Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
2.3. Recombinant Peptide Production
2.4. Extraction of Biological Samples from Catheters
2.5. Antimicrobial Activity Assays
2.6. Analysis of Antibiofilm Activity by Confocal Laser Scanning Microscopy (CLSM)
2.7. Statistical Analyses
3. Results
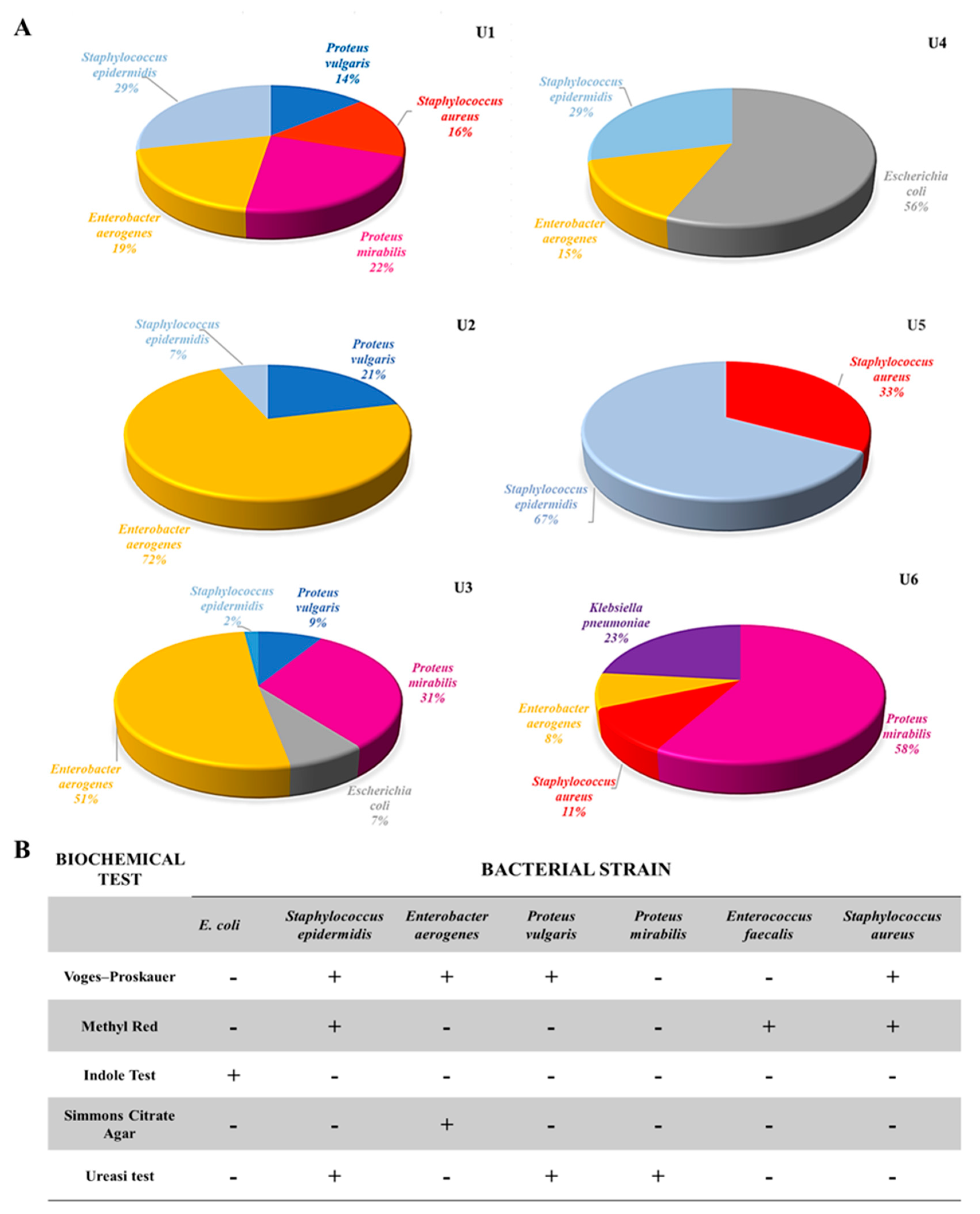
3.1. Catheter Collection, Microbial Strain Extraction, and Assessment
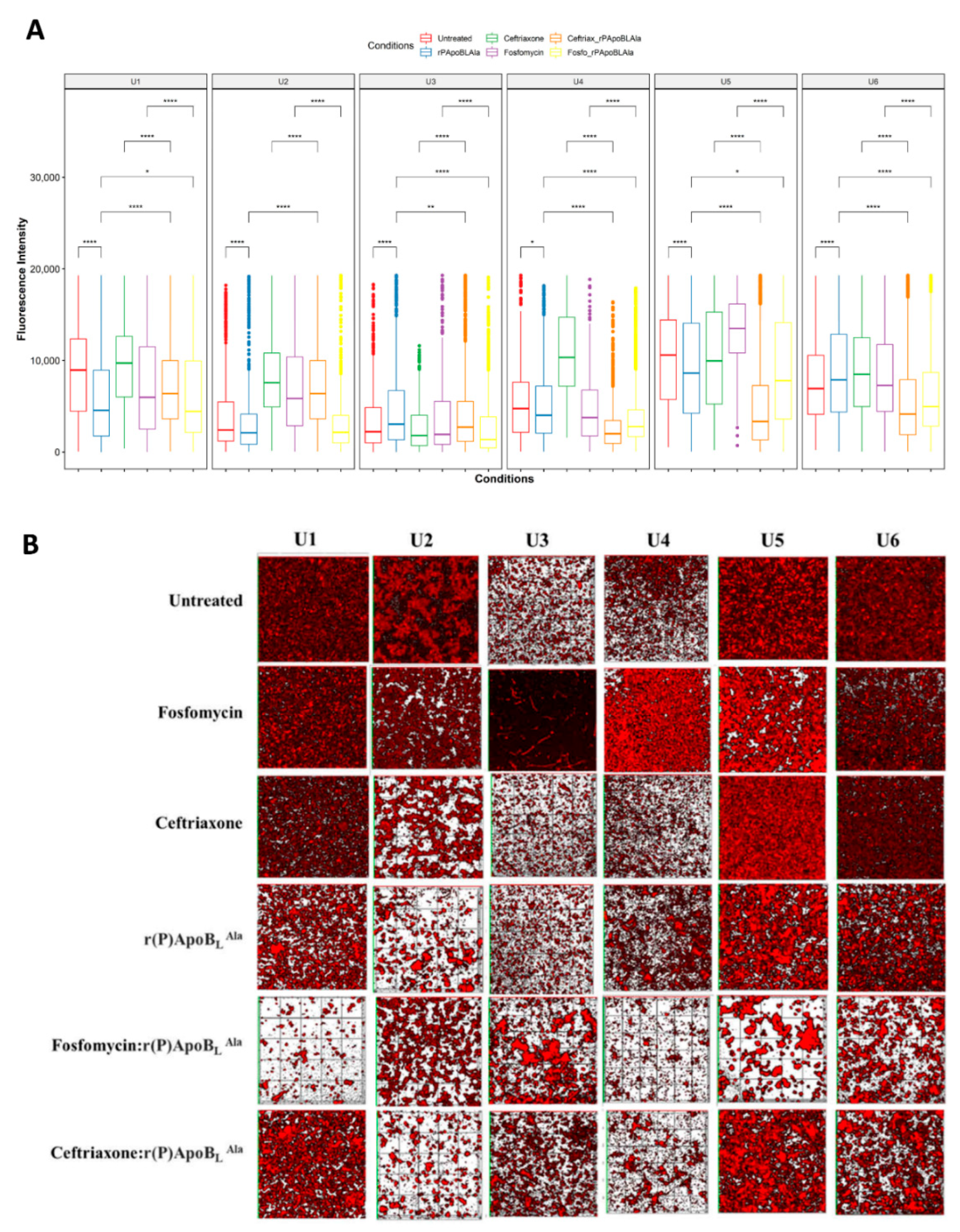
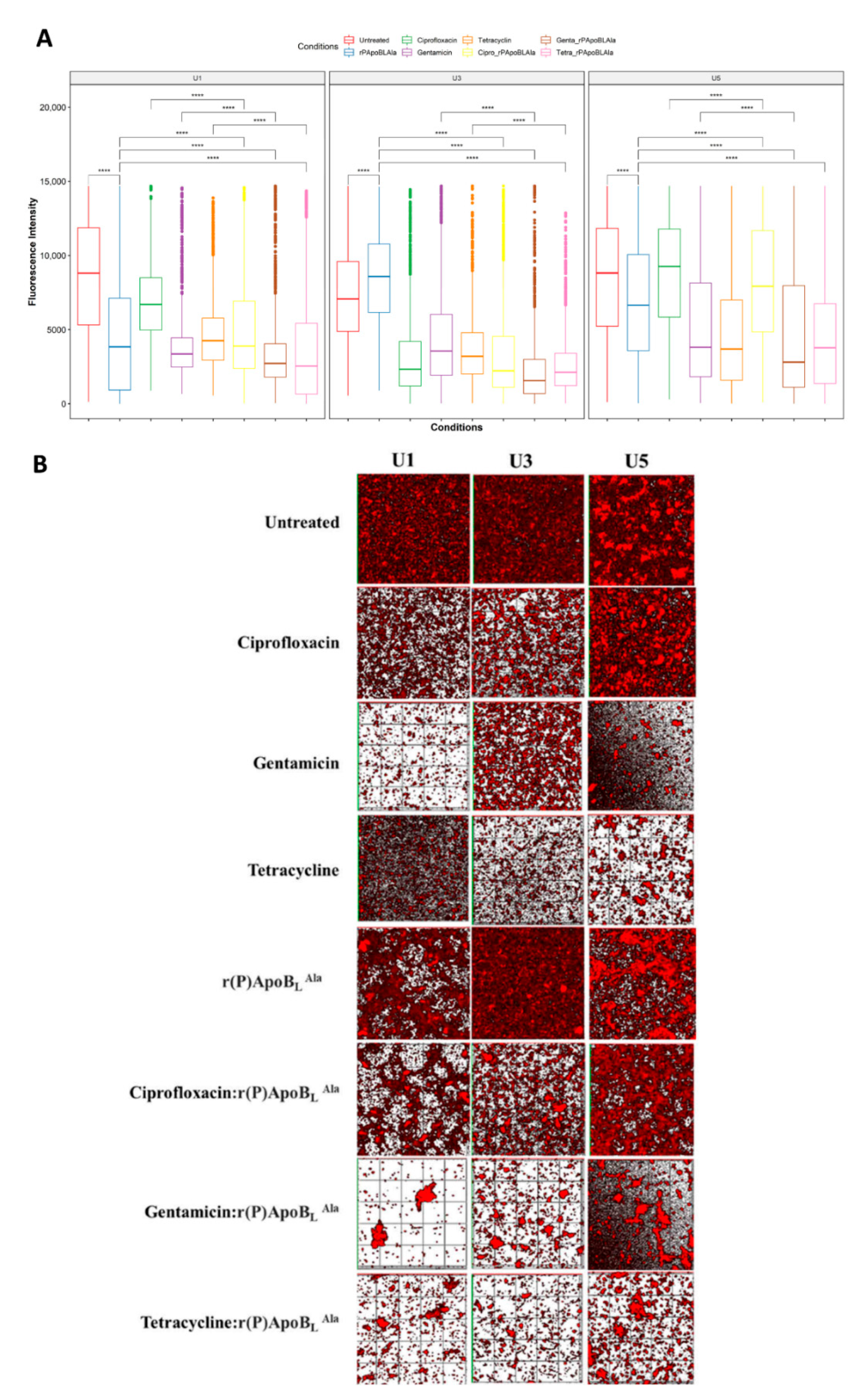
