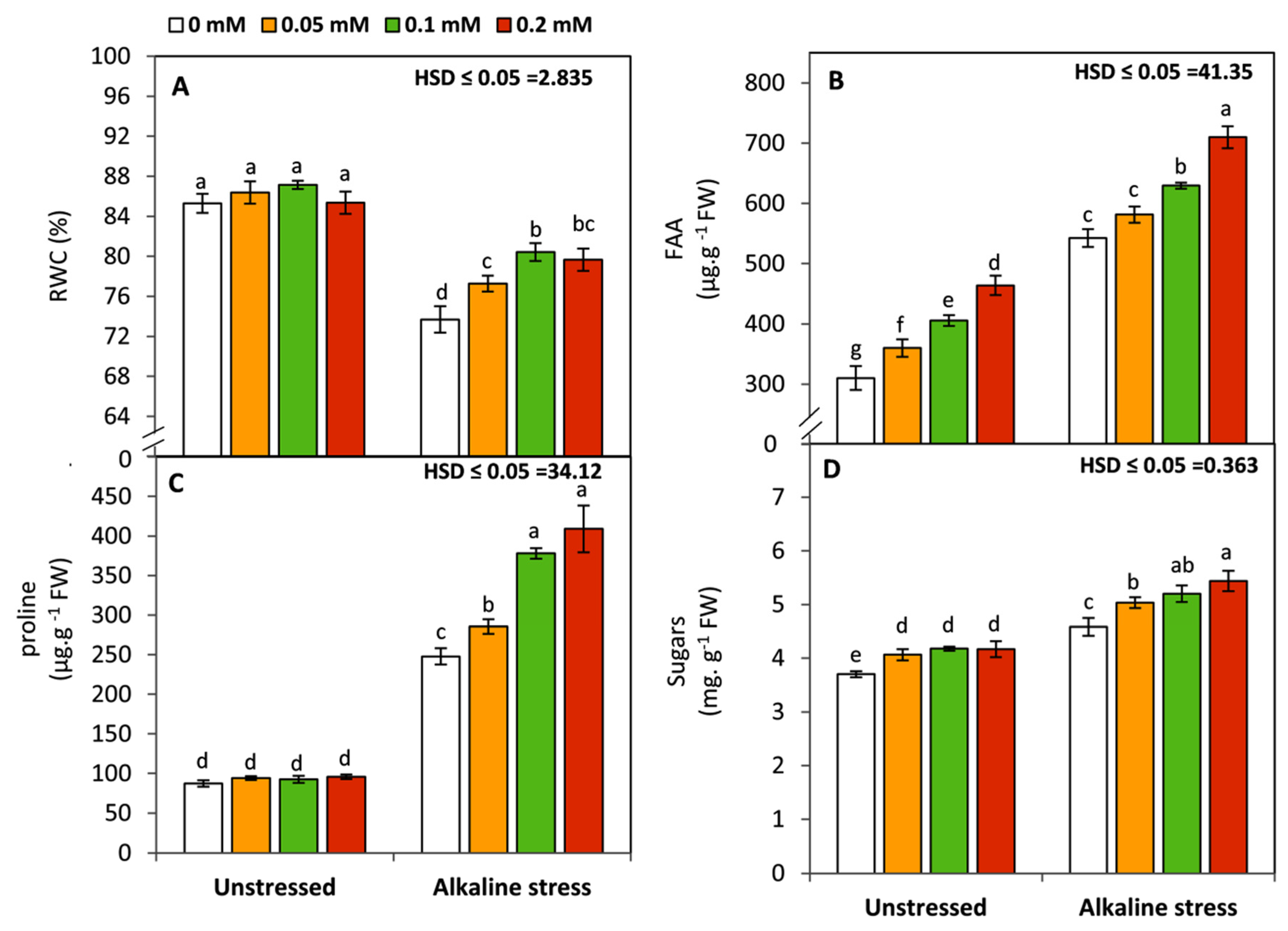
2.6. Determination of Leaf Relative Water Content and Osmotic Compounds
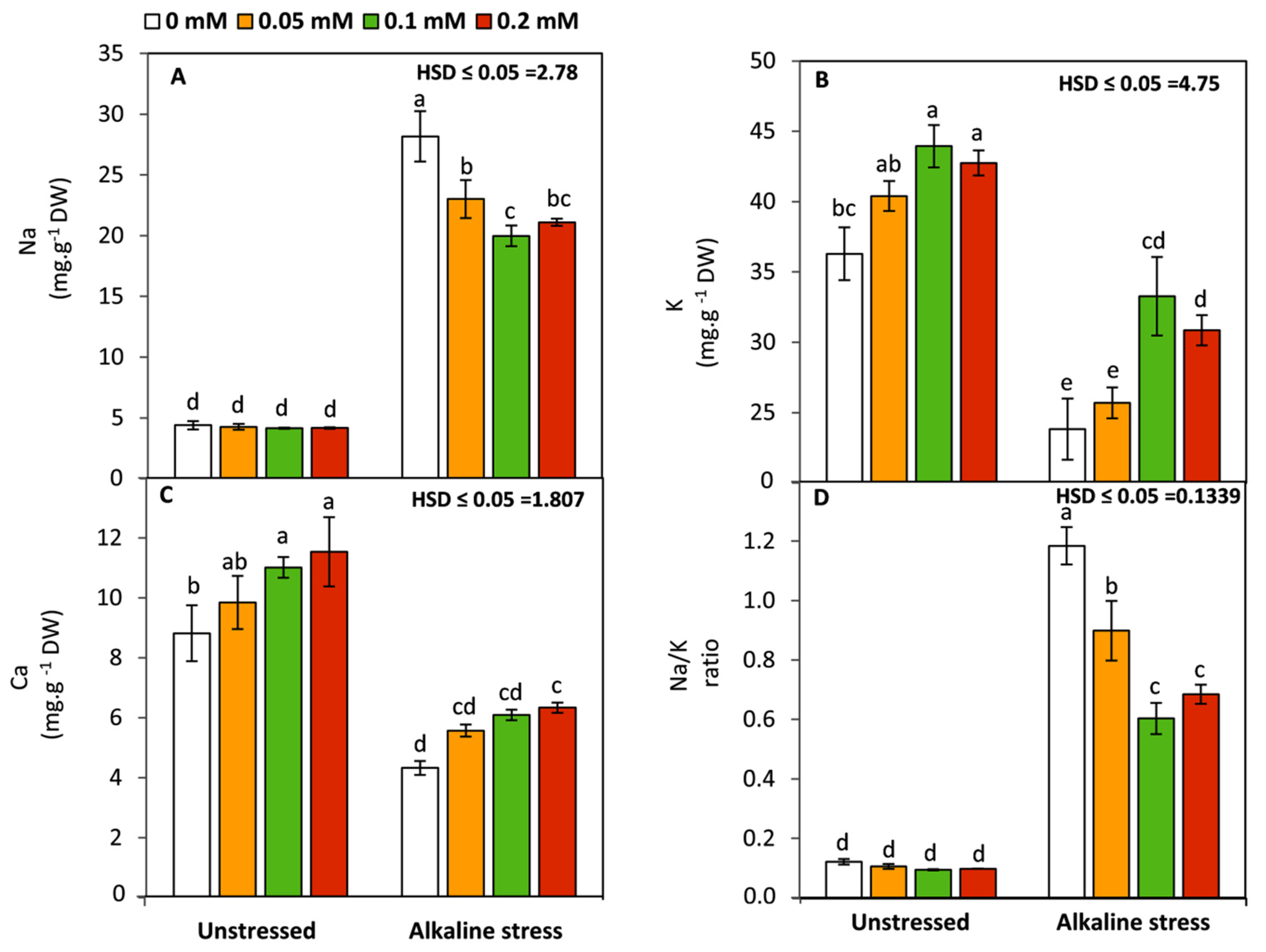
2.7. Determination of Na, K, and Ca
2.8. Gene Expression
2.9. Statistical Analysis
3. Results
3.1. Effect of Folic Acid on Plant Growth and Photosynthetic Pigments
3.2. Effect of Folic Acid on Membrane Stability Index and Oxidative-Damage Markers
3.3. Effect of Folic Acid on the Activities of Antioxidant Enzymes
3.4. Effect of Folic Acid on Leaf Relative Water Content and Osmolytes
3.5. Effect of Folic Acid on Na, K, and Ca Concentration
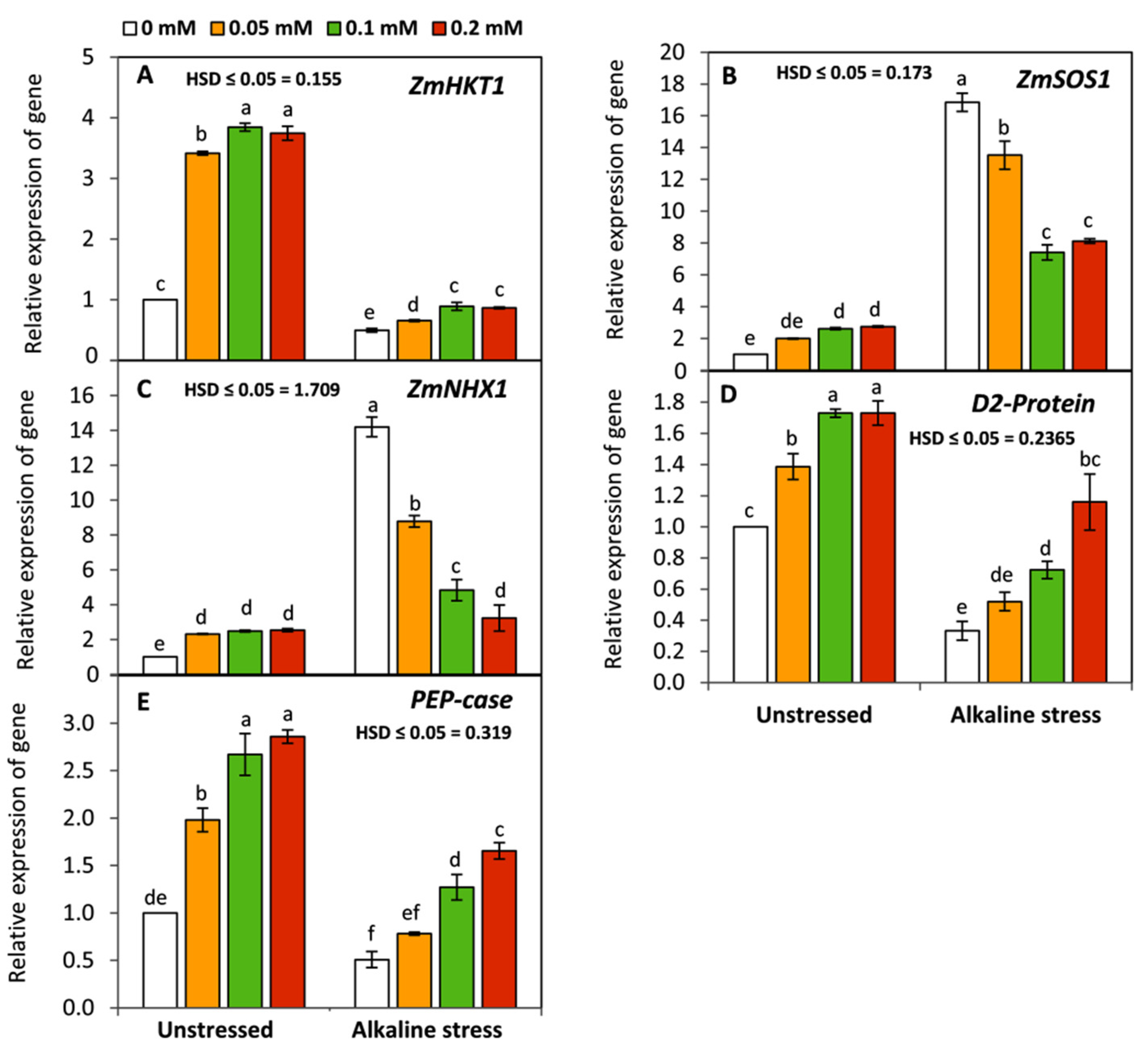
3.6. Effect of Folic Acid on the Expression of Photosynthesis and Salt-Stress-Responsive Genes
4. Discussion
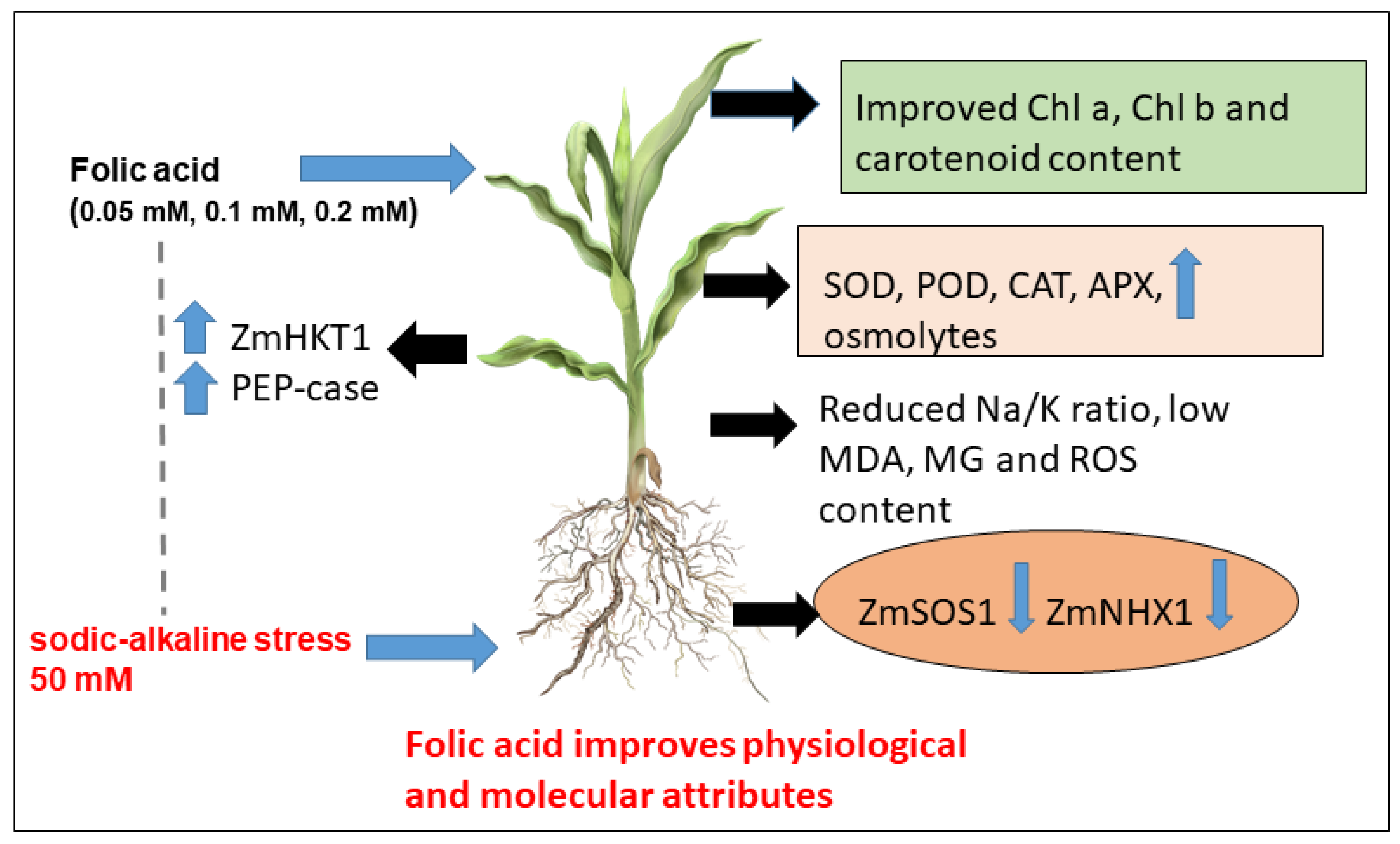
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci 2019, 1, 1–3. [Google Scholar]
- Gong, B.; Wen, D.; Bloszies, S.; Li, X.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stresses on respiratory metabolism, antioxidant system, nutritional status, and organic acid metabolism in tomato roots. Acta Physiol. Plant. 2014, 36, 2167–2181. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Yilmaz, O.; Uzİlday, B.; Uzİlday, r.Ö.; TÜrkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar]
- Joseph, B.; Jini, D. Salinity induced programmed cell death in plants: Challenges and opportunities for salt-tolerant plants. J. Plant Sci. 2010, 5, 376–390. [Google Scholar] [CrossRef][Green Version]
- Paz, R.C.; Rocco, R.A.; Reinoso, H.; Menéndez, A.B.; Pieckenstain, F.L.; Ruiz, O.A. Comparative study of alkaline, saline, and mixed saline–alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of Lotus tenuis. J. Plant Growth Regul. 2012, 31, 448–459. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, A.T.; El-Gabry, Y.A.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef]
