The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms
Abstract
1. Introduction
1.1. MS-Related Symptoms
1.1.1. Spasticity in MS
1.1.2. MS-Related Pain
1.1.3. MS-Related Tremor and Ataxia
1.1.4. Bladder Dysfunction in MS
1.1.5. MS-Related Sleep Disorders
1.1.6. Health-Related Quality of Life in MS Patients
1.1.7. MS-Related Disability and Disability Progression
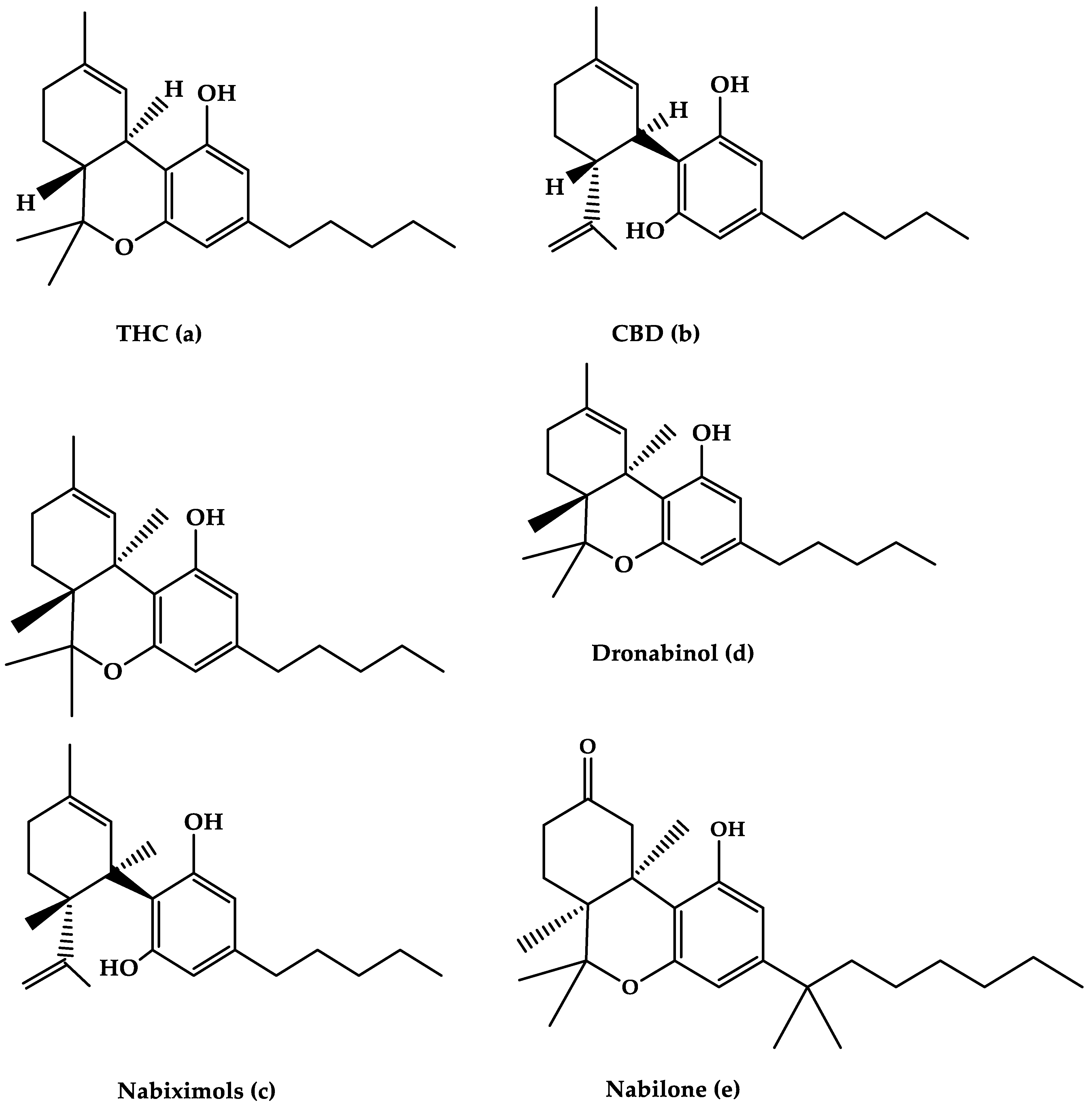
2. Cannabinoid Agents for the Treatment of MS
2.1. Nabiximols
2.1.1. Effects of Nabiximols on MS-Related Spasticity
2.1.2. Effect of Nabiximols on MS-Related Pain
2.1.3. Effects of Nabiximols on MS-Related Tremor
2.1.4. Effect of Nabiximols on MS-Related Bladder Function
2.1.5. Effects of Nabiximols on MS-Related Sleep Disturbances
2.1.6. Effects of Nabiximols on MS-Related HRQoL
2.1.7. Effects of Nabiximols on MS-Related Disability and Disability Progression
2.2. Dronabinol
2.2.1. Effects of Dronabinol on MS-Related Spasticity
2.2.2. Effects of Dronabinol on MS-Related Pain
2.2.3. Effects of Dronabinol on MS-Related Tremor
2.2.4. Effects of Dronabinol on MS-Related Bladder Functions
2.2.5. Effects of Dronabinol on MS-Related Sleep Disorders
2.2.6. Effects of Dronabinol on MS-Related HRQoL
2.2.7. Effects of Dronabinol on MS-Related MS Disability and Disability Progression
2.3. Nabilone
2.3.1. Effects of Nabilone on MS-Related Spasticity
2.3.2. Nabilone Effects on MS-Related Pain
2.3.3. Effects of Nabilone on MS-Related Tremor
2.3.4. Effects of Nabilone on MS-Related Bladder Function
2.3.5. Effects of Nabilone on MS-Related Sleep Disorders
2.3.6. Effects of Nabilone on MS-Related HRQol
2.3.7. Effects of Nabilone on MS Disability and Disability Progression
3. Adverse Effects
3.1. Nabiximols
3.2. Oral Cannabinoids and THC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CBD | cannabidiol |
| C. Sativa | Cannabis sativa |
| GPCRs | G protein-coupled receptors |
| HRQoL | Health-related Quality of life |
| MS | multiple sclerosis |
| NRS | numerical rating scale |
| THC | Δ9-tetrahydrocannabinol |
References
- Trapp, D.B.; Nave, K.-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Schwab, N.; Schneider-Hohendorf, T.; Wiendl, H. Therapeutic uses of anti-α4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int. Immunol. 2015, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.P.; Incorvaia, B.; Munari, L.M.; Ebers, G.; Polman, C.; D’Amico, R.; Parmelli, E.; Filippini, G. Interferon in relapsing-remitting multiple sclerosis. Cochrane Database Syst. Rev. 2001, 2001, CD002002. [Google Scholar] [CrossRef] [PubMed]
- Kraft, G.H.; Freal, J.E.; Coryell, J.K. Disability, disease duration, and rehabilitation service needs in multiple sclerosis: Patient perspectives. Arch. Phys. Med. Rehabil. 1986, 67, 164–168. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Bufo, S.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An update of current cannabis-based pharmaceuticals in pain medicine. Pain Ther. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Brunt, T.M.; van Genugten, M.; Höner-Snoeken, K.; van de Velde, M.J.; Niesink, R.J. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J. Clin. Psychopharmacol. 2014, 34, 344–349. [Google Scholar] [CrossRef]
- Kumar, R.; Chambers, W.; Pertwee, R. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 2001, 56, 1059–1068. [Google Scholar]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef]