3.2. Evaluation of Antimicrobial Activity towards Isolated Bacterial Communities
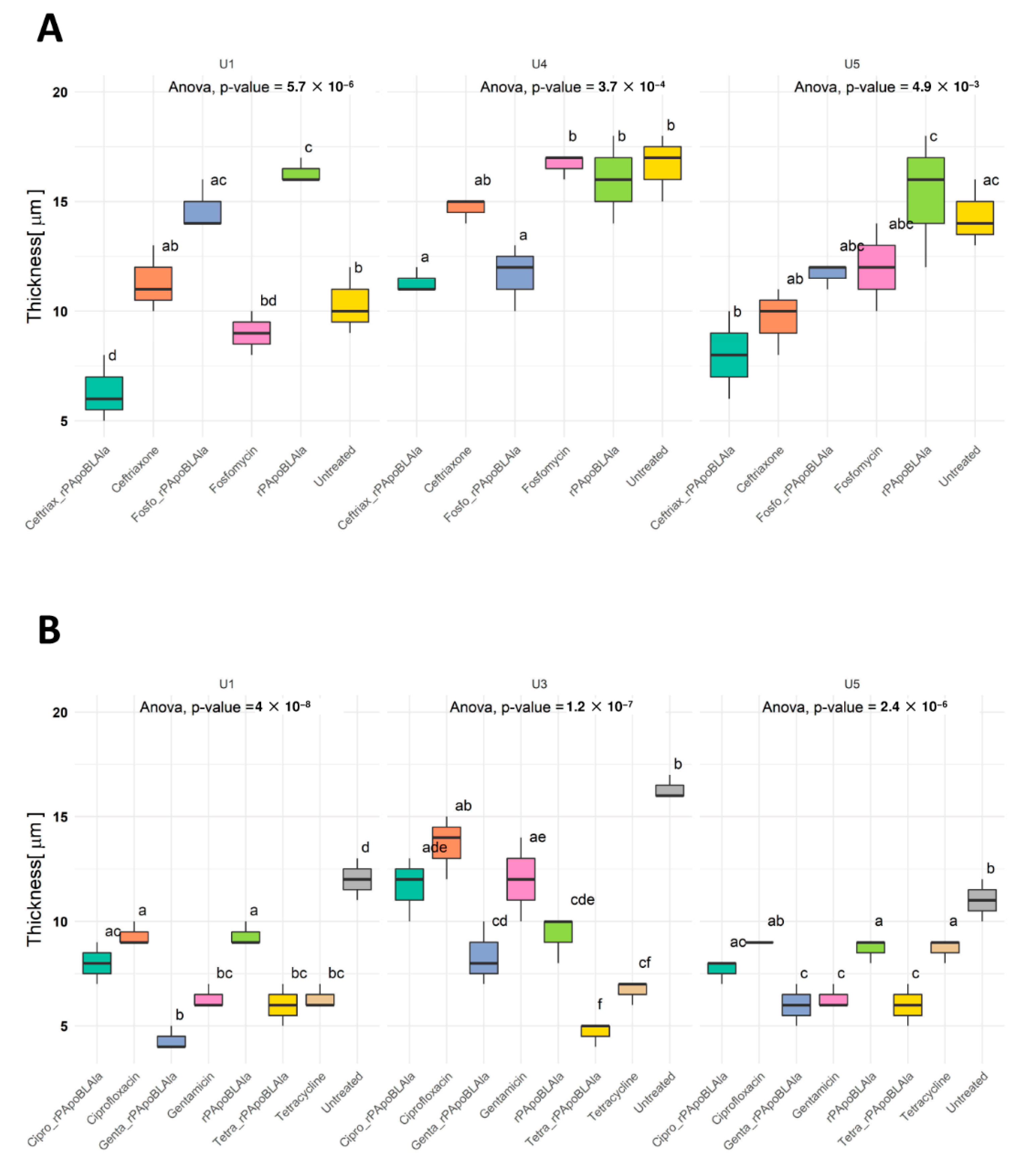
3.3. Evaluation of Antimicrobial Activity by CLSM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Mirabelli, P.; Coppola, L.; Illiano, E.; Salvatore, M.; Franzese, M. New Roadmaps for Non-muscle-invasive Bladder Cancer with Unfavorable Prognosis. Front. Chem. 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2020, 79, 62–79. [Google Scholar] [CrossRef]
- Saint, S.; Lipsky, B.A. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch. Intern. Med. 1999, 159, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am. J. Med. 2002, 113, 14–19. [Google Scholar] [CrossRef]
- Berrondo, C.; Feng, C.; Kukreja, J.B.; Messing, E.M.; Joseph, J.V. Antibiotic prophylaxis at the time of catheter removal after radical prostatectomy: A prospective randomized clinical trial. Urol. Oncol. Semin. Orig. Investig. 2018, 37, 181.e7–181.e14. [Google Scholar] [CrossRef]
- Köves, B.; Cai, T.; Veeratterapillay, R.; Pickard, R.; Seisen, T.; Lam, T.B.; Yuan, Y.; Bruyere, F.; Wagenlehner, F.; Bartoletti, R.; et al. Benefits and Harms of Treatment of Asymptomatic Bacteriuria: A Systematic Review and Meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur. Urol. 2017, 72, 865–868. [Google Scholar] [CrossRef]
- EAU Guidelines: Urological Infections | Uroweb. Available online: https://uroweb.org/guideline/urological-infections/ (accessed on 18 May 2021).
