Proteomics for Early Detection of Non-Muscle-Invasive Bladder Cancer: Clinically Useful Urine Protein Biomarkers
Abstract
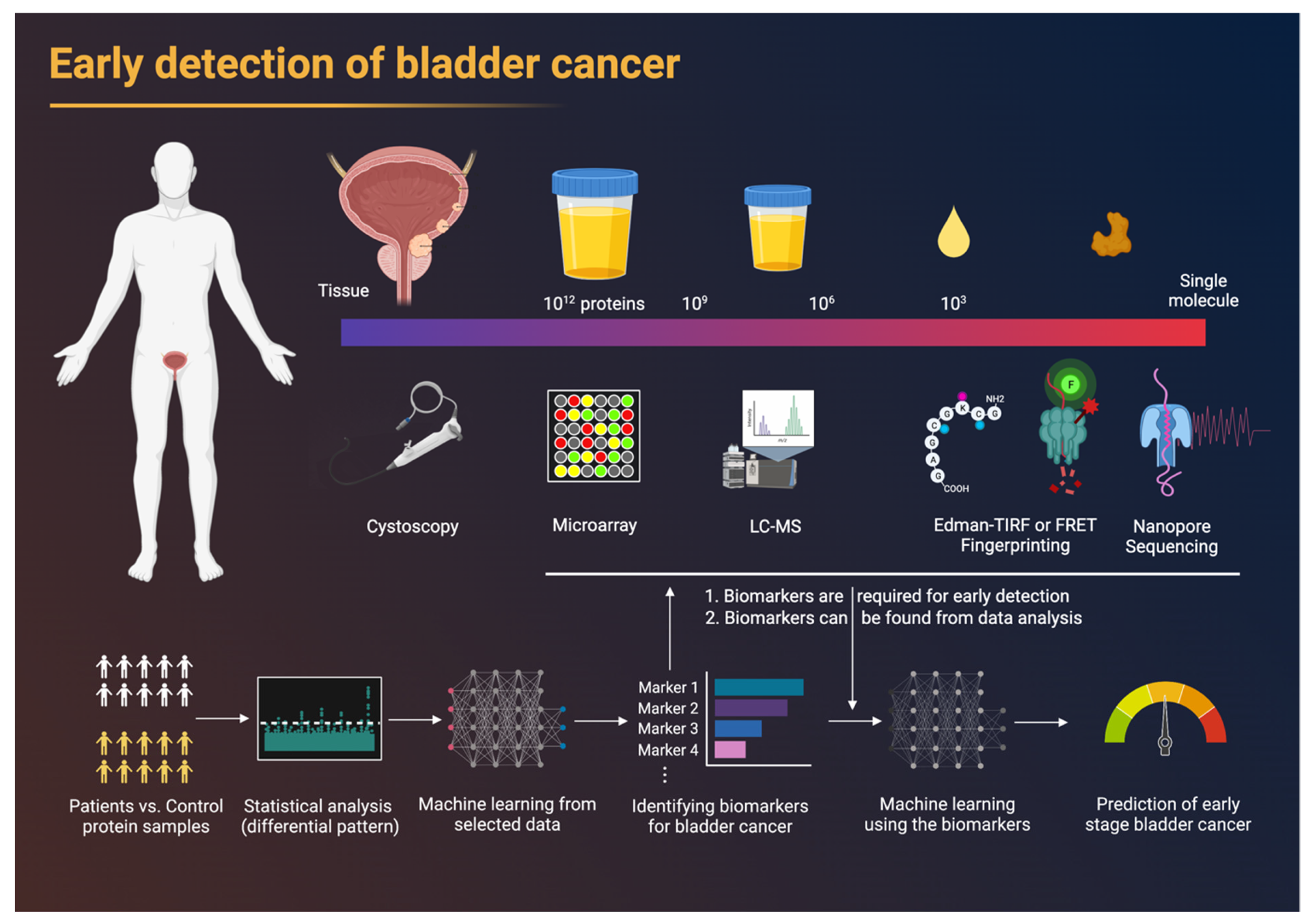
:1. Introduction
1.1. Aim and Methods of the Study
1.2. Prevalence
1.3. Grade and Stage of Bladder Cancer
1.4. Molecular Characteristics of Bladder Cancer
1.5. Presentation and Diagnosis
1.6. The Urgent Need to Develop Urinary Biomarkers
2. Proteomics
2.1. Antibody-Based Methods
2.2. Mass-Spectrometry-Based Methods
2.3. Statistical Analysis and Machine-Learning-Based Diagnosis
3. Urine Protein Biomarkers
3.1. Urinary Biomarker Tests for Diagnosis and Screening of NMIBC
3.2. Diagnostic Tests for Determining the Accuracy of FDA-Approved Biomarkers in Detecting NMIBC Recurrence
3.2.1. NMP22
3.2.2. BTA
3.2.3. ImmunoCyt
3.2.4. UroVysion (Multi-Target Fluorescence In Situ Hybridization)
4. New Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor, J.A.; et al. What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur. Urol. Focus 2020, 6, 653–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder Cancer. Lancet Lond. Engl. 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Matulay, J.T.; Kamat, A.M. Advances in Risk Stratification of Bladder Cancer to Guide Personalized Medicine. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [Green Version]
- Van Batavia, J.; Yamany, T.; Molotkov, A.; Dan, H.; Mansukhani, M.; Batourina, E.; Schneider, K.; Oyon, D.; Dunlop, M.; Wu, X.-R.; et al. Bladder Cancers Arise from Distinct Urothelial Sub-Populations. Nat. Cell Biol. 2014, 16, 982–991. [Google Scholar] [CrossRef]
- Theodorescu, D.; Cech, T.R. Telomerase in Bladder Cancer: Back to a Better Future? Eur. Urol. 2014, 65, 370–371. [Google Scholar] [CrossRef]
- Edwards, T.J.; Dickinson, A.J.; Natale, S.; Gosling, J.; McGrath, J.S. A Prospective Analysis of the Diagnostic Yield Resulting from the Attendance of 4020 Patients at a Protocol-Driven Haematuria Clinic. BJU Int. 2006, 97, 301–305. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Benayoun, S.; Zippe, C.; Lüdecke, G.; Boman, H.; Sanchez-Carbayo, M.; Casella, R.; Mian, C.; Friedrich, M.G.; Eissa, S.; et al. Institutional Variability in the Accuracy of Urinary Cytology for Predicting Recurrence of Transitional Cell Carcinoma of the Bladder. BJU Int. 2006, 97, 997–1001. [Google Scholar] [CrossRef]
- Wallace, E.; Higuchi, R.; Satya, M.; McCann, L.; Sin, M.L.Y.; Bridge, J.A.; Wei, H.; Zhang, J.; Wong, E.; Hiar, A.; et al. Development of a 90-Minute Integrated Noninvasive Urinary Assay for Bladder Cancer Detection. J. Urol. 2018, 199, 655–662. [Google Scholar] [CrossRef]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; et al. The IDENTIFY Study: The Investigation and Detection of Urological Neoplasia in Patients Referred with Suspected Urinary Tract Cancer—A Multicentre Observational Study. BJU Int. 2021, 128, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Osunkoya, A.O.; Siddiqui, M.T.; Looney, S.W. Accuracy of Grading of Urothelial Carcinoma on Urine Cytology: An Analysis of Interobserver and Intraobserver Agreement. Int. J. Clin. Exp. Pathol. 2012, 5, 882–891. [Google Scholar]
