Management Strategies to Mitigate N2O Emissions in Agriculture
Abstract
:1. Introduction
2. N2O Production and Emission
- The decomposition of hydroxylamine during the process of autotrophic as well as heterotrophic nitrification;
- The chemical DNF of soil NO2− and abiotic decomposition of ammonium nitrate in the presence of light, humidity and reacting surfaces;
- The production of N2O by nitrifier denitrification within the same nitrifying micro-organisms;
- The coupled nitrification–denitrification by different micro-organisms (the nitrite oxidizers produce nitrate, which is denitrified by denitrifiers in situ);
- The DNF conducted by microbes capable of using nitrogen oxides as alternative electron acceptors under O2 limited conditions;
- The co-denitrification of organic N compounds with NO and nitrate ammonification or dissimilatory nitrate reduction to ammonium [51].
3. Environmental and Anthropic Factors Affecting N2O Emission from Agricultural Soils
3.1. Soil pH
3.2. Soil Moisture and Temperature
3.3. Application of Crop Residues
3.4. Nitrogen Application
3.5. Soil Micro-Organisms
3.6. Soil Characteristics
4. Management Options to Mitigate N2O Emission
4.1. Modification of Irrigation Pattern
4.2. Tillage Practices
4.3. Crop Residue Management
4.4. Fertilizer Management
4.4.1. Adjusting Fertilizer Dose and Matching N Supply with Demand
4.4.2. Time of Fertilizer Application
| Crop | N Sources | N2O Emission (kg ha−1) | References |
|---|---|---|---|
| Rice | Control (no fertilizers) | 0.04 | [164] |
| AS (100 kg ha−1) | 0.17 | ||
| Urea (100 kg ha−1) | 0.15 | ||
| Rice | Control (no fertilizers) | 0.67 | [116] |
| NPK (210:105:240 kg ha−1) | 6.51 | ||
| Rice | Control (no fertilizers) | 0.64 | [165] |
| Urea (300 kg ha−1) | 1.39 | ||
| Maize | Control (no fertilizers) | 1.53 (kg N Mg−1) | [77] |
| UAN (150 kg ha−1) | 1.92 (kg N Mg−1) | ||
| CAN (150 kg ha−1) | 1.81 (kg N Mg−1) | ||
| Maize | Control (no fertilizers) | 0.16 | [166] |
| Urea (145 kg ha−1) | 0.30 | ||
| AN (145 kg ha−1) | 0.29 |
4.4.3. Improving N Fertilizer Placement
4.4.4. Selection of Suitable Fertilizers
4.5. Biochar Application
| Biochar Application | N2O Mitigation Potential (%) | Reference |
|---|---|---|
| BBC: 5 tons/ha | 38 | [204] |
| BBC: 10 tons/ha | 48 | |
| BBC: 15 tons/ha | 61 | |
| RCHBC: 50 tons/ha | 36 | [205] |
| MSBC: 16.77 tons/ha | 10.8 | [206] |
| BBC: 5 tons/ha | 24.25 | [207] |
| BBC: 15 tons/ha | 30.7 | |
| RSBC: 22.4 tons/ha | 72.95 | [208] |
| RSBC: 44.8 tons/ha | 235.1 | |
| RSBC: 36 tons/ha | 50 | [209] |
| RSBC: 72 tons/ha | 83 | |
| WSBC: 10 tons/ha | 101.68 | [210] |
| CSBC: 9 tons/ha | 46.3 | [211] |
| CSBC:13 tons/ha | 33.3 | |
| RSBC: 1% (w/w) | 82.28 | [212] |
| RSBC: 5% (w/w) | 185.21 |
4.6. Lime Application
4.7. Use of Nitrification Inhibitors or Slow-Release Fertilizers
4.8. Use of Organic Amendments
4.9. Fermented Organic Manures
4.10. Composting
4.11. Role of Arbuscular Mycorrhizal Fungi
4.12. Selection of Plant Genotypes
4.13. Modifying Cropping Schemes and Crop Rotations
4.14. Integrated Nutrient Management
5. Role of Regulatory Authorities in Implementing Environment-Friendly Management Practices to Reduce GHGs Emissions
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Santanen, A.; Jaakkola, S.; Ekholm, P.; Hartikainen, H.; Stoddard, F.L.; Mäkelä, P.S.A. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass Bioenerg. 2013, 59, 477–485. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Kheir, A.M.S.; Al-Dhumri, S.; Alghamdi, A.G.; Omar, E.S.H.; Aboelsoud, H.M.; Abdella, K.A.; Abou El Hassan, W.H. Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy 2019, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.E.; Seleiman, M.F.; Kheir, A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alotaibi, M.; Alhammad, B.A.; Rady, M.M.; Mahdi, A.H.A. Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. By boosting antioxidant defense system under actual saline field conditions. Agronomy 2020, 10, 1741. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Tomer, R.; Harit, R.C.; Jain, N.; Bhowmik, A.; Kaushik, R. Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ. Sci. Poll. Res. 2021, 28, 51425–51439. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Hassan, M.U.; Aamer, M.; Batool, M.; Fang, S.; WU, Z.; LI, H. A Critical Review on the Improvement of Drought Stress Tolerance in Rice (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1756–1788. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Tellez-Rio, A.; Vallejo, A.; García-Marco, S.; Martin-Lammerding, D.; Tenorio, J.L.; Rees, R.M.; Guardia, G. Conservation Agriculture practices reduce the global warming potential of rainfed low N input semi-arid agriculture. Eur. J. Agron. 2017, 84, 95–104. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Fagodiya, R.K.; Kumar, S.S.; Kumar, A.; Gupta, D.K.; Tomer, R.; Harit, R.C.; Kumar, V.; Jain, N.; et al. Plummeting global warming potential by chemicals interventions in irrigated rice: A lab to field assessment. Agric. Ecosyst. Environ. 2021, 319, 107545. [Google Scholar] [CrossRef]
- Sekoai, T.T.; Yoro, K.O. Biofuel development initiatives in sub-Saharan Africa: Opportunities and challenges. Climate 2016, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xu, H.; Jiang, Y.; Zhang, K.; Hu, Y.; Zeng, Z. Methane emissions and microbial communities as influenced by dual cropping of Azolla along with early rice. Sci. Rep. 2017, 7, 40635. [Google Scholar] [CrossRef] [PubMed]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate Change 2014 Mitigation of Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; pp. 1–164. [Google Scholar]
- Anenberg, S.C.; Schwartz, J.; Shindell, D.; Amann, M.; Faluvegi, G.; Klimont, Z.; Janssens-Maenhout, G.; Pozzoli, L.; van Dingenen, R.; Vignati, E.; et al. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ. Health Perspect. 2012, 120, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos. Environ. 2011, 45, 2284–2296. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf (accessed on 5 May 2021).
- Mannina, G.; Capodici, M.; Cosenza, A.; Ditrapani, D.; Vanloosdrecht, M.C.J.J. Nitrous oxide emission in a University of Cape Town membrane bioreactor: The effect of carbon to nitrogen ratio. J. Clean. Prod. 2017, 149, 180–190. [Google Scholar] [CrossRef]
- Haider, A.; Bashir, A.; Husnain, M.I. Impact of agricultural land use and economic growth on nitrous oxide emissions: Evidence from developed and developing countries. Sci. Total Environ. 2020, 741, 140421. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.; Ippolito, J.; Hagemann, N.; Borchard, N.; Cayuela, M.L.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Jeffery, S.; Kern, J.; Novak, J.; et al. Biochar as a tool to reduce the agricultural greenhouse-gas burden–knowns, unknowns and future research needs. J. Environ. Eng. Landsc. Manag. 2017, 25, 114–139. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, B.; Akiyama, H. Seasonal variations in indirect N2O emissions from an agricultural headwater ditch. Biol. Fertil. Soils 2017, 53, 651–662. [Google Scholar] [CrossRef]
- Baggs, E.M. Soil microbial sources of nitrous oxide: Recent advances in knowledge, emerging challenges and future direction. Curr. Opin. Environ. Sustain. 2011, 3, 321–327. [Google Scholar] [CrossRef]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Phil. Trans. R. Soc. B 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Aamer, M.; Hassan, M.U.; Shaaban, M.; Rasul, F.; Haiying, T.; Qiaoying, M.; Batool, M.; Rasheed, A.; Chuan, Z.; Qitao, S.; et al. Rice straw biochar mitigates N2O emissions under alternate wetting and drying conditions in paddy soil. J. Saudi Chem. Soc. 2021, 25, 101172. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Bonin, P.C.; Michotey, V.D.; Garcia, N.; LokaBharathi, P.A. Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium. Sci. Rep. 2012, 2, 419. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [Green Version]
