Imaging of Cerebral Venous Thrombosis
Abstract
1. Introduction
2. Pathophysiology
3. Clinical Presentations
4. Cerebral Venous Anatomy
MR Anatomy of the Superior Sagittal, Straight, Lateral and Torcular Sinuses
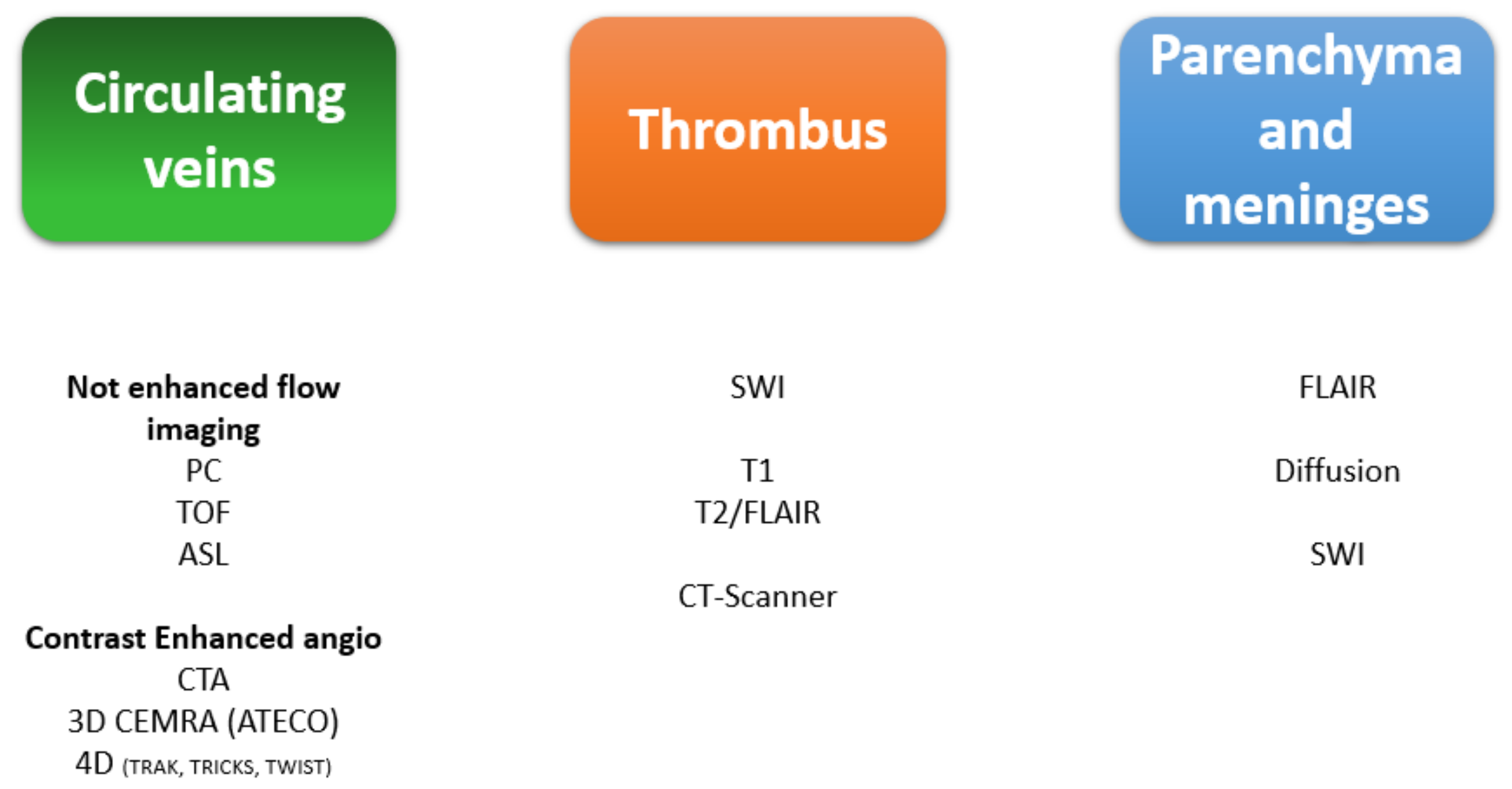
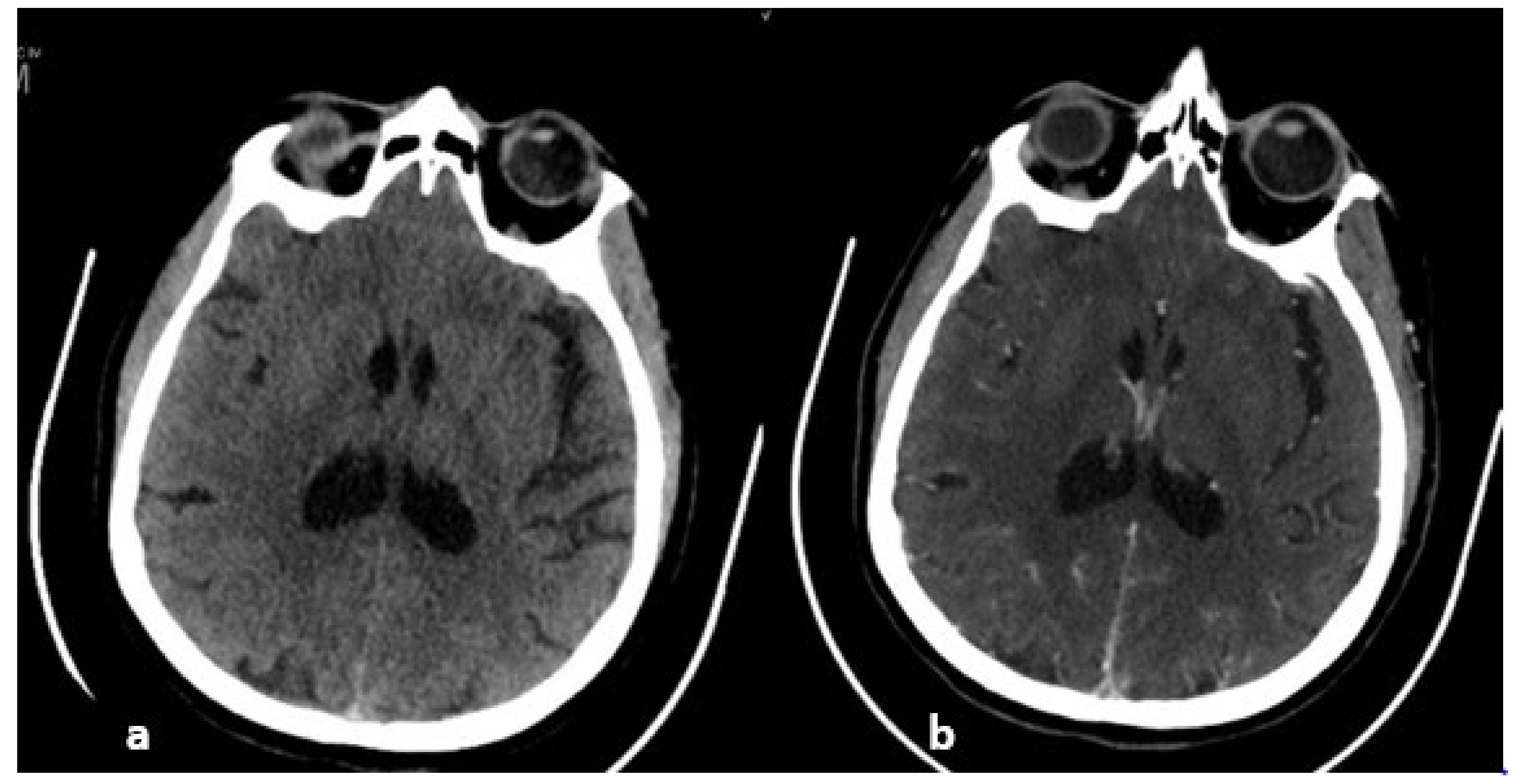
5. Brain Imaging
6. MRI
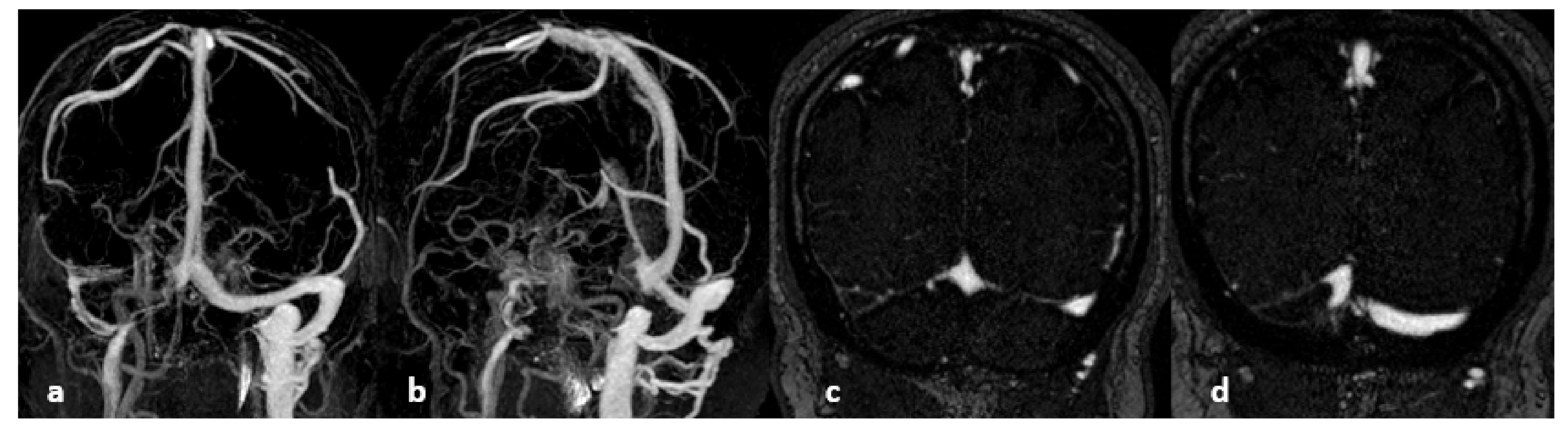
7. MRI Sequences and Semiology
What Is the Place of 3D Anatomical Sequences?
8. CT and Venous CT Scanning
Technique and Results
9. Sensitivity and specificity
Digital Subtraction Angiography (DSA)
10. Summary of Imaging Support
11. Special Cases
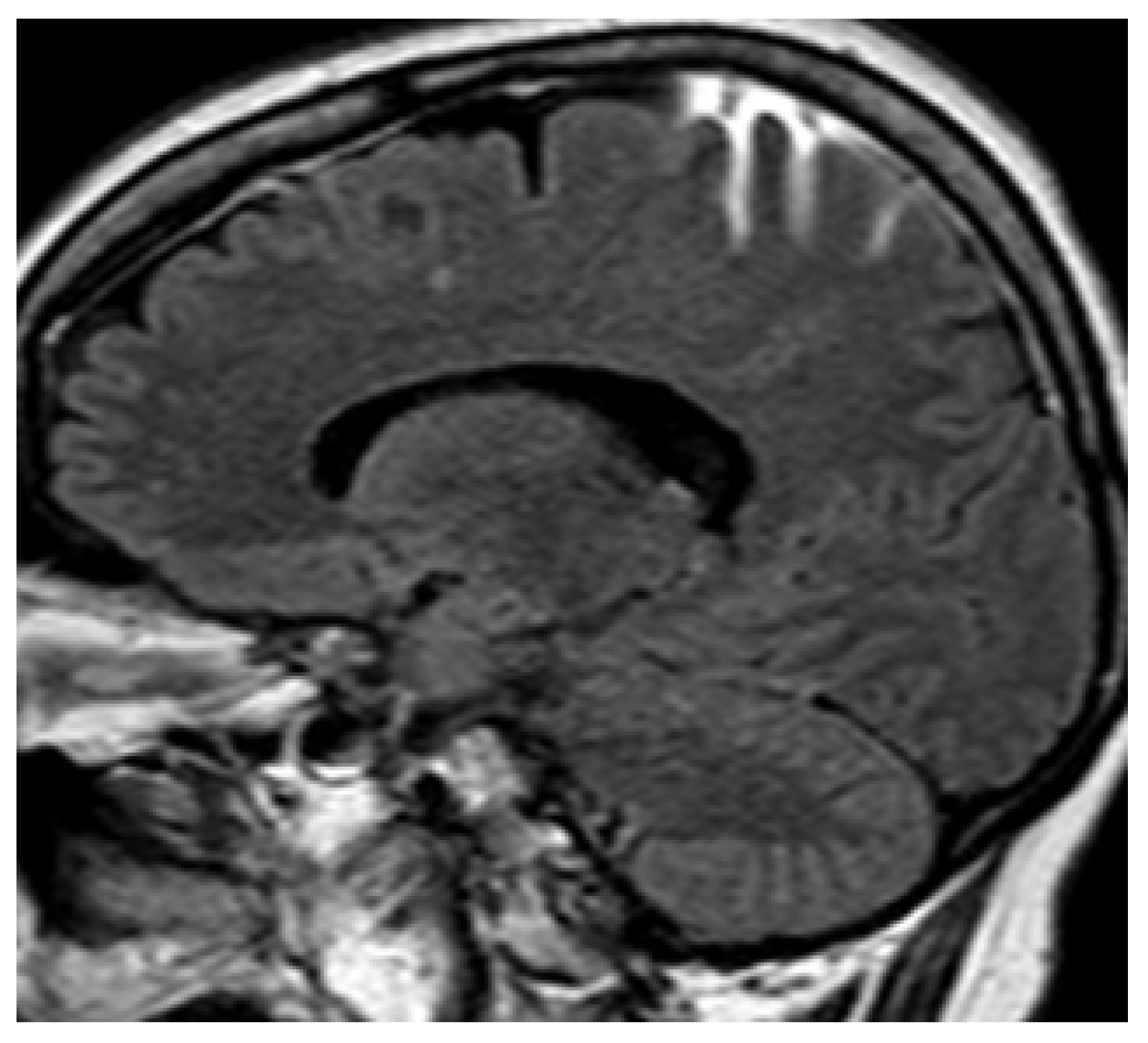
11.1. Thrombosis of the Straight Sinus
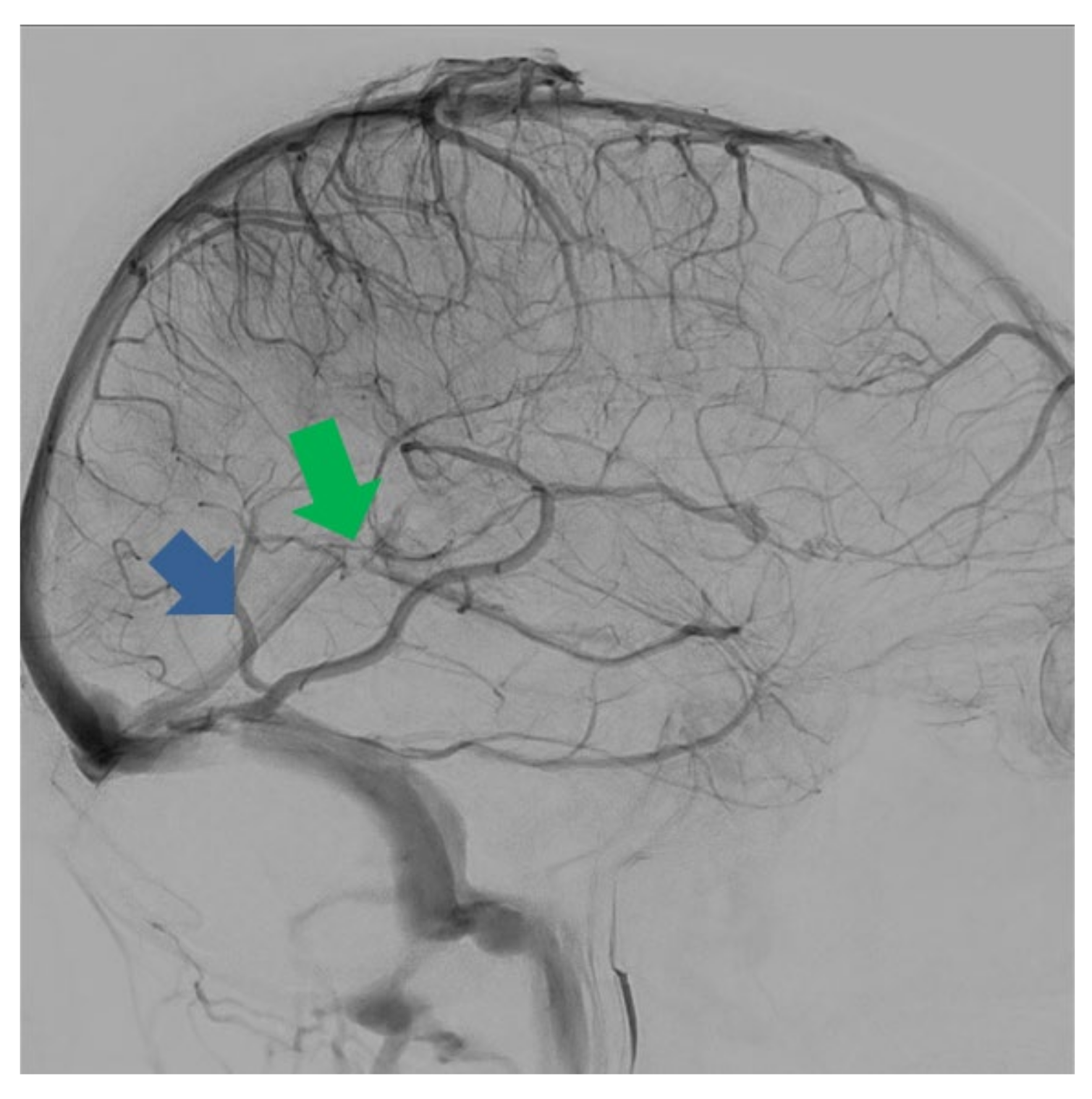
11.2. Thrombosis of Labbé’s Vein
11.3. Differential Diagnoses
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ulivi, L.; Squitieri, M.; Cohen, H.; Cowley, P.; Werring, D.J. Cerebral venous thrombosis: A practical guide. Pract. Neurol. 2020, 20, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Jianu, D.C.; Jianu, S.N.; Munteanu, G.; Flavius, T.D.; Brasan, C. Ischemic Stroke of Brain; IntechOpen: London, UK, 2018; Volume 1, pp. 45–76. ISBN 978-1-83881-709-1. [Google Scholar]
- Stam, J. Thrombosis of the cerebral veins and sinuses. N. Engl. J. Med. 2005, 352, 1791–1798. [Google Scholar] [CrossRef]
- Bousser, M.-G.; Ferro, J.M. Cerebral venous thrombosis: An update. Lancet Neurol. 2007, 6, 162–170. [Google Scholar] [CrossRef]