- Pearce, D.D.; Mitsouras, K.; Irizarry, K.J. Discriminating the effects of Cannabis sativa and Cannabis indica: A web survey of medical cannabis users. J. Altern. Complementary Med. 2014, 20, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Honce, J.M. Cannabis and multiple sclerosis—The way forward. Front. Neurol. 2017, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Ingram, G.; Pearson, O.R. Cannabis and multiple sclerosis. Pract. Neurol. 2019, 19, 310–315. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid receptors, and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Phytocannabinoids 2017, 103, 1–36. [Google Scholar]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.; Zielińska, A.; Souto, E.; Wielgus, K. Cannabidiol in neurological and neoplastic diseases: Latest developments on the molecular mechanism of action. Int. J. Mol. Sci. 2021, 22, 4294. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T. Identification of terpenoid chemotypes among high (−)-trans-Δ9-tetrahydrocannabinol-producing Cannabis sativa L. cultivars. Cannabis Cannabinoid Res. 2017, 2, 34–47. [Google Scholar] [CrossRef] [PubMed]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Regehr, W.G. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 2002, 12, 324–330. [Google Scholar] [CrossRef]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef]
- Rudroff, T.; Sosnoff, J. Cannabidiol to improve mobility in people with multiple sclerosis. Front. Neurol. 2018, 9, 183. [Google Scholar] [CrossRef]
- Zwibel, H.L. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther. 2009, 26, 1043–1057. [Google Scholar] [CrossRef]
- Jensen, C.J.; Stankovich, J.; Van Der Walt, A.; Bahlo, M.; Taylor, B.V.; van der Mei, I.; Foote, S.; Kilpatrick, T.; Johnson, L.J.; Wilkins, E.; et al. Multiple sclerosis susceptibility-associated SNPs do not influence disease severity measures in a cohort of Australian MS patients. PLoS ONE 2010, 5, e10003. [Google Scholar] [CrossRef]
- Briggs, F.B.S.; Shao, X.; A Goldstein, B.; Oksenberg, J.R.; Barcellos, L.F.; De Jager, P.L.; International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes Immun. 2011, 12, 615–625. [Google Scholar]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory potential of cannabidiol in multiple sclerosis: A systematic review. J. Neuroimmune Pharmacol. 2021, 16, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Hugos, C.L.; Cameron, M.H. Assessment and Measurement of Spasticity in MS: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.A.; Hadjimichael, O.C.; Preiningerova, J.L.; Vollmer, T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult. Scler. J. 2004, 10, 589–595. [Google Scholar] [CrossRef]
- Milinis, K.; Tennant, A.; Young, C. Spasticity in multiple sclerosis: Associations with impairments and overall quality of life. Mult. Scler. Relat. Disord. 2016, 5, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, F.; Nielsen, J.B.; Klinge, K. Spasticity-assessment: A review. Spinal Cord 2006, 44, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Haselkorn, J.K.; Richer, C.B.; Welch, D.F.; Herndon, R.M.; Johnson, B.; Little, J.W.; Miller, J.R.; Rosenberg, J.H.; E Seidle, M.; Guidelines, M.S.C.F.C.P. Overview of spasticity management in multiple sclerosis. Evidence-based management strategies for spasticity treatment in multiple sclerosis. J. Spinal Cord Med. 2005, 28, 167–199. [Google Scholar] [CrossRef]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Spasticity in patients with multiple sclerosis–clinical characteristics, treatment and quality of life. Acta Neurol. Scand. 2014, 129, 154–162. [Google Scholar] [CrossRef]
- Lapeyre, E.; Kuks, J.B.; Meijler, W.J. Spasticity: Revisiting the role and the individual value of several pharmacological treatments. NeuroRehabilitation 2010, 27, 193–200. [Google Scholar] [CrossRef]
- Halpern, R.; Gillard, P.; Graham, G.D.; Varon, S.F.; Zorowitz, R.D. Adherence associated with oral medications in the treatment of spasticity. PMR 2013, 5, 747–756. [Google Scholar] [CrossRef]
- Koppel, B.S.; Brust, J.C.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef]
- Yadav, V.; Bever, C.; Bowen, J.; Bowling, A.; Weinstock-Guttman, B.; Cameron, M.; Bourdette, D.; Gronseth, G.S.; Narayanaswami, P. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.S.; Wolff, K.; Wise, K.; Tanton, C.; Winstock, A.; Silber, E. Cannabis use in patients with multiple sclerosis. Mult. Scler. J. 2006, 12, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.; Adams, H.; Guy, G. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pract. 2005, 59, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Amar, M.B. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Rowland, M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: A systematic review. BMC Neurol. 2009, 9, 1–6. [Google Scholar] [CrossRef]
- Karst, M.; Wippermann, S.; Ahrens, J.; Karst, M. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 2010, 70, 2409–2438. [Google Scholar] [CrossRef]
- Hadjimichael, O.; Kerns, R.D.; Rizzo, M.A.; Cutter, G.; Vollmer, T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain 2007, 127, 35–41. [Google Scholar] [CrossRef]
- Forbes, A.; While, A.; Mathes, L.; Griffiths, P. Health problems and health-related quality of life in people with multiple sclerosis. Clin. Rehabil. 2006, 20, 67–78. [Google Scholar] [CrossRef]
- O’Connor, A.B.; Schwid, S.R.; Herrmann, D.N.; Markman, J.D.; Dworkin, R.H. Pain associated with multiple sclerosis: Systematic review and proposed classification. PAIN 2008, 137, 96–111. [Google Scholar] [CrossRef]
- Iskedjian, M.; Bereza, B.; Gordon, A.; Piwko, C.; Einarson, T.R. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr. Med. Res. Opin. 2007, 23, 17–24. [Google Scholar] [CrossRef]
- Solaro, C.; Lunardi, G.L.; Mancardi, G.L. Pain and MS. Int. MS J. 2003, 10, 14–19. [Google Scholar] [PubMed]
- Wilkins, A. Cerebellar dysfunction in multiple sclerosis. Front. Neurol. 2017, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Swingler, R.; Compston, D. The morbidity of multiple sclerosis. QJM Int. J. Med. 1992, 83, 325–337. [Google Scholar]
- Mills, R.J.; Yap, L.; Young, C.A. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst. Rev. 2007, 1, CD005029. [Google Scholar] [CrossRef]
- Fox, S.H.; Kellett, M.; Moore, A.P.; Crossman, A.R.; Brotchie, J. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov. Disord. 2002, 17, 145–149. [Google Scholar] [CrossRef]
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. J. 2004, 10, 434–441. [Google Scholar] [CrossRef]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- De Sèze, M.; Ruffion, A.; Denys, P.; Joseph, P.-A.; Perrouin-Verbe, B.; International Francophone Neuro-Urological expert Study Group (GENULF). The neurogenic bladder in multiple sclerosis: Review of the literature and proposal of management guidelines. Mult. Scler. J. 2007, 13, 915–928. [Google Scholar] [CrossRef]
- Gallien, P.; Robineau, S.; Nicolas, B.; Le Bot, M.-P.; Brissot, R.; Verin, M. Vesicourethral Dysfunction and Urodynamic Findings in Multiple Sclerosis: A Study of 149 Cases. J. Urol. 1999, 161, 2032–2033. [Google Scholar] [CrossRef]
- Giannantoni, A.; Scivoletto, G.; Di Stasi, S.M.; Grasso, M.G.; Vespasiani, G.; Castellano, V. Urological dysfunctions and upper urinary tract involvement in multiple sclerosis patients. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 1998, 17, 89–98. [Google Scholar] [CrossRef]
- Koldewijn, E.L.; Hommes, O.R.; Lemmens, W.A.J.G.; Debruyne, F.M.J.; Van Kerrebroeck, P.E.V. Relationship between lower urinary tract abnormalities and disease-related parameters in multiple sclerosis. J. Urol. 1995, 154, 169–173. [Google Scholar] [CrossRef]
- Andersson, K.-E. Current and future drugs for treatment of MS-associated bladder dysfunction. Ann. Phys. Rehabil. Med. 2014, 57, 321–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Fratticci, L.; Lenchig, C.; Valente, M.; Cargnelutti, D.; Picello, M.; Serafini, A.; Dolso, P.; Gigli, G. Prevalence of ‘poor sleep’among patients with multiple sclerosis: An independent predictor of mental and physical status. Sleep Med. 2009, 10, 26–34. [Google Scholar] [CrossRef]
- Brass, S.D.; Duquette, P.; Proulx-Therrien, J.; Auerbach, S. Sleep disorders in patients with multiple sclerosis. Sleep Med. Rev. 2010, 14, 121–129. [Google Scholar] [CrossRef]
- Crayton, H.; Heyman, R.A.; Rossman, H.S. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology 2004, 63, S12–S18. [Google Scholar] [CrossRef]
- Attarian, H. Importance of sleep in the quality of life of multiple sclerosis patients: A long under-recognized issue. Sleep Med. 2008, 10, 7–8. [Google Scholar] [CrossRef]
- Kaminska, M.; Kimoff, R.J.; Benedetti, A.; Robinson, A.; Bar-Or, A.; Lapierre, Y.; Schwartzman, K.; A Trojan, D. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult. Scler. J. 2012, 18, 1159–1169. [Google Scholar] [CrossRef]
- Group, W. The World Health Organization quality of life assessment: Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar]
- Amtmann, D.; Bamer, A.M.; Kim, J.; Chung, H.; Salem, R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil. Health J. 2018, 11, 99–107. [Google Scholar] [CrossRef]
- McCabe, M.P.; McKern, S. Quality of life and multiple sclerosis: Comparison between people with multiple sclerosis and people from the general population. J. Clin. Psychol. Med. Settings 2002, 9, 287–295. [Google Scholar] [CrossRef]
- Nielsen, S.; Germanos, R.; Weier, M.; Pollard, J.; Degenhardt, L.; Hall, W.; Buckley, N.; Farrell, M. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Marta, M.; Giovannoni, G. Disease modifying drugs in multiple sclerosis: Mechanisms of action and new drugs in the horizon. CNS Neurol. Disord. Drug Targets 2012, 11, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Vermersch, P. Sativex®(tetrahydrocannabinol+ cannabidiol), an endocannabinoid system modulator: Basic features and main clinical data. Expert Rev. Neurother. 2011, 11, 15–19. [Google Scholar] [CrossRef]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G.; et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef]
- Perez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today 2006, 42, 495–503. [Google Scholar] [CrossRef]
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’Leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459. [Google Scholar] [CrossRef]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef]
- Leocani, L.; Nuara, A.; Houdayer, E. Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: A double-blind, placebo-controlled, crossover study. In Proceedings of the Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting, Boston, MA, USA, 10–13 September 2014. [Google Scholar]