- Venterea, R.T.; Halvorson, A.D.; Kitchen, N.; Liebig, M.A.; Cavigelli, M.A.; Del Grosso, S.J.; Motavalli, P.P.; Nelson, K.A.; Spokas, K.A.; Singh, B.P. Challenges and opportunities for mitigating nitrous oxide emissions from fertilized cropping systems. Front. Ecol. Environ. 2012, 10, 562–570. [Google Scholar] [CrossRef]
- Seleiman, M.; Abdel-Aal, M. Response of growth, productivity and quality of some Egyptian wheat cultivars to different irrigation regimes. Egypt. J. Agron. 2018, 40, 313–330. [Google Scholar] [CrossRef]
- Seleiman, M.; Abdel-Aal, M. Effect of organic, inorganic and bio-fertilization on growth, yield and quality traits of some chickpea (Cicer arietinum L.) varieties. Egypt. J. Agron. 2018, 40, 105–117. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Kheir, A.M.S. Maize productivity, heavy metals uptake and their availability in contaminated clay and sandy alkaline soils as affected by inorganic and organic amendments. Chemosphere 2018, 204, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Hardan, A.N. Importance of mycorrhizae in crop productivity. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Awaad, H., Abu-hashim, M., Negm, A., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 471–484. [Google Scholar]
- Seleiman, M.F.; Hafez, E.M. Optimizing inputs management for sustainable agricultural development. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Awaad, H., Abu-hashim, M., Negm, A., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 487–507. [Google Scholar]
- Seleiman, M.F.; Refay, Y.; Al-Suhaibani, N.; Al-Ashkar, I.; El-Hendawy, S.; Hafez, E.M. Hafez integrative effects of rice-straw biochar and silicon on oil and seed quality, yield and physiological traits of Helianthus annuus L. grown under water deficit stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Santanen, A.; Kleemola, J.; Stoddard, F.L.; Mäkelä, P.S.A. Improved sustainability of feedstock production with sludge and interacting mycorrhiza. Chemosphere 2013, 91, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.P.; Zhang, L.M.; Di, H.J.; He, J.Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [Green Version]
- Lehtovirta-Morley, L.E.; Sayavedra-Soto, L.A.; Gallois, N.; Schouten, S.; Stein, L.Y.; Prosser, J.I.; Nicol, G.W. Identifying potential mechanisms enabling acidophily in the ammonia-oxidizing archaeon “Candidatus Nitrosotalea devanaterra”. Appl. Environ. Microbiol. 2016, 82, 2608–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, R. Metabolism of nitric oxide in soil and soil microorganisms and regulation of flux into the atmosphere. In Microbiology of Atmospheric Trace Gases; Murrell, J.C., Kelly, D.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 167–203. [Google Scholar]
- Mushinski, R.M.; Phillips, R.P.; Payne, Z.C.; Abney, R.B.; Jo, I.; Fei, S.; Pusede, S.E.; White, J.R.; Rusch, D.B.; Raff, J.D. Microbial mechanisms and ecosystem flux estimation for aerobic NOy emissions from deciduous forest soils. Proc. Natl. Acad. Sci. USA 2019, 116, 2138–2145. [Google Scholar] [CrossRef] [Green Version]
- Abeliovich, A. The Nitrite-oxidizing bacteria introduction. Prokaryotes 2006, 5, 861–872. [Google Scholar]
- Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R. Soc. B 2013, 368, 20130126. [Google Scholar] [CrossRef] [Green Version]
- Moreira, F.M.S.; Siqueira, J.O. Microbiology and Soil Bio-Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Čuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Zhuge, Y.; Zhang, J.; Cai, Z. Soil pH was the main controlling factor of the denitrification rates and N2/N2O emission ratios in forest and grassland soils along the northeast China transect. Soil Sci. Plant Nutr. 2012, 58, 517–525. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Khan, S.; Clough, T.J.; Goh, K.M.; Sherlock, R.R. Influence of soil pH on NOx and N2O emissions from bovine urine applied to soil columns. N. Z. J. Agric. Res. 2011, 54, 285–301. [Google Scholar] [CrossRef]
- Groffman, P.M.; Altabet, M.A.; Böhlke, J.K.; Butterbach-Bahl, K.; David, M.B.; Firestone, M.K.; Giblin, A.E.; Kana, T.M.; Nielsen, L.P.; Voytek, M.A. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef]