- Ren, A.H.; Diamandis, E.P.; Kulasingam, V. Uncovering the Depths of the Human Proteome: Antibody-Based Technologies for Ultrasensitive Multiplexed Protein Detection and Quantification. Mol. Cell. Proteom. 2021, 20, 100155. [Google Scholar] [CrossRef] [PubMed]
- Macklin, A.; Khan, S.; Kislinger, T. Recent Advances in Mass Spectrometry Based Clinical Proteomics: Applications to Cancer Research. Clin. Proteom. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Tsurusawa, N.; Chang, J.; Namba, M.; Makioka, D.; Yamura, S.; Iha, K.; Kyosei, Y.; Watabe, S.; Yoshimura, T.; Ito, E. Modified ELISA for Ultrasensitive Diagnosis. J. Clin. Med. 2021, 10, 5197. [Google Scholar] [CrossRef]
- Shah, K.; Maghsoudlou, P. Enzyme-Linked Immunosorbent Assay (ELISA): The Basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef]
- Soloway, M.S.; Briggman, V.; Carpinito, G.A.; Chodak, G.W.; Church, P.A.; Lamm, D.L.; Lange, P.; Messing, E.; Pasciak, R.M.; Reservitz, G.B.; et al. Use of a New Tumor Marker, Urinary NMP22, in the Detection of Occult or Rapidly Recurring Transitional Cell Carcinoma of the Urinary Tract Following Surgical Treatment. J. Urol. 1996, 156, 363–367. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Giacoia, E.G.; Rizwani, W.; Ross, S.; Rosser, C.J. Influencing Factors on the NMP-22 Urine Assay: An Experimental Model. BMC Urol. 2012, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Kinders, R.; Jones, T.; Root, R.; Bruce, C.; Murchison, H.; Corey, M.; Williams, L.; Enfield, D.; Hass, G.M. Complement Factor H or a Related Protein Is a Marker for Transitional Cell Cancer of the Bladder. Clin. Cancer Res. 1998, 4, 2511–2520. [Google Scholar]
- Morita, T.; Kikuchi, T.; Hashimoto, S.; Kobayashi, Y.; Tokue, A. Cytokeratin-19 Fragment (CYFRA 21-1) in Bladder Cancer. Eur. Urol. 1997, 32, 237–244. [Google Scholar] [CrossRef]
- Mungan, N.A.; Vriesema, J.L.J.; Thomas, C.M.G.; Kiemeney, L.A.L.M.; Witjes, J.A. Urinary Bladder Cancer Test: A New Urinary Tumor Marker in the Follow-up of Superficial Bladder Cancer. Urology 2000, 56, 787–791. [Google Scholar] [CrossRef]
- Stoeber, K.; Swinn, R.; Prevost, A.T.; de Clive-Lowe, P.; Halsall, I.; Dilworth, S.M.; Marr, J.; Turner, W.H.; Bullock, N.; Doble, A.; et al. Diagnosis of Genito-Urinary Tract Cancer by Detection of Minichromosome Maintenance 5 Protein in Urine Sediments. JNCI J. Natl. Cancer Inst. 2002, 94, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Nisman, B.; Barak, V.; Shapiro, A.; Golijanin, D.; Peretz, T.; Pode, D. Evaluation of Urine CYFRA 21-1 for the Detection of Primary and Recurrent Bladder Carcinoma. Cancer 2002, 94, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Soukup, V.; Pešl, M.; Koštířová, M.; Drncová, E.; Smolová, H.; Szakacsová, M.; Getzenberg, R.; Pavlík, I.; Dvořáček, J. Urinary Cytology and Quantitative BTA and UBC Tests in Surveillance of Patients with PTapT1 Bladder Urothelial Carcinoma. Urology 2008, 71, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, E.; Vlahou, A.; Petrolekas, A.; Stravodimos, K.; Tauber, R.; Geschwend, J.E.; Neuhaus, J.; Stolzenburg, J.-U.; Conaway, M.R.; Mischak, H.; et al. Prediction of Muscle-Invasive Bladder Cancer Using Urinary Proteomics. Clin. Cancer Res. 2009, 15, 4935–4943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, L.I.; Song, W.J.; Qing, H.M.; Yan, S.; Song, F.L. CYFRA21-1 Levels Could Be a Biomarker for Bladder Cancer: A Meta-Analysis. Genet. Mol. Res. GMR 2015, 14, 3921–3931. [Google Scholar] [CrossRef]