- Ferro, J.M.; Bousser, M.; Canhão, P.; Coutinho, J.M.; Crassard, I.; Dentali, F.; di Minno, M.; Maino, A.; Martinelli, I.; Masuhr, F.; et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—Endorsed by the European Academy of Neurology. Eur. J. Neurol. 2017, 24, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; Tong, E.; Yedavalli, V.S. A case-based review of cerebral venous infarcts with perfusion imaging and comparison to arterial ischemic stroke. Front. Radiol. 2021, 1, 687045. [Google Scholar] [CrossRef]
- Arquizan, C. Thrombophlébites cérébrales: Aspects cliniques, diagnostic et traitement. Réanimation 2001, 10, 383–391. [Google Scholar] [CrossRef]
- Canedo-Antelo, M.; Baleato-González, S.; Mosqueira, A.J.; Casas-Martínez, J.; Oleaga, L.; Vilanova, J.C.; Luna-Alcalá, A.; García-Figueiras, R. Radiologic clues to cerebral venous thrombosis. RadioGraphics 2019, 39, 1611–1628. [Google Scholar] [CrossRef]
- Qu, H.; Yang, M. Early imaging characteristics of 62 cases of cerebral venous sinus thrombosis. Exp. Ther. Med. 2013, 5, 233–236. [Google Scholar] [CrossRef][Green Version]
- Gaikwad, A.B.; Mudalgi, B.A.; Patankar, K.B.; Patil, J.K.; Ghongade, D.V. Diagnostic role of 64-slice multidetector row CT scan and CT venogram in cases of cerebral venous thrombosis. Emerg. Radiol. 2008, 15, 325–333. [Google Scholar] [CrossRef]
- Linn, J.; Ertl-Wagner, B.; Seelos, K.C.; Strupp, M.; Reiser, M.; Brückmann, H.; Brüning, R. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. Am. J. Neuroradiol. 2007, 28, 946–952. [Google Scholar]