- Tate, K.R.; Ross, D.J.; Saggar, S.; Hedley, C.B.; Dando, J.; Singh, B.K.; Lambie, S.M. Methane uptake in soils from Pinus radiata plantations, a reverting shrubland and adjacent pastures: Effects of land-use change, and soil texture, water and mineral nitrogen. Soil Biol. Biochem. 2007, 39, 1437–1449. [Google Scholar] [CrossRef]
- Granli, T.; Bøckman, O.C. Nitrous oxide from agriculture. Norwegian J. Agric. Sci. 1994, 3, 14–21. [Google Scholar]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. Adv. Agron. 2011, 110, 1–75. [Google Scholar]
- Stres, B.; Danevčič, T.; Pal, L.; Fuka, M.M.; Resman, L.; Leskovec, S.; Hacin, J.; Stopar, D.; Mahne, I.; Mandic-Mulec, I. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol. 2008, 66, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braker, G.; Schwarz, J.; Conrad, R. Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol. Ecol. 2010, 73, 134–148. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.C.J.; Hödl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef]
- Lemke, R.L.; Izaurralde, R.C.; Nyborg, M.; Solberg, E.D. Tillage and N source influence soil-emitted nitrous oxide in the Alberta Parkland region. Can. J. Soil Sci. 1999, 79, 15–24. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Eichner, M.J. Nitrous oxide emissions from fertilized soils: Summary of available data. J. Environ. Qual. 1990, 19, 272–280. [Google Scholar] [CrossRef]
- Patten, D.K.; Bremner, J.M.; Blackmer, A.M. Effects of drying and air-dry storage of soils on their capacity for denitrification of nitrate. Soil Sci. Soc. Am. J. 1980, 44, 67–70. [Google Scholar] [CrossRef]
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- Shelp, M.L.; Beauchamp, E.G.; Thurtell, G.W. Nitrous oxide emissions from soil amended with glucose, alfalfa, or corn residues. Commun. Soil Sci. Plant Anal. 2000, 31, 877–892. [Google Scholar] [CrossRef]
- Rochette, P.; Worth, D.E.; Lemke, R.L.; McConkey, B.G.; Pennock, D.J.; Wagner-Riddle, C.; Desjardins, R.L. Estimation of N2O emissions from agricultural soils in Canada. I. Development of a country-specific methodology. Can. J. Soil Sci. 2008, 88, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecu. Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R. Tillage, crop residue and N fertilizer effects on crop yield, nutrient uptake, soil quality and nitrous oxide gas emissions in a second 4-yr rotation cycle. Soil Tillage Res. 2007, 90, 171–183. [Google Scholar] [CrossRef]
- De Boer, W.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Venterea, R.T.; Hyatt, C.R.; Rosen, C.J. Fertilizer management effects on nitrate leaching and indirect nitrous oxide emissions in irrigated potato production. J. Environ. Qual. 2011, 40, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, B.; Ziadi, N.; Rochette, P.; Chantigny, M.H.; Angers, D.A. Fertilizer source influenced nitrous oxide emissions from a clay soil under corn. Soil Sci. Soc. Am. J. 2011, 75, 595–604. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhu, T.B.; Cai, Z.C.; Qin, S.W.; Müller, C. Effects of long-term repeated mineral and organic fertilizer applications on soil nitrogen transformations. Eur. J. Soil Sci. 2012, 63, 75–85. [Google Scholar] [CrossRef]
- Bremner, J.M.; Blackmer, A.M. Effects of acetylene and soil water content on emission of nitrous oxide from soils. Nature 1979, 280, 380–381. [Google Scholar] [CrossRef]
- Khalil, M.I.; Baggs, E.M. CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol. Biochem. 2005, 37, 1785–1794. [Google Scholar] [CrossRef]