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Clifford, T.G.; Katebian, B.; Van Horn, C.M.; Bazargani, S.T.; Cai, J.; Miranda, G.; Daneshmand, S.; Djaladat, H. Urinary tract infections following radical cystectomy and urinary diversion: A review of 1133 patients. World J. Urol. 2018, 36, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2018, 11, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martinez-de-Tejada, G. AMPs as Anti-biofilm Agents for Human Therapy and Prophylaxis. Adv. Exp. Med. Biol. 2019, 1117, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Li, J.; Fernández-Millán, P.; Boix, E. Synergism between Host Defence Peptides and Antibiotics Against Bacterial Infections. Curr. Top. Med. Chem. 2020, 20, 1238–1263. [Google Scholar] [CrossRef]
- Sousa, M.G.C.; Xavier, P.D.; Cantuária, A.P.D.C.; Porcino, R.A.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb. Pathog. 2020, 152, 104634. [Google Scholar] [CrossRef]
- Gaglione, R.; Pizzo, E.; Notomista, E.; De La Fuente-Nunez, C.; Arciello, A. Host Defence Cryptides from Human Apolipoproteins: Applications in Medicinal Chemistry. Curr. Top. Med. Chem. 2020, 20, 1324–1337. [Google Scholar] [CrossRef]
- Gaglione, R.; Dell’Olmo, E.; Bosso, A.; Chino, M.; Pane, K.; Ascione, F.; Itri, F.; Caserta, S.; Amoresano, A.; Lombardi, A.; et al. Novel human bioactive peptides identified in Apolipoprotein B: Evaluation of their therapeutic potential. Biochem. Pharmacol. 2017, 130, 34–50. [Google Scholar] [CrossRef]
- Gaglione, R.; Pane, K.; Dell’Olmo, E.; Cafaro, V.; Pizzo, E.; Olivieri, G.; Notomista, E.; Arciello, A. Cost-effective production of recombinant peptides in Escherichia coli. New Biotechnol. 2019, 51, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gaglione, R.; Cesaro, A.; Dell’Olmo, E.; DELLA Ventura, B.; Casillo, A.; Di Girolamo, R.; Velotta, R.; Notomista, E.; Veldhuizen, E.J.A.; Corsaro, M.M.; et al. Effects of human antimicrobial cryptides identified in apolipoprotein B depend on specific features of bacterial strains. Sci. Rep. 2019, 9, 6728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaglione, R.; Cesaro, A.; Dell’Olmo, E.; Di Girolamo, R.; Tartaglione, L.; Pizzo, E.; Arciello, A. Cryptides Identified in Human Apolipoprotein B as New Weapons to Fight Antibiotic Resistance in Cystic Fibrosis Disease. Int. J. Mol. Sci. 2020, 21, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaglione, R.; Smaldone, G.; Cesaro, A.; Rumolo, M.; De Luca, M.; Di Girolamo, R.; Petraccone, L.; Del Vecchio, P.; Oliva, R.; Notomista, E.; et al. Impact of a Single Point Mutation on the Antimicrobial and Fibrillogenic Properties of Cryptides from Human Apolipoprotein B. Pharmaceuticals 2021, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Dell’Olmo, E.; Gaglione, R.; Cesaro, A.; Cafaro, V.; Teertstra, W.R.; de Cock, H.; Notomista, E.; Haagsman, H.P.; Veldhuizen, E.J.A.; Arciello, A. Host defence peptides identified in human apolipoprotein B as promising antifungal agents. Appl. Microbiol. Biotechnol. 2021, 105, 1953–1964. [Google Scholar] [CrossRef]
- Dell’Olmo, E.; Gaglione, R.; Sabbah, M.; Schibeci, M.; Cesaro, A.; Di Girolamo, R.; Porta, R.; Arciello, A. Host defense peptides identified in human apolipoprotein B as novel food biopreservatives and active coating components. Food Microbiol. 2021, 99, 103804. [Google Scholar] [CrossRef]
- Pane, K.; Durante, L.; Pizzo, E.; Varcamonti, M.; Zanfardino, A.; Sgambati, V.; Di Maro, A.; Carpentieri, A.; Izzo, V.; Di Donato, A.; et al. Rational Design of a Carrier Protein for the Production of Recombinant Toxic Peptides in Escherichia coli. PLoS ONE 2016, 11, e0146552. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, E.; Pane, K.; Bosso, A.; Landi, N.; Ragucci, S.; Russo, R.; Gaglione, R.; Torres, M.D.; de la Fuente-Nunez, C.; Arciello, A.; et al. Novel bioactive peptides from PD-L1/2, a type 1 ribosome inactivating protein from Phytolacca dioica L. Evaluation of their antimicrobial properties and anti-biofilm activities. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1425–1435. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 18 May 2021).