- Rosser, C.J.; Chang, M.; Dai, Y.; Ross, S.; Mengual, L.; Alcaraz, A.; Goodison, S. Urinary Protein Biomarker Panel for the Detection of Recurrent Bladder Cancer. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1340–1345. [Google Scholar] [CrossRef] [Green Version]
- Pichler, R.; Tulchiner, G.; Fritz, J.; Schaefer, G.; Horninger, W.; Heidegger, I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med. Sci. 2017, 14, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Ecke, T.H.; Weiß, S.; Stephan, C.; Hallmann, S.; Arndt, C.; Barski, D.; Otto, T.; Gerullis, H. UBC® Rapid Test—A Urinary Point-of-Care (POC) Assay for Diagnosis of Bladder Cancer with a Focus on Non-Muscle Invasive High-Grade Tumors: Results of a Multicenter-Study. Int. J. Mol. Sci. 2018, 19, 3841. [Google Scholar] [CrossRef] [Green Version]
- Gontero, P.; Montanari, E.; Roupret, M.; Longo, F.; Stockley, J.; Kennedy, A.; Rodriguez, O.; McCracken, S.R.C.; Dudderidge, T.; Sieverink, C.; et al. Comparison of the Performances of the ADXBLADDER Test and Urinary Cytology in the Follow-up of Non-Muscle-Invasive Bladder Cancer: A Blinded Prospective Multicentric Study. BJU Int. 2021, 127, 198–204. [Google Scholar] [CrossRef]
- Yang, N.; Feng, S.; Shedden, K.; Xie, X.; Liu, Y.; Rosser, C.J.; Lubman, D.M.; Goodison, S. Urinary Glycoprotein Biomarker Discovery for Bladder Cancer Detection Using LC/MS-MS and Label-Free Quantification. Clin. Cancer Res. 2011, 17, 3349–3359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, B.B.; López-Cortés, R.; Casas-Nebra, F.J.; Vázquez-Estévez, S.; Pérez-Fentes, D.; Chantada-Vázquez, M.D.P.; Bravo, S.B.; Núñez, C. Detection of Circulating Serum Protein Biomarkers of Non-Muscle Invasive Bladder Cancer after Protein Corona-Silver Nanoparticles Analysis by SWATH-MS. Nanomaterials 2021, 11, 2384. [Google Scholar] [CrossRef] [PubMed]
- Schwamborn, K.; Krieg, R.C.; Grosse, J.; Reulen, N.; Weiskirchen, R.; Knuechel, R.; Jakse, G.; Henkel, C. Serum Proteomic Profiling in Patients with Bladder Cancer. Eur. Urol. 2009, 56, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nedjadi, T.; Benabdelkamal, H.; Albarakati, N.; Masood, A.; Al-Sayyad, A.; Alfadda, A.A.; Alanazi, I.O.; Al-Ammari, A.; Al-Maghrabi, J. Circulating Proteomic Signature for Detection of Biomarkers in Bladder Cancer Patients. Sci. Rep. 2020, 10, 10999. [Google Scholar] [CrossRef]
- Thomas, S.; Hao, L.; Ricke, W.A.; Li, L. Biomarker Discovery in Mass Spectrometry-Based Urinary Proteomics. Proteom.-Clin. Appl. 2016, 10, 358–370. [Google Scholar] [CrossRef]
- Alberice, J.V.; Amaral, A.F.S.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Márquez, M.; García, A.; Malats, N.; Barbas, C. Searching for Urine Biomarkers of Bladder Cancer Recurrence Using a Liquid Chromatography–Mass Spectrometry and Capillary Electrophoresis–Mass Spectrometry Metabolomics Approach. J. Chromatogr. A 2013, 1318, 163–170. [Google Scholar] [CrossRef]