- Dmytriw, A.A.; Song, J.S.A.; Yu, E.; Poon, C.S. Cerebral venous thrombosis: State of the art diagnosis and management. Neuroradiology 2018, 60, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Barinagarrementeria, F.; Brown, R.D.; Bushnell, C.D.; Cucchiara, B.; Cushman, M.; DeVeber, G.; Ferro, J.; Tsai, F.Y. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 1158–1192. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Haider, C.R.; Campeau, N.G.; Huston, J., III; Riederer, S.J. Intracranial contrast-enhanced magnetic resonance venography with 6.4-fold sensitivity encoding at 1.5 and 3.0 Tesla. J. Magn. Reson. Imaging 2008, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, F. Imaging of cerebral venous thrombosis. Diagn. Interv. Imaging 2014, 95, 1145–1150. [Google Scholar] [CrossRef]
- Lv, B.; Tian, C.-L.; Cao, X.-Y.; Liu, X.-F.; Wang, J.; Yu, S.-Y. Role of diffusion-weighted imaging in the diagnosis of cerebral venous thrombosis. J. Int. Med. Res. 2020, 48, 300060520933448. [Google Scholar] [CrossRef]
- Lv, B.; Jing, F.; Tian, C.-L.; Liu, J.-C.; Wang, J.; Cao, X.-Y.; Liu, X.-F.; Yu, S.-Y. Diffusion-weighted magnetic resonance imaging in the diagnosis of cerebral venous thrombosis: A meta-analysis. J. Korean Neurosurg. Soc. 2021, 64, 418–426. [Google Scholar] [CrossRef]