- dplyr: Dplyr: A Grammar of Data Manipulation in Sctyner/dplyr050: A Grammar of Data Manipulation. Available online: https://rdrr.io/github/sctyner/dplyr050/man/dplyr.html (accessed on 18 May 2021).
- rstatix: Pipe-Friendly Framework for Basic Statistical Tests Version 0.7.0 from CRAN. Available online: https://rdrr.io/cran/rstatix/ (accessed on 18 May 2021).
- ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics Version 3.3.3 from CRAN. Available online: https://rdrr.io/cran/ggplot2/ (accessed on 18 May 2021).
- RVAideMemoire: Testing and Plotting Procedures for Biostatistics Version 0.9-79 from CRAN. Available online: https://rdrr.io/cran/RVAideMemoire/ (accessed on 18 May 2021).
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2016, 129, 242–258. [Google Scholar] [CrossRef]
- Clark, J.Y.; Raman, J.D. Urinary tract infection after radical cystectomy: A vexing problem despite prophylactic antibiotics. Transl. Androl. Urol. 2019, 8, S510–S513. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, P.I.; Afferi, L.; Antonelli, A.; Cerruto, M.A.; Mordasini, L.; Mattei, A.; Baumeister, P.; Marra, G.; Krajewski, W.; Mari, A.; et al. Frailty impact on postoperative complications and early mortality rates in patients undergoing radical cystectomy for bladder cancer: A systematic review. Arab J. Urol. 2020, 19, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Gaglione, R.; Della Ventura, B.; Cesaro, A.; Di Girolamo, R.; Velotta, R.; Arciello, A. Loading of Polydimethylsiloxane with a Human ApoB-Derived Antimicrobial Peptide to Prevent Bacterial Infections. Int. J. Mol. Sci. 2022, 23, 5219. [Google Scholar] [CrossRef]
- Cesaro, A.; Torres, M.D.T.; Gaglione, R.; Dell’Olmo, E.; Di Girolamo, R.; Bosso, A.; Pizzo, E.; Haagsman, H.P.; Veldhuizen, E.J.A.; de la Fuente-Nunez, C.; et al. Synthetic Antibiotic Derived from Sequences Encrypted in a Protein from Human Plasma. ACS Nano 2022, 16, 1880–1895. [Google Scholar] [CrossRef]
| Compound (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| Catheter Extracts | Fosfomycin | Ceftriaxone | Cyprofloxacin | Tetracyclin | Gentamycin | r(P)ApoBLAla |
| U1 | 0.15–0.3 | 2.5 | 0.625 (MIC90) | 1 | 1 | >0.4 |
| U2 | 0.15–0.3 | 2.5 | 0.625 (MIC90) | 2 (MIC90) | 2 (MIC90) | >0.4 |
| U3 | 0.6 | 0.3–0.6 | 0.625 (MIC90) | 2 (MIC90) | 2 (MIC90) | >0.4 |
| U4 | 0.3–0.6 | 2.5 | 0.625 (MIC90) | 2 (MIC90) | 2 (MIC90) | >0.4 |
| U5 | 0.15–0.3 | 2.5 | 0.625 (MIC90) | 2 (MIC90) | 2 (MIC90) | >0.4 |
| U6 | 0.6 | 0.3–0.6 | 0.625 (MIC90) | 2 | 2 (MIC90) | >0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaglione, R.; Pane, K.; De Luca, M.; Franzese, M.; Arciello, A.; Trama, F.; Brancorsini, S.; Salvatore, M.; Illiano, E.; Costantini, E. Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study. Life 2022, 12, 802. https://doi.org/10.3390/life12060802
Gaglione R, Pane K, De Luca M, Franzese M, Arciello A, Trama F, Brancorsini S, Salvatore M, Illiano E, Costantini E. Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study. Life. 2022; 12(6):802. https://doi.org/10.3390/life12060802
Chicago/Turabian StyleGaglione, Rosa, Katia Pane, Maria De Luca, Monica Franzese, Angela Arciello, Francesco Trama, Stefano Brancorsini, Marco Salvatore, Ester Illiano, and Elisabetta Costantini. 2022. "Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study" Life 12, no. 6: 802. https://doi.org/10.3390/life12060802
APA StyleGaglione, R., Pane, K., De Luca, M., Franzese, M., Arciello, A., Trama, F., Brancorsini, S., Salvatore, M., Illiano, E., & Costantini, E. (2022). Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study. Life, 12(6), 802. https://doi.org/10.3390/life12060802