- Goodison, S.; Rosser, C.J.; Urquidi, V. Urinary Proteomic Profiling for Diagnostic Bladder Cancer Biomarkers. Expert Rev. Proteom. 2009, 6, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Bitzios, A.; Kassa, E.; Shevchenko, G.; Falk, A.; Malmström, P.-U.; Dragomir, A.; Segersten, U.; Lind, S.B. Proteomic Comparison between Different Tissue Preservation Methods for Identification of Promising Biomarkers of Urothelial Bladder Cancer. Sci. Rep. 2021, 11, 7595. [Google Scholar] [CrossRef]
- Guan, S.; Taylor, P.P.; Han, Z.; Moran, M.F.; Ma, B. Data Dependent–Independent Acquisition (DDIA) Proteomics. J. Proteome Res. 2020, 19, 3230–3237. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Z.; Gao, Y.; Chen, Y.; Hang, W.; Xing, J.; Yan, X. LC-MS-Based Serum Metabolic Profiling for Genitourinary Cancer Classification and Cancer Type-Specific Biomarker Discovery. Proteomics 2012, 12, 2238–2246. [Google Scholar] [CrossRef]
- Lindén, M.; Lind, S.B.; Mayrhofer, C.; Segersten, U.; Wester, K.; Lyutvinskiy, Y.; Zubarev, R.; Malmström, P.-U.; Pettersson, U. Proteomic Analysis of Urinary Biomarker Candidates for Nonmuscle Invasive Bladder Cancer. Proteomics 2012, 12, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Frantzi, M.; van Kessel, K.E.; Zwarthoff, E.C.; Marquez, M.; Rava, M.; Malats, N.; Merseburger, A.S.; Katafigiotis, I.; Stravodimos, K.; Mullen, W.; et al. Development and Validation of Urine-Based Peptide Biomarker Panels for Detecting Bladder Cancer in a Multi-Center Study. Clin. Cancer Res. 2016, 22, 4077–4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinitcyn, P.; Rudolph, J.D.; Cox, J. Computational Methods for Understanding Mass Spectrometry–Based Shotgun Proteomics Data. Annu. Rev. Biomed. Data Sci. 2018, 1, 207–234. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Tsai, C.-H.; Chen, C.-L.; Yu, J.-S.; Chang, Y.-H. Development of Biomarkers of Genitourinary Cancer Using Mass Spectrometry-Based Clinical Proteomics. J. Food Drug Anal. 2019, 27, 387–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroggilos, R.; Mokou, M.; Latosinska, A.; Makridakis, M.; Lygirou, V.; Mavrogeorgis, E.; Drekolias, D.; Frantzi, M.; Mullen, W.; Fragkoulis, C.; et al. Proteome-Based Classification of Nonmuscle Invasive Bladder Cancer. Int. J. Cancer 2020, 146, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Dal Moro, F.; Valotto, C.; Guttilla, A.; Zattoni, F. Urinary Markers in the Everyday Diagnosis of Bladder Cancer. Urologia 2013, 80, 265–275. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. Proteome and Proteomics: New Technologies, New Concepts, and New Words. Electrophoresis 1998, 19, 1853–1861. [Google Scholar] [CrossRef]
- Wang, Z.; Que, H.; Suo, C.; Han, Z.; Tao, J.; Huang, Z.; Ju, X.; Tan, R.; Gu, M. Evaluation of the NMP22 BladderChek Test for Detecting Bladder Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 100648–100656. [Google Scholar] [CrossRef]
- Guo, A.; Wang, X.; Gao, L.; Shi, J.; Sun, C.; Wan, Z. Bladder Tumour Antigen (BTA Stat) Test Compared to the Urine Cytology in the Diagnosis of Bladder Cancer: A Meta-Analysis. Can. Urol. Assoc. J. 2014, 8, E347–E352. [Google Scholar] [CrossRef] [Green Version]
- Glas, A.S.; Roos, D.; Deutekom, M.; Zwinderman, A.H.; Bossuyt, P.M.; Kurth, K.H. Tumor Markers in the Diagnosis of Primary Bladder Cancer. A Systematic Review. J. Urol. 2003, 169, 1975–1982. [Google Scholar] [CrossRef]
- He, H.; Han, C.; Hao, L.; Zang, G. ImmunoCyt Test Compared to Cytology in the Diagnosis of Bladder Cancer: A Meta-Analysis. Oncol. Lett. 2016, 12, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Hajdinjak, T. UroVysion FISH Test for Detecting Urothelial Cancers: Meta-Analysis of Diagnostic Accuracy and Comparison with Urinary Cytology Testing. Urol. Oncol. 2008, 26, 646–651. [Google Scholar] [CrossRef]
- Gleichenhagen, J.; Arndt, C.; Casjens, S.; Meinig, C.; Gerullis, H.; Raiko, I.; Brüning, T.; Ecke, T.; Johnen, G. Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. Int. J. Mol. Sci. 2018, 19, E226. [Google Scholar] [CrossRef] [Green Version]
- Myers-Irvin, J.M.; Landsittel, D.; Getzenberg, R.H. Use of the Novel Marker BLCA-1 for the Detection of Bladder Cancer. J. Urol. 2005, 174, 64–68. [Google Scholar] [CrossRef]
- Alavi, A.; Izadpanahi, M.-H.; Haghshenas, L.; Faridizad, R.; Eslami, M.-J.; Ghadimi, K. Comparing Urine Levels of BLCA-4 Nuclear Matrix Protein in Patients with Bladder Cancer and Non-Bladder Cancer. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 289–292. [Google Scholar]
- Cai, Q.; Wu, Y.; Guo, Z.; Gong, R.; Tang, Y.; Yang, K.; Li, X.; Guo, X.; Niu, Y.; Zhao, Y. Urine BLCA-4 Exerts Potential Role in Detecting Patients with Bladder Cancers: A Pooled Analysis of Individual Studies. Oncotarget 2015, 6, 37500–37510. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.; Shtabsky, A.; Lew, S.; Rona, R.; Leibovitch, I.; Nativ, O.; Cohen, M.; Mor, Y.; Lindner, U.; Glickman, Y.; et al. A Novel Urine-Based Assay for Bladder Cancer Diagnosis: Multi-Institutional Validation Study. Eur. Urol. Focus 2018, 4, 388–394. [Google Scholar] [CrossRef]
- Nativ, O.; Halachmi, S.; Biton, K.; Zlotnik, M.; Yoffe, C.; Davis, N.; Glickman, Y.; Bejar, J. Pd19-09 Performance of a Novel Urine-Based Biomarker for the Monitoring of Bladder Cancer Recurrence. J. Urol. 2017, 197, e368. [Google Scholar] [CrossRef]
- Goodison, S.; Chang, M.; Dai, Y.; Urquidi, V.; Rosser, C.J. A Multi-Analyte Assay for the Non-Invasive Detection of Bladder Cancer. PLoS ONE 2012, 7, e47469. [Google Scholar] [CrossRef]
- Pham, H.T.; Block, N.L.; Lokeshwar, V.B. Tumor-Derived Hyaluronidase: A Diagnostic Urine Marker for High-Grade Bladder Cancer. Cancer Res. 1997, 57, 778–783. [Google Scholar] [CrossRef]
- Eissa, S.; Matboli, M.; Essawy, N.O.E.; Kotb, Y.M. Integrative Functional Genetic-Epigenetic Approach for Selecting Genes as Urine Biomarkers for Bladder Cancer Diagnosis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 9545–9552. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Wang, C.; Shi, F.; Cao, H.; Yu, Y.; Zhang, M.; Liu, X. Hyaluronic Acid/Hyaluronidase as Biomarkers for Bladder Cancer: A Diagnostic Meta-Analysis. Neoplasma 2017, 64, 901–908. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Schroeder, G.L.; Selzer, M.G.; Hautmann, S.H.; Posey, J.T.; Duncan, R.C.; Watson, R.; Rose, L.; Markowitz, S.; Soloway, M.S. Bladder Tumor Markers for Monitoring Recurrence and Screening Comparison of Hyaluronic Acid-Hyaluronidase and BTA-Stat Tests. Cancer 2002, 95, 61–72. [Google Scholar] [CrossRef]
- Schroeder, G.L.; Lorenzo-Gomez, M.-F.; Hautmann, S.H.; Friedrich, M.G.; Ekici, S.; Huland, H.; Lokeshwar, V. A Side by Side Comparison of Cytology and Biomarkers for Bladder Cancer Detection. J. Urol. 2004, 172, 1123–1126. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, P.K.; Singh, D.; Dalela, D.; Rath, S.K.; Bhatt, M.L.B. Clinical Utility of Urinary Soluble Fas in Screening for Bladder Cancer. Asia Pac. J. Clin. Oncol. 2016, 12, e215–e221. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Wang, Z.; Gao, J.; Guo, Y. Is Urinary Soluble Fas an Independent Predictor of Non-Muscle-Invasive Bladder Cancer? A Prospective Chart Study. Urol. Int. 2013, 91, 456–461. [Google Scholar] [CrossRef]
- Margulis, V.; Lotan, Y.; Shariat, S.F. Survivin: A Promising Biomarker for Detection and Prognosis of Bladder Cancer. World J. Urol. 2008, 26, 59–65. [Google Scholar] [CrossRef]
- Smith, S.D.; Wheeler, M.A.; Plescia, J.; Colberg, J.W.; Weiss, R.M.; Altieri, D.C. Urine Detection of Survivin and Diagnosis of Bladder Cancer. JAMA 2001, 285, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Kenney, D.M.; Geschwindt, R.D.; Kary, M.R.; Linic, J.M.; Sardesai, N.Y.; Li, Z.-Q. Detection of Newly Diagnosed Bladder Cancer, Bladder Cancer Recurrence and Bladder Cancer in Patients with Hematuria Using Quantitative Rt-PCR of Urinary Survivin. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2007, 28, 57–62. [Google Scholar] [CrossRef]
- Wolfs, J.R.E.; Hermans, T.J.N.; Koldewijn, E.L.; van de Kerkhof, D. Novel Urinary Biomarkers ADXBLADDER and Bladder EpiCheck for Diagnostics of Bladder Cancer: A Review. Urol. Oncol. 2021, 39, 161–170. [Google Scholar] [CrossRef]
- Babu, S.; Kim, N.W.; Wu, M.; Chan, I.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 Is a Novel Cytologic Biomarker for Urothelial Carcinoma Diagnosis. Am. J. Clin. Pathol. 2021, 156, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Mockler, D.C.; Roa-Peña, L.; Szygalowicz, A.; Kim, N.W.; Jahanfard, S.; Gholami, S.S.; Moffitt, R.; Fitzgerald, J.P.; Escobar-Hoyos, L.F.; et al. Keratin 17 Is a Sensitive and Specific Biomarker of Urothelial Neoplasia. Mod. Pathol. 2019, 32, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Hampson, A.; Agarwal, S.; Swamy, R.; Chilvers, M.; Hampson, A.; Jahanfard, S.; Kim, N. The Role of URO17TM Biomarker to Enhance Diagnosis of Urothelial Cancer in New Hematuria Patients—First European Data. BJUI Compass 2021, 2, 46–52. [Google Scholar] [CrossRef]
- Gourin, C.G.; Zhi, W.; Adam, B.-L. Proteomic Identification of Serum Biomarkers for Head and Neck Cancer Surveillance. Laryngoscope 2009, 119, 1291–1302. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Hu, W.; Lv, D.; Ding, W.; Tang, L.; Jiang, J.; Ye, M. Lipoprotein(a) Level and Its Association with Tumor Stage in Male Patients with Primary Lung Cancer. Clin. Chem. Lab. Med. 2009, 47, 452–457. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wu, H.; Zhang, T.; Wang, J.; Wang, S.; Chang, J. Identification of Apo-A1 as a Biomarker for Early Diagnosis of Bladder Transitional Cell Carcinoma. Proteome Sci. 2011, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Chen, C.-L.; Chen, H.-W.; Chung, T.; Wu, C.-C.; Chen, C.-D.; Hsu, C.-W.; Chen, M.-C.; Tsui, K.-H.; Chang, P.-L.; et al. Discovery of Novel Bladder Cancer Biomarkers by Comparative Urine Proteomics Using ITRAQ Technology. J. Proteome Res. 2010, 9, 5803–5815. [Google Scholar] [CrossRef]
- Frantzi, M.; Vlahou, A. Ten Years of Proteomics in Bladder Cancer: Progress and Future Directions. Bladder Cancer 2017, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Konety, B.R. Molecular Markers in Bladder Cancer: A Critical Appraisal. Urol. Oncol. 2006, 24, 326–337. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Shariat, S.F.; Marberger, M.J.; Lotan, Y.; Sanchez-Carbayo, M.; Zippe, C.; Lüdecke, G.; Boman, H.; Sawczuk, I.; Friedrich, M.G.; Casella, R.; et al. Variability in the Performance of Nuclear Matrix Protein 22 for the Detection of Bladder Cancer. J. Urol. 2006, 176, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.E.; Pruthi, R.S. Value of Urinary Cytology in the Diagnosis and Management of Urinary Tract Malignancies. Urology 2004, 63, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; O’Sullivan, P.; Raman, J.D.; Shariat, S.F.; Kavalieris, L.; Frampton, C.; Guilford, P.; Luxmanan, C.; Suttie, J.; Crist, H.; et al. Clinical Comparison of Noninvasive Urine Tests for Ruling out Recurrent Urothelial Carcinoma. Urol. Oncol. 2017, 35, 531.e15–531.e22. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Rizwani, W.; Ross, S.; Bart Grossman, H.; Rosser, C.J. Urinary BTA: Indicator of Bladder Cancer or of Hematuria. World J. Urol. 2012, 30, 869–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarosdy, M.F.; Hudson, M.A.; Ellis, W.J.; Soloway, M.S.; deVere White, R.; Sheinfeld, J.; Jarowenko, M.V.; Schellhammer, P.F.; Schervish, E.W.; Patel, J.V.; et al. Improved Detection of Recurrent Bladder Cancer Using the Bard BTA Stat Test. Urology 1997, 50, 349–353. [Google Scholar] [CrossRef]
- Sharma, S.; Zippe, C.D.; Pandrangi, L.; Nelson, D.; Agarwal, A. Exclusion Criteria Enhance the Specificity and Positive Predictive Value of NMP22 and BTA Stat. J. Urol. 1999, 162, 53–57. [Google Scholar] [CrossRef]
- Miyanaga, N.; Akaza, H.; Tsukamoto, S.; Shimazui, T.; Ohtani, M.; Ishikawa, S.; Noguchi, R.; Manabe, F.; Nishijima, Y.; Kikuchi, K.; et al. Usefulness of Urinary NMP22 to Detect Tumor Recurrence of Superficial Bladder Cancer after Transurethral Resection. Int. J. Clin. Oncol. 2003, 8, 369–373. [Google Scholar] [CrossRef]
- Mian, C.; Maier, K.; Comploj, E.; Lodde, M.; Berner, L.; Lusuardi, L.; Palermo, S.; Vittadello, F.; Pycha, A. UCyt+/ImmunoCyt in the Detection of Recurrent Urothelial Carcinoma: An Update on 1991 Analyses. Cancer 2006, 108, 60–65. [Google Scholar] [CrossRef]
- Zellweger, T.; Benz, G.; Cathomas, G.; Mihatsch, M.J.; Sulser, T.; Gasser, T.C.; Bubendorf, L. Multi-Target Fluorescence in Situ Hybridization in Bladder Washings for Prediction of Recurrent Bladder Cancer. Int. J. Cancer 2006, 119, 1660–1665. [Google Scholar] [CrossRef]
- Kim, J.-S.; Fillmore, T.L.; Liu, T.; Robinson, E.; Hossain, M.; Champion, B.L.; Moore, R.J.; Camp, D.G.; Smith, R.D.; Qian, W.-J. 18O-Labeled Proteome Reference as Global Internal Standards for Targeted Quantification by Selected Reaction Monitoring-Mass Spectrometry. Mol. Cell. Proteom. 2011, 10, M110.007302. [Google Scholar] [CrossRef] [Green Version]
- Wingren, C.; Borrebaeck, C.A. Antibody Microarray Analysis of Directly Labelled Complex Proteomes. Curr. Opin. Biotechnol. 2008, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, J.; Boulgakov, A.A.; Marcotte, E.M. A Theoretical Justification for Single Molecule Peptide Sequencing. PLoS Comput. Biol. 2015, 11, e1004080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ginkel, J.; Filius, M.; Szczepaniak, M.; Tulinski, P.; Meyer, A.S.; Joo, C. Single-Molecule Peptide Fingerprinting. Proc. Natl. Acad. Sci. USA 2018, 115, 3338–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restrepo-Pérez, L.; Joo, C.; Dekker, C. Paving the Way to Single-Molecule Protein Sequencing. Nat. Nanotechnol. 2018, 13, 786–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, J.A.; Bohländer, P.; Dai, M.; Filius, M.; Howard, C.J.; van Kooten, X.F.; Ohayon, S.; Pomorski, A.; Schmid, S.; Aksimentiev, A.; et al. The Emerging Landscape of Single-Molecule Protein Sequencing Technologies. Nat. Methods 2021, 18, 604–617. [Google Scholar] [CrossRef]
- Brinkerhoff, H.; Kang, A.S.W.; Liu, J.; Aksimentiev, A.; Dekker, C. Multiple Rereads of Single Proteins at Single–Amino Acid Resolution Using Nanopores. Science 2021, 374, 1509–1513. [Google Scholar] [CrossRef]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
| Biomarker | Method | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| UBC | ELISA Immunoradiometric assay | 64.4 | 80.3 |
| CYFRA21-1 | ELISA | 82 | 80 |
| BLCA-1 | ELISA | 80 | 87 |
| BLCA-4 | ELISA | 93 | 97 |
| CellDetect | Immunostaining | 84 | 70 |
| Hyaluronic acid | ELISA RT-qPCR | 87–100 | 89–98 |
| sFas | ELISA | 51.2 | 85.9 |
| Survivin | Bio-dot test | 79 | 93 |
| MCM5-ADXBLADDER | ELISA | 51.9 | 66.4 |
| URO17 | Immunocytochemistry | 97 | AUC: 90 |
| Apo-A1 | ELISA | 83.7–95 | 85–97 |
| ANG, APOE, CA9, IL-8, MMP9, MMP10, PAI-1, and VEGF | ELISA | 92 | 97 |
| Test | Biomarker | Method | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| NMP22 | NMP-22 | Sandwich immunoassay | 52–59 | 87–89 |
| BTA stat® | Complement factor H-related protein | Colorimetric immunoassay | 64–69 | 73–77 |
| BTA TRAK® | Complement factor H-related protein | Sandwich immunoassay | 62–71 | 45–81 |
| ImmunoCytTM | Carcinoembryonic antigen and two mucins (M344, LDQ10, and 19A11) | Immunofluorescence cytology | 78 | 78 |
| UroVysionTM | Aneuploidy of chromosomes 3, 7, and 17 and loss of the 9p21 locus | Multi-target FISH | 63 (30–86) | 87 (63–95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-H.; Kang, C.-K.; Kim, E.-M.; Kim, A.-R.; Kim, A. Proteomics for Early Detection of Non-Muscle-Invasive Bladder Cancer: Clinically Useful Urine Protein Biomarkers. Life 2022, 12, 395. https://doi.org/10.3390/life12030395
Ahn J-H, Kang C-K, Kim E-M, Kim A-R, Kim A. Proteomics for Early Detection of Non-Muscle-Invasive Bladder Cancer: Clinically Useful Urine Protein Biomarkers. Life. 2022; 12(3):395. https://doi.org/10.3390/life12030395
Chicago/Turabian StyleAhn, Jae-Hak, Chan-Koo Kang, Eun-Mee Kim, Ah-Ram Kim, and Aram Kim. 2022. "Proteomics for Early Detection of Non-Muscle-Invasive Bladder Cancer: Clinically Useful Urine Protein Biomarkers" Life 12, no. 3: 395. https://doi.org/10.3390/life12030395
APA StyleAhn, J.-H., Kang, C.-K., Kim, E.-M., Kim, A.-R., & Kim, A. (2022). Proteomics for Early Detection of Non-Muscle-Invasive Bladder Cancer: Clinically Useful Urine Protein Biomarkers. Life, 12(3), 395. https://doi.org/10.3390/life12030395