- Rychel, K.; Meurer, K.H.; Börjesson, G.; Strömgren, M.; Getahun, G.T.; Kirchmann, H.; Kätterer, T. Deep N fertilizer placement mitigated N2O emissions in a Swedish field trial with cereals. Nutr. Cycl. Agroecosyst. 2020, 118, 133–148. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Zhang, M.; Fan, P.; Ashraf, U.; Liu, H.; Chen, X.; Duan, M.; Tang, X.; Wang, Z.; et al. Deep placement of nitrogen fertilizer increases rice yield and nitrogen use efficiency with fewer greenhouse gas emissions in a mechanical direct-seeded cropping system. Crop J. 2021, 9, 1386–1396. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Brentrup, F.; Kusters, J.; Lammel, J.; Kuhlmann, H. Methods to estimate on-field nitrogen emissions from crop production as an input to LCA studies in the agricultural sector. Int. J. Life Cycle Assess. 2000, 5, 349–357. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Ciarlo, E.A.; Conti, M.E. Nitrous oxide emissions from soil during soybean [(Glycine max (L.) Merrill] crop phenological stages and stubbles decomposition period. Biol. Fertil. Soils 2008, 44, 581–588. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosyst. 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Lesschen, J.P.; Velthof, G.L.; De Vries, W.; Kros, J. Differentiation of nitrous oxide emission factors for agricultural soils. Environ. Pollut. 2011, 159, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Valentine, D.W.; Schimel, D.S.; Weier, K.; Kulmala, A.E. Generalized model for N2 and N2O production from nitrification and denitrification. Glob. Biogeochem. Cycles 1996, 10, 401–412. [Google Scholar] [CrossRef]
- Gaillard, R.; Duval, B.D.; Osterholz, W.R.; Kucharik, C.J. Simulated effects of soil texture on nitrous oxide emission factors from corn and soybean agroecosystems in Wisconsin. J. Environ. Qual. 2016, 45, 1540–1548. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Meurer, K.H.; Franko, U.; Stange, C.F.; Dalla Rosa, J.; Madari, B.E.; Jungkunst, H.F. Direct nitrous oxide (N2O) fluxes from soils under different land use in Brazil—A critical review. Environ. Res. Lett. 2016, 11, 23001. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Z.; Cai, Z.; Reverchon, F. Review of denitrification in tropical and subtropical soils of terrestrial ecosystems. J. Soils Sediments 2013, 13, 699–710. [Google Scholar] [CrossRef]
- Hefting, M.M.; Bobbink, R.; De Caluwe, H. Nitrous oxide emission and denitrification in chronically nitrate-loaded riparian buffer zones. J. Environ. Qual. 2003, 32, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Munir, H.; Nawaz, M.; Mahmood, A.; Usman, M.; Kharal, M. Alternate skip irrigation strategy ensure sustainable sugarcane yield. J. Anim. Plant Sci. 2017, 27, 1604–1610. [Google Scholar]
- Liu, S.; Zhang, Y.; Lin, F.; Zhang, L.; Zou, J. Methane and nitrous oxide emissions from direct-seeded and seedling-transplanted rice paddies in southeast China. Plant Soil 2014, 374, 285–297. [Google Scholar] [CrossRef]
- Naghedifar, S.M.; Ziaei, A.N.; Ansari, H. Simulation of irrigation return flow from a triticale farm under sprinkler and furrow irrigation systems using experimental data: A case study in arid region. Agric. Water Manag. 2018, 210, 185–197. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Lassaletta, L.; Aguilera, E.; del Prado, A.; Garnier, J.; Billen, G.; Iglesias, A.; Sánchez, B.; Guardia, G.; Abalos, D.; et al. Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: A review. Agric. Ecosyst. Environ. 2017, 238, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Niu, H.D.; Zhou, P.; Jia, R.J.; Gao, M. Effects of biochar on the net greenhouse gas emissions under continuous flooding and water-saving irrigation conditions in paddy soils. Sustainablity 2018, 10, 1403. [Google Scholar] [CrossRef] [Green Version]
- LaHue, G.T.; Chaney, R.L.; Adviento-Borbe, M.A.; Linquist, B.A. Alternate wetting and drying in high yielding direct-seeded rice systems accomplishes multiple environmental and agronomic objectives. Agric. Ecosyst. Environ. 2016, 229, 30–39. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Agnelli, A.E.; Linquist, B.; Adviento-Borbe, M.A.; Agnelli, A.; Gavina, G.; Ravaglia, S.; Ferrara, R.M. Alternate wetting and drying of rice reduced CH4 emissions but triggered N2O peaks in a clayey soil of Central Italy. Pedosphere 2016, 26, 533–548. [Google Scholar] [CrossRef]