- Meckel, S.; Reisinger, C.; Bremerich, J.; Damm, D.; Wolbers, M.; Engelter, S.; Scheffler, K.; Wetzel, S. Cerebral venous thrombosis: Diagnostic accuracy of combined, dynamic and static, contrast-enhanced 4D MR venography. Am. J. Neuroradiol. 2010, 31, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.L.; Fortuna, R.B.; Jones, B.V.; Gaskill-Shipley, M.F. Imaging of cerebral venous thrombosis: Current techniques, spectrum of findings, and diagnostic pitfalls. RadioGraphics 2006, 26, S19–S41. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Duarte, J.; Dalaqua, M.; Jarry, V.M.; Pereira, F.V.; Reis, F. Cerebral venous thrombosis: Imaging patterns. Radiol. Bras. 2022, 55, 54–61. [Google Scholar] [CrossRef]
- Boukobza, M.; Crassard, I.; Bousser, M.; Chabriat, H. MR imaging features of isolated cortical vein thrombosis: Diagnosis and follow-up. Am. J. Neuroradiol. 2009, 30, 344–348. [Google Scholar] [CrossRef]
- Linn, J.; Pfefferkorn, T.; Ivanicova, K.; Müller-Schunk, S.; Hartz, S.; Wiesmann, M.; Dichgans, M.; Brückmann, H. Noncontrast CT in deep cerebral venous thrombosis and sinus thrombosis: Comparison of its diagnostic value for both entities. Am. J. Neuroradiol. 2009, 30, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, L.F.; van Walderveen, M.A.; Kroft, L.J.; Kruyt, N.D.; Wermer, M.J.; van Osch, M.J.; Huisman, M.V.; Klok, E. Current imaging modalities for diagnosing cerebral vein thrombosis—A critical review. Thromb. Res. 2020, 189, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.M. Cerebral venous thrombosis. J. Thromb. Haemost. 2015, 13, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Bousser, M.G.; Barnett, H.J.M. Chapter 12: Cerebral venous thrombosis. In Stroke: Pathophysiology, Diagnosis, and Management, 4th ed.; Mohr, J.P., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2004; pp. 301–325. ISBN 9780443066009. [Google Scholar]
- Lafitte, F.; Boukobza, M.; Guichard, J.; Hoeffel, C.; Reizine, D.; Ille, O.; Woimant, F.; Merland, J. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin. Radiol. 1997, 52, 672–679. [Google Scholar] [CrossRef]
- Liang, L.; Korogi, Y.; Sugahara, T.; Onomichi, M.; Shigematsu, Y.; Yang, D.; Kitajima, M.; Hiai, Y.; Takahashi, M. Evaluation of the intracranial dural sinuses with a 3D contrast-enhanced MP-RAGE sequence: Prospective comparison with 2D-TOF MR venography and digital subtraction angiography. Am. J. Neuroradiol. 2001, 22, 481–492. [Google Scholar]
- Hatami, H.; Danesh, N.; Shojaei, M.; Hamedani, A.R. Evaluation of diagnostic values in NCCT and MRI of the patients with cerebral venous or sinus thrombosis in Loghman Hakim Hospital in Tehran 2014–2018. Int. Clin. Neurosci. J. 2019, 6, 17–21. [Google Scholar] [CrossRef]
- Wong, G.K.C.; Siu, D.Y.W.; Abrigo, J.M.; Ahuja, A.T.; Poon, W.S. Computed tomographic angiography for patients with acute spontaneous intracerebral hemorrhage. J. Clin. Neurosci. 2012, 19, 498–500. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Li, T.; Ramdoyal, N.D.; Zhang, J.; Shao, A. The performance of CT versus MRI in the differential diagnosis of cerebral venous thrombosis. Thromb. Haemost. 2018, 118, 1067–1077. [Google Scholar] [CrossRef]