- Wade, D.T.; Makela, P.M.; House, H.; Bateman, C.; Robson, P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult. Scler. J. 2006, 12, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S.; for the Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: A double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci. 2019, 129, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Henze, T.; Essner, U.; Trompke, C.; Silván, C.V. Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: Consistency of response across subgroups from the savant randomized clinical trial. Int. J. Neurosci. 2020, 130, 1199–1205. [Google Scholar] [CrossRef]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Rice, J.; Cameron, M. Cannabinoids for treatment of MS symptoms: State of the evidence. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Dykukha, I.; Malessa, R.; Essner, U.; Überall, M.A. Nabiximols in chronic neuropathic pain: A meta-analysis of randomized placebo-controlled trials. Pain Med. 2021, 22, 861–874. [Google Scholar] [CrossRef]
- Kavia, R.; De Ridder, D.; Constantinescu, C.; Stott, C.; Fowler, C.; Constantinescu, C. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. J. 2010, 16, 1349–1359. [Google Scholar] [CrossRef]
- Maniscalco, G.T.; Aponte, R.; Bruzzese, D.; Guarcello, G.; Manzo, V.; Napolitano, M.; Moreggia, O.; Chiariello, F.; Florio, C. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: A pilot prospective study. Neurol. Sci. 2018, 39, 97–102. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex®, a cannabis-based medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice-results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur. Neurol. 2014, 71, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur. Neurol. 2014, 72, 95–102. [Google Scholar] [CrossRef]
- Gado, F.; Digiacomo, M.; Macchia, M.; Bertini, S.; Manera, C. Traditional uses of cannabinoids and new perspectives in the treatment of multiple sclerosis. Medicines 2018, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Vickery, J.; Hobart, J.; Wright, D.; Green, C.; Shearer, J.; Nunn, A.; Cano, M.G.; MacManus, D.; Miller, D.; et al. The CUPID trial: A randomised double-blind placebo-controlled parallel-group multi-centre trial of cannabinoids to slow progression in multiple sclerosis. Health Technol. Assess. 2015, 19, 1–187. [Google Scholar] [CrossRef]
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen, A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M.; Polman, C.H. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002, 58, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1983, 13, 669–671. [Google Scholar] [CrossRef]
- Killestein, J. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J. Neuroimmunol. 2003, 137, 140–143. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef]
- Zajicek, J.P. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1664–1669. [Google Scholar] [CrossRef]
- Petro, D.J.; Ellenberger, C.J. Treatment of Human Spasticity with Δ9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1981, 21, 413S–416S. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, J.T.; Andyrsiak, T.; Fairbanks, L.; Ellison, G.W.; Myers, L.W. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv. Alcohol Subst. Abus. 1987, 7, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.; Wright, D.; Zajicek, J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. 2006, 17, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Rosse, R.B.; Connor, J.M.; Burket, J.A.; Murphy, M.E.; Fox, F.J. Current status of cannabis treatment of multiple sclerosis with an illustrative case presentation of a patient with MS, complex vocal tics, paroxysmal dystonia, and marijuana dependence treated with dronabinol. CNS Spectr. Int. J. Neuropsychiatr. Med. 2008, 13, 393–403. [Google Scholar] [CrossRef]
- Martyn, C.; Illis, L.; Thom, J. Nabilone in the treatment of multiple sclerosis. Lancet 1995, 345, 579. [Google Scholar] [CrossRef]
- Wissel, J.; Haydn, T.; Müller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain. J. Neurol. 2006, 253, 1337–1341. [Google Scholar] [CrossRef]
- Turcotte, D.; Doupe, M.; Torabi, M.; Gomori, A.; Ethans, K.; Esfahani, F.; Galloway, K.; Namaka, M. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Med. 2015, 16, 149–159. [Google Scholar] [CrossRef]
- Centonze, D.; Mori, F.; Koch, G.; Buttari, F.; Codecà, C.; Rossi, S.; Cencioni, M.T.; Bari, M.; Fiore, S.; Bernardi, G.; et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol. Sci. 2009, 30, 531–534. [Google Scholar] [CrossRef]
- Zajicek, J.P.; Hobart, J.C.; Slade, A.; Barnes, D.; Mattison, P.G.; on behalf of the MUSEC Research Group. Multiple sclerosis and extract of cannabis: Results of the MUSEC trial. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, S.; Søndergaard, H.; Linnet, K.; Thomsen, R.; Rasmussen, B.; Sorensen, P.; Sellebjerg, F.; Oturai, A. Safety and efficacy of low-dose medical cannabis oils in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 48, 102708. [Google Scholar]
- Meza, R.; Peña, J.; García, K.; Corsi, O.; Rada, G. Are cannabinoids effective in multiple sclerosis? Medwave 2017, 17, e6865. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Prosperini, L.; Pozzilli, C. Balance worsening associated with nabiximols in multiple sclerosis. Mult. Scler. J. 2019, 25, 113–117. [Google Scholar] [CrossRef] [PubMed]
- De Blasiis, P.; Siani, M.F.; Fullin, A.; Sansone, M.; Melone, M.A.B.; Sampaolo, S.; Signoriello, E.; Lus, G. Short and long term effects of Nabiximols on balance and walking assessed by 3D-gait analysis in people with Multiple Sclerosis and spasticity. Mult. Scler. Relat. Disord. 2021, 51, 102805. [Google Scholar] [CrossRef]
- Carotenuto, A.; Costabile, T.; De Lucia, M.; Moccia, M.; Falco, F.; Petruzzo, M.; De Angelis, M.; Russo, C.V.; Saccà, F.; Lanzillo, R.; et al. Predictors of Nabiximols (Sativex®) discontinuation over long-term follow-up: A real-life study. J. Neurol. 2020, 267, 1737–1743. [Google Scholar] [CrossRef]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. J. Clin. Rehab. 2003, 17, 21–29. [Google Scholar] [CrossRef]
- Torres-Moreno, M.C.; Papaseit, E.; Torrens, M.; Farré, M. Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: A systematic review and meta-analysis. JAMA Netw. Open 2018, 1, e183485. [Google Scholar] [CrossRef]
| Cannabinoid Agents | Therapeutic Actions | Adverse Effects |
|---|---|---|
| Nabiximols |
|
|
| Dronabinol |
|
|
| Nabilone |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682. https://doi.org/10.3390/life12050682
Haddad F, Dokmak G, Karaman R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life. 2022; 12(5):682. https://doi.org/10.3390/life12050682
Chicago/Turabian StyleHaddad, Fatma, Ghadeer Dokmak, and Rafik Karaman. 2022. "The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms" Life 12, no. 5: 682. https://doi.org/10.3390/life12050682
APA StyleHaddad, F., Dokmak, G., & Karaman, R. (2022). The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life, 12(5), 682. https://doi.org/10.3390/life12050682