- Hou, H.; Peng, S.; Xu, J.; Yang, S.; Mao, Z. Seasonal variations of CH4 and N2O emissions in response to water management of paddy fields located in Southeast China. Chemosphere 2012, 89, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Friedl, J.; De Rosa, D.; Rowlings, D.W.; Grace, P.R.; Müller, C.; Scheer, C. Dissimilatory nitrate reduction to ammonium (DNRA), not denitrification dominates nitrate reduction in subtropical pasture soils upon rewetting. Soil Biol. Biochem. 2018, 125, 340–349. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Singh, U.; Islam, S.M.M.; Huda, A.; Islam, M.R.; Sanabria, J.; Satter, M.A.; Islam, M.R.; Biswas, J.C.; Jahiruddin, M. Nitrous oxide and nitric oxide emissions and nitrogen use efficiency as affected by nitrogen placement in lowland rice fields. Nutr. Cycl. Agroecosyst. 2018, 110, 277–291. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, X.; Xie, B.; Liu, C.; Song, T.; Han, S.; Zhu, J. Nitric oxide emissions from rice-wheat rotation fields in eastern China: Effect of fertilization, soil water content, and crop residue. Plant Soil 2010, 336, 87–98. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Zhang, L.; Anderson, C.J.; Altor, A.E.; Hernández, M.E. Creating riverine wetlands: Ecological succession, nutrient retention, and pulsing effects. Ecol. Eng. 2005, 25, 510–527. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Zheng, X.; Wang, Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos. Environ. 2007, 41, 8030–8042. [Google Scholar] [CrossRef]
- Yang, W.; Kang, Y.; Feng, Z.; Gu, P.; Wen, H.; Liu, L.; Jia, Y. Sprinkler Irrigation Is Effective in Reducing Nitrous Oxide Emissions from a Potato Field in an Arid Region: A Two-Year Field Experiment. Atmosphere 2019, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Kang, Y.; Jiang, S. Effects of water application intensity, drop size and water application amount on the characteristics of topsoil pores under sprinkler irrigation. Agric. Water Manag. 2008, 95, 869–876. [Google Scholar] [CrossRef]
- Wang, X.J.; Wei, C.Z.; Zhang, J.; Dong, P.; Wang, J.; Zhu, Q.C.; Wang, J.X. Effects of irrigation mode and N application rate on cotton field fertilizer N use efficiency and N losses. J. Appl. Ecol. 2012, 23, 2751–2758. [Google Scholar]
- Lv, G.; Kang, Y.; Li, L.; Wan, S. Effect of irrigation methods on root development and profile soil water uptake in winter wheat. Irrig. Sci. 2010, 28, 387–398. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Vallejo, A.; Dick, J.; Skiba, U.M. The influence of soluble carbon and fertilizer nitrogen on nitric oxide and nitrous oxide emissions from two contrasting agricultural soils. Soil Biol. Biochem. 2008, 40, 142–151. [Google Scholar] [CrossRef]
- Sanchez-Martín, L.; Meijide, A.; Garcia-Torres, L.; Vallejo, A. Combination of drip irrigation and organic fertilizer for mitigating emissions of nitrogen oxides in semiarid climate. Agric. Ecosyst. Environ. 2010, 137, 99–107. [Google Scholar] [CrossRef]
- Maqsood, M.; Chattha, M.U.; Chattha, M.B.; Khan, I.; Fayyaz, M.A.; Hassan, M.U.; Zaman, Q.U.; Chattha, M.U. Influence of foliar applied potassium and deficit irrigation under different tillage systems on productivity of hybrid maize. Pak. J. Agric. Sci. 2017, 69, 317–322. [Google Scholar]
- Li, C.; Zhang, Z.; Guo, L.; Cai, M.; Cao, C. Emissions of CH4 and CO2 from double rice cropping systems under varying tillage and seeding methods. Atmos. Environ. 2013, 80, 438–444. [Google Scholar] [CrossRef]
- Sainju, U.M. Tillage, cropping sequence, and nitrogen fertilization influence dryland soil nitrogen. Agron. J. 2013, 105, 1253–1263. [Google Scholar] [CrossRef]
- Beare, M.H.; Gregorich, E.G.; St-Georges, P. Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biol. Biochem. 2009, 41, 611–621. [Google Scholar] [CrossRef]
- Xiao, X.P.; Wu, F.L.; Huang, F.Q.; Li, Y.; Sun, G.F.; Hu, Q.; He, Y.Y.; Chen, F.; Yang, G.L. Greenhouse air emission under different pattern of rice straw returned to field in double rice area. Res. Agric. Mod. 2007, 28, 629–632. [Google Scholar]