- Lazzaro, N.; Wright, B.; Castillo, M.; Fischbein, N.; Glastonbury, C.; Hildenbrand, P.; Wiggins, R.; Quigley, E.; Osborn, A. Artery of percheron infarction: Imaging patterns and clinical spectrum. Am. J. Neuroradiol. 2010, 31, 1283–1289. [Google Scholar] [CrossRef]
- Kheiralla, O.; Alghamdi, S.; Aljondi, R.; Tajaldeen, A.; Bakheet, A. Artery of percheron infarction: A characteristic pattern of ischemia and variable clinical presentation: A literature review. Curr. Med. Imaging 2021, 17, 669–674. [Google Scholar] [CrossRef]
- Shivaprasad, S.; Shroff, G.; Kumar, V. Vein of Labbe thrombosis by CT and MRI. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1168–1169. [Google Scholar] [CrossRef] [PubMed]



















| Thrombus-Veins | Parenchyma | Optic Nerves | Sella | |
|---|---|---|---|---|
| TI | Loss of flow hyposignal Thrombus in iso/hypersignal | Hypo/isosignaledema Hemorrhages-Petechiae | ||
| T2 | Thrombus in hypo/iso signal at the acute stage then hypersignal | High signal edema Sometimes: Small ventricles and ptosis of the cerebellar tonsils. Parenchymal hematoma | Intracranial hypertension: dilation of the optic nerve sheaths. Papilledema | intracranial hypertension: “Empty” sella turcica. |
| Axial T2* or SWI | Frank hyposignal. High sensitivity for cortical veins. | Hemorrhages-Petechiae. | ||
| Axial DWI. | Thrombus in hypersignal | Hypersignal B1000 and decreased ADC in ischemic lesions. | ||
| Enhanced 3DT1EG | Intraluminal defect | |||
| Non-enhanced Venous MRA | Specifies the location of the occlusion. | |||
| CE-MRA | Defect in the vein. Not very sensitive to slow flows. Good filling of the cortical veins. | |||
| 3D Flair | Thrombus in hypersignal. | Edema. Intra parenchymal hematoma. Subarachnoid hemorrhage. |
| Risk | Solution |
|---|---|
| Acquisition too early: part of the venous system is not opacified | Immediately launch a new acquisition |
| Using anatomical sequences: thrombus signal | |
| Complete with CT angiography | |
| Missing a partial thrombosis on the MIP | Watch native images |
| Missing an old thrombosis (enhancement of the thrombus) | Do not use long and/or delayed injected sequences |
| Confusing Pacchionian granulation with thrombosis | Semiological knowledge |
| Sequence | Gado | Duration | Advantages | Disadvantages |
|---|---|---|---|---|
| 2D TOF | No | 4 min | No contrast injection Arteries removed | Slow flow Cortical veins Spatial resolution |
| 3D PC | No | 6 min | No contrast injection Less sensitive to slow flows than 2DTOF | Slow flows Long Acquisition Spatial resolution |
| 2D PC | No | 2 min | No contrast injection Fast Acquisition No reconstructions | Slow flows Cortical vein Spatial resolution |
| Elliptic-centric 3D T1 | Yes | 1 min | Insensitive to slow flows (no false positives) Cortical veins | Not feasible if injection contraindicated Arteries not removed May contain fat interposition |
| Normal Sinus | Thrombus Less than 5 Days Old | Thrombus from D5 to D30 | Thrombus Older than 1 Month | |
|---|---|---|---|---|
| T1 | Hyposignal | Isosignal | Hypersignal | Iso/Hypersignal |
| T2 | Hyposignal | Hypo/Isosignal | Iso/Hypersignal | Iso/Hypersignal |
| Contrast-enhanced T1 | Homogeneous because the fresh thrombus can be enhanced | Empty Delta sign | Empty Delta sign | Empty Delta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadik, J.-C.; Jianu, D.C.; Sadik, R.; Purcell, Y.; Novaes, N.; Saragoussi, E.; Obadia, M.; Lecler, A.; Savatovsky, J. Imaging of Cerebral Venous Thrombosis. Life 2022, 12, 1215. https://doi.org/10.3390/life12081215
Sadik J-C, Jianu DC, Sadik R, Purcell Y, Novaes N, Saragoussi E, Obadia M, Lecler A, Savatovsky J. Imaging of Cerebral Venous Thrombosis. Life. 2022; 12(8):1215. https://doi.org/10.3390/life12081215
Chicago/Turabian StyleSadik, Jean-Claude, Dragos Catalin Jianu, Raphaël Sadik, Yvonne Purcell, Natalia Novaes, Edouard Saragoussi, Michaël Obadia, Augustin Lecler, and Julien Savatovsky. 2022. "Imaging of Cerebral Venous Thrombosis" Life 12, no. 8: 1215. https://doi.org/10.3390/life12081215
APA StyleSadik, J.-C., Jianu, D. C., Sadik, R., Purcell, Y., Novaes, N., Saragoussi, E., Obadia, M., Lecler, A., & Savatovsky, J. (2022). Imaging of Cerebral Venous Thrombosis. Life, 12(8), 1215. https://doi.org/10.3390/life12081215