- Alsamadany, H.; Mansour, H.; Elkelish, A.; Ibrahim, M.F. Folic Acid Confers Tolerance against Salt Stress-Induced Oxidative Damages in Snap Beans through Regulation Growth, Metabolites, Antioxidant Machinery and Gene Expression. Plants 2022, 11, 1459. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.D.; Abdelaal, K.A.; Al Husnain, L.; AlGwaiz, H.I.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef]
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gong, B.; Jin, Z.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J. Plant Physiol. 2015, 186, 68–77. [Google Scholar] [CrossRef]
- Baranova, E.; Gulevich, A. Structural organization of nuclei and nucleoli of wheat shoot and root meristem during germination under alkaline pH conditions. Russ. Agric. Sci. 2009, 35, 11–14. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Stakhova, L.; Stakhov, L.; Ladygin, V. Effects of exogenous folic acid on the yield and amino acid content of the seed of Pisum sativum L. and Hordeum vulgare L. Appl. Biochem. Microbiol. 2000, 36, 85–89. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Ramos-Parra, P.A.; Bedair, M.; Robledo-Hernández, A.L.; Tang, Y.; Sumner, L.W.; Díaz de la Garza, R.I.; Blancaflor, E.B. The folylpolyglutamate synthetase plastidial isoform is required for postembryonic root development in Arabidopsis. Plant Physiol. 2011, 155, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Neilson, K.A.; Mariani, M.; Haynes, P.A. Quantitative proteomic analysis of cold-responsive proteins in rice. Proteomics 2011, 11, 1696–1706. [Google Scholar] [CrossRef]
- Powell, J.J.; Fitzgerald, T.L.; Stiller, J.; Berkman, P.J.; Gardiner, D.M.; Manners, J.M.; Henry, R.J.; Kazan, K. The defence-associated transcriptome of hexaploid wheat displays homoeolog expression and induction bias. Plant Biotechnol. J. 2017, 15, 533–543. [Google Scholar] [CrossRef]
- Storozhenko, S.; Navarrete, O.; Ravanel, S.; De Brouwer, V.; Chaerle, P.; Zhang, G.-F.; Bastien, O.; Lambert, W.; Rébeillé, F.; Van Der Straeten, D. Cytosolic Hydroxymethyldihydropterin Pyrophosphokinase/Dihydropteroate Synthase from Arabidopsis thaliana a specific role in early development and stress response. J. Biol. Chem. 2007, 282, 10749–10761. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.A.; Abd El-Gawad, H. Folic acid as a protective agent in snap bean plants under water deficit conditions. J. Hortic. Sci. Biotechnol. 2021, 96, 94–109. [Google Scholar] [CrossRef]
- Khan, M.T.; Ahmed, S.; Shah, A.A. Regulatory role of folic acid in biomass production and physiological activities of Coriandrum sativum L. under irrigation regimes. Int. J. Phytoremediation 2021, 24, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Javadi, A.; Esfandiari, E.; Pourmohammad, A. Evaluation the effects of ascorbate and folic acid on improving some germination indicators of wheat (Triticum aestivume L.) under salt and drought stress. Appl. Biol. 2018, 30, 46–57. [Google Scholar]
- Kilic, S.; Aca, H.T. Role of exogenous folic acid in alleviation of morphological and anatomical inhibition on salinity-induced stress in barley. Ital. J. Agron. 2016, 11, 246–251. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, S.; Wang, Y.; Zhong, Q.; Feng, C. Effects of folic acid and methionine on chelating agents in the removal of heavy metals. J. Agro-Environ. Sci. 2018, 37, 1667–1675. [Google Scholar]
- El-Yazied, A.A.; Ibrahim, M.F.; Ibrahim, M.A.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53. [Google Scholar]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.; Sopory, S. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Abd El-Samad, G.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of agronomic traits, nutrient uptake, osmolytes and antioxidants of maize as influenced by exogenous potassium silicate under deficit irrigation and semiarid conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Dhugga, K.S. Maize biomass yield and composition for biofuels. Crop Sci. 2007, 47, 2211–2227. [Google Scholar] [CrossRef]
- Kleinmans, J.; Densley, R.; Hurley, T.; Williams, I. BRIEF COMMUNICATION: Feed value of maize silage in New Zealand-a review. N. Z. Soc. Anim. Prod. 2016, 76, 100–102. [Google Scholar]
- Ostrander, B.M. Maize Starch for Industrial Applications. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; pp. 171–189. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective Effect of γ-Aminobutyric Acid Against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 1917. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D.; Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide: Release, 6.03 ed.; SAS Inst. Inc.: Cary, NC, USA, 1988. [Google Scholar]
- Alnusairi, G.S.; Mazrou, Y.S.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.; ElNahhas, N. Exogenous nitric oxide reinforces photosynthetic efficiency, osmolyte, mineral uptake, antioxidant, expression of stress-responsive genes and ameliorates the effects of salinity stress in wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Ferjani, A.; Mustardy, L.; Sulpice, R.; Marin, K.; Suzuki, I.; Hagemann, M.; Murata, N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003, 131, 1628–1637. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Gambonnet, B.; Jabrin, S.; Ravanel, S.; Karan, M.; Douce, R.; Rébeillé, F. Folate distribution during higher plant development. J. Sci. Food Agric. 2001, 81, 835–841. [Google Scholar] [CrossRef]
- Jabrin, S.; Ravanel, S.; Gambonnet, B.; Douce, R.; Rébeillé, F. One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant Physiol. 2003, 131, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- ÖZMEN, S.; Tabur, S. Functions of Folic Acid (Vitamin B9) Against Cytotoxic Effects of Salt Stress in Hordeum Vulgare L. Pak. J. Bot. 2020, 52, 17–22. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen peroxide supplementation in irrigation water alleviates drought stress and boosts growth and productivity of potato plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Elkelish, A.; Ibrahim, M.F.; Ashour, H.; Bondok, A.; Mukherjee, S.; Aftab, T.; Hikal, M.; El-Yazied, A.A.; Azab, E.; Gobouri, A.A. Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation. Agronomy 2021, 11, 87. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Methylglyoxal: An emerging signaling molecule in plant abiotic stress responses and tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Li, W.; Li, Z.; Liu, H.; Gao, Y.; Huang, G. Folic acid modulates VPO1 DNA methylation levels and alleviates oxidative stress-induced apoptosis in vivo and in vitro. Redox Biol. 2018, 19, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A. Folates as antioxidants. Food Chem. 2007, 101, 1480–1483. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.; Hamada, M.M.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.; Bendary, E.S.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A. Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Burguieres, E.; McCue, P.; Kwon, Y.-I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Emam, M.; Helal, N. Vitamins minimize the salt-induced oxidative stress hazards. Aust. J. Basic Appl. Sci. 2008, 2, 110–1119. [Google Scholar]
- Vicuna, D. The Role of Peroxidases in the Development of Plants and Their Responses to Abiotic Stresses. Ph.D. Thesis, Technological University Dublin, Dublin, Ireland, 2005. [Google Scholar]
- Basset, G.J.; Quinlivan, E.P.; Gregory, J.F.; Hanson, A.D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop Sci. 2005, 45, 449–453. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; Abd-El-Gawad, H.G.; Bondok, A. Physiological impacts of potassium citrate and folic acid on growth, yield and some viral diseases of potato plants. Middle East J. Agric. Res. 2015, 4, 577–589. [Google Scholar]

| Primer Name | Sequence | Tm | |
|---|---|---|---|
| ZmHKT1 | F | 5′-TGCTAATGTTTATCGTGCTG-3′ | 56 °C |
| R | 5′-AGGCTGATCCTCTTCCTAAC-3′ | ||
| ZmSOS1 | F | 5′-ACTTGCAGGAGGAATACAAC-3′ | |
| R | 5′- CGAGAAGAGAAGACCACATC-3′ | ||
| ZmNHX | F | 5′-CGTGATGTCGCATTACACCT-3′ | |
| R | 5′-CTGGCAAACTCCCACTTCTC-3′ | ||
| D2 protein | F | 5′-GGAAGATCAATCGACCGAAA-3′ | |
| R | 5′-CCTTATGCACCCATTTCACA-3′ | ||
| PEP case | F | 5′-AGCCTTCAGAACCGATGAAATC-3′ | |
| R | 5′-CATCCCATAGCGCATTTCG-3′ | ||
| β-Actin | F | 5’-GTGCCCATTTACGAAGGATA-3′ | |
| R | 5′-GAAGACTCCATGCCGATCAT-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljuaid, B.S.; Mukherjee, S.; Sayed, A.N.; El-Gabry, Y.A.E.-G.; Omar, M.M.A.; Mahmoud, S.F.; Alsubeie, M.S.; Darwish, D.B.E.; Al-Qahtani, S.M.; Al-Harbi, N.A.; et al. Folic Acid Reinforces Maize Tolerance to Sodic-Alkaline Stress through Modulation of Growth, Biochemical and Molecular Mechanisms. Life 2022, 12, 1327. https://doi.org/10.3390/life12091327
Aljuaid BS, Mukherjee S, Sayed AN, El-Gabry YAE-G, Omar MMA, Mahmoud SF, Alsubeie MS, Darwish DBE, Al-Qahtani SM, Al-Harbi NA, et al. Folic Acid Reinforces Maize Tolerance to Sodic-Alkaline Stress through Modulation of Growth, Biochemical and Molecular Mechanisms. Life. 2022; 12(9):1327. https://doi.org/10.3390/life12091327
Chicago/Turabian StyleAljuaid, Bandar S., Soumya Mukherjee, Amany N. Sayed, Yasser Abd El-Gawad El-Gabry, Mohamed M. A. Omar, Samy F. Mahmoud, Moodi Saham Alsubeie, Doaa Bahaa Eldin Darwish, Salem Mesfir Al-Qahtani, Nadi Awad Al-Harbi, and et al. 2022. "Folic Acid Reinforces Maize Tolerance to Sodic-Alkaline Stress through Modulation of Growth, Biochemical and Molecular Mechanisms" Life 12, no. 9: 1327. https://doi.org/10.3390/life12091327
APA StyleAljuaid, B. S., Mukherjee, S., Sayed, A. N., El-Gabry, Y. A. E.-G., Omar, M. M. A., Mahmoud, S. F., Alsubeie, M. S., Darwish, D. B. E., Al-Qahtani, S. M., Al-Harbi, N. A., Alzuaibr, F. M., Basahi, M. A., & Hamada, M. M. A. (2022). Folic Acid Reinforces Maize Tolerance to Sodic-Alkaline Stress through Modulation of Growth, Biochemical and Molecular Mechanisms. Life, 12(9), 1327. https://doi.org/10.3390/life12091327