- Liang, W.; Shi, Y.; Zhang, H.; Yue, J.; Huang, G.H. Greenhouse gas emissions from northeast China rice fields in fallow season. Pedosphere 2007, 17, 630–638. [Google Scholar] [CrossRef]
- Mei, K.; Wang, Z.; Huang, H.; Zhang, C.; Shang, X.; Dahlgren, R.A.; Zhang, M.; Xia, F. Stimulation of N2O emission by conservation tillage management in agricultural lands: A meta-analysis. Soil Tillage Res. 2018, 182, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, F.; Zhou, X.; Xu, C.; Ji, L.; Chen, Z.; Fang, F. Impact of agronomy practices on the effects of reduced tillage systems on CH4 and N2O emissions from agricultural fields: A global meta-analysis. PLoS ONE 2018, 13, e0196703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurer, K.H.; Haddaway, N.R.; Bolinder, M.A.; Kätterer, T. Tillage intensity affects total SOC stocks in boreo-temperate regions only in the topsoil—A systematic review using an ESM approach. Earth-Sci. Rev. 2018, 177, 613–622. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Wuta, M.; Chirinda, N.; Mujuru, L.; Smith, J.L. Greenhouse gas emissions from intermittently flooded (dambo) rice under different tillage practices in chiota smallholder farming area of Zimbabwe. Atmos. Clim. Sci. 2013, 3, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Li, C.; Dai, G.; Zhan, M.; Wang, J.; Pan, S.; Cao, C. Greenhouse gas emission from direct seeding paddy field under different rice tillage systems in central China. Soil Tillage Res. 2009, 106, 54–61. [Google Scholar] [CrossRef]
- Lampurlanés, J.; Cantero-Martínez, C. Soil bulk density and penetration resistance under different tillage and crop management systems and their relationship with barley root growth. Agron. J. 2003, 95, 526–536. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Plaza-Bonilla, D.; Ramos, M.C.; Lampurlanés, J.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Long-term no-till as a means to maintain soil surface structure in an agroecosystem transformed into irrigation. Soil Tillage Res. 2017, 174, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Grandy, A.S.; Loecke, T.D.; Parr, S.; Robertson, G.P. Long-Term Trends in nitrous oxide emissions, soil nitrogen, and crop yields of till and no-till cropping systems. J. Environ. Qual. 2006, 35, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Ball, B.C.; Crichton, I.; Horgan, G.W. Dynamics of upward and downward N2O and CO2 fluxes in ploughed or no-tilled soils in relation to water-filled pore space, compaction and crop presence. Soil Tillage Res. 2008, 1, 20–30. [Google Scholar] [CrossRef]
- Elder, J.W.; Lal, R. Tillage effects on gaseous emissions from an intensively farmed organic soil in North Central Ohio. Soil Tillage Res. 2008, 98, 45–55. [Google Scholar] [CrossRef]
- Van Kessel, C.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; Van Groenigen, K.J. Climate, duration, and N placement determine N2O emissions in reduced tillage systems: A meta-analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Farooq, T.H.; Sahar, N.E.; Shahzad, S.M.; Altaf, M.M.; Ashraf, M. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total Environ. 2021, 750, 142299. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Dar, A.A.; Arif, M.S.; Farooq, T.H.; Yasmeen, T.; Shahzad, S.M.; Tufail, M.A.; Ahmed, W.; Albasher, G.; Ashraf, M. Do soil conservation practices exceed their relevance as a countermeasure to greenhouse gases emissions and increase crop productivity in agriculture? Sci. Total Environ. 2022, 805, 150337. [Google Scholar] [CrossRef]
- Memon, M.; Guo, J.; Tagar, A.; Perveen, N.; Ji, C.; Memon, S.; Memon, N. The effects of tillage and straw incorporation on soil organic carbon status, rice crop productivity, and sustainability in the rice-wheat cropping system of eastern China. Sustainability 2018, 10, 961. [Google Scholar] [CrossRef] [Green Version]
- Turmel, M.S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Gregorutti, V.C.; Caviglia, O.P. Nitrous oxide emission after the addition of organic residues on soil surface. Agric. Ecosyst. Environ. 2017, 246, 234–242. [Google Scholar] [CrossRef]
- Henderson, S.L.; Dandie, C.E.; Patten, C.L.; Zebarth, B.J.; Burton, D.L.; Trevors, J.T.; Goyer, C. Changes in denitrifier abundance, denitrification gene mRNA levels, nitrous oxide emissions, and denitrification in anoxic soil microcosms amended with glucose and plant residues. Appl. Environ. Microbiol. 2010, 76, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; Oswald, C. Closing the global N2O budget: Nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Shang, Q.; Yang, X.; Gao, C.; Wu, P.; Liu, J.; Xu, Y.; Shen, Q.; Zou, J.; Guo, S. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: A 3-year field measurement in long-term fertilizer experiments. Glob. Chang. Biol. 2011, 17, 2196–2210. [Google Scholar] [CrossRef]
- Xia, L.; Wang, S.; Yan, X. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice-wheat cropping system in China. Agric. Ecosyst. Environ. 2014, 197, 118–127. [Google Scholar] [CrossRef]
- Sander, B.O.; Samson, M.; Buresh, R.J. Methane and nitrous oxide emissions from flooded rice fields as affected by water and straw management between rice crops. Geoderma 2014, 235, 355–362. [Google Scholar] [CrossRef]
- Ma, E.; Zhang, G.; Ma, J.; Xu, H.; Cai, Z.; Yagi, K. Effects of rice straw returning methods on N2O emission during wheat-growing season. Nutr. Cycl. Agroecosyst. 2010, 88, 463–469. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Hu, N.; Chen, Q.; Zhu, L. The responses of soil N2O emissions to residue returning systems: A meta-analysis. Sustainability 2019, 11, 748. [Google Scholar] [CrossRef] [Green Version]
- Kudo, Y.; Noborio, K.; Shimoozono, N.; Kurihara, R. The effective water management practice for mitigating greenhouse gas emissions and maintaining rice yield in central Japan. Agric. Ecosyst. Environ. 2014, 186, 77–85. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Peng, Q.; Wu, L.; Van Zwieten, L.; Khalid, M.S.; Younas, A.; Lin, S.; Zhao, J.; Bashir, S.; et al. The interactive effects of dolomite application and straw incorporation on soil N2O emissions. Eur. J. Soil Sci. 2018, 69, 502–511. [Google Scholar] [CrossRef]
- Weitz, A.M.; Linder, E.; Frolking, S.; Crill, P.M.; Keller, M. N2O emissions from humid tropical agricultural soils: Effects of soil moisture, texture and nitrogen availability. Soil Biol. Biochem. 2001, 33, 1077–1093. [Google Scholar] [CrossRef]
- Burger, M.; Jackson, L.E. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 2003, 35, 29–36. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Rochette, P.; Angers, D.A.; Bittman, S.; Buckley, K.; Massé, D.; Bélanger, G.; Eriksen-Hamel, N.; Gasser, M.-O. Soil nitrous oxide emissions following band-incorporation of fertilizer nitrogen and swine manure. J. Environ. Qual. 2010, 39, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Pelster, D.E.; Chantigny, M.H.; Rochette, P.; Angers, D.A.; Rieux, C.; Vanasse, A. Nitrous oxide emissions respond differently to mineral and organic nitrogen sources in contrasting soil types. J. Environ. Qual. 2012, 41, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, F.; Shi, W. Plant material addition affects soil nitrous oxide production differently between aerobic and oxygen-limited conditions. Appl. Soil Ecol. 2013, 64, 91–98. [Google Scholar] [CrossRef]
- Duan, Y.F.; Kong, X.W.; Schramm, A.; Labouriau, R.; Eriksen, J.; Petersen, S.O. Microbial N transformations and N2O emission after simulated grassland cultivation: Effects of the nitrification inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Appl. Environ. Microbiol. 2017, 83, 0201916. [Google Scholar] [CrossRef] [Green Version]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; Hill, J.E.; Six, J.; van Kessel, C.; Linquist, B.A. Yield-scaled global warming potential of annual nitrous oxide and methane emissions from continuously flooded rice in response to nitrogen input. Agric. Ecosyst. Environ. 2013, 177, 10–20. [Google Scholar] [CrossRef]
- IPCC Guidelines for National Greenhouse Gas Inventories. Workbook. In IPCC Guidelines of National Greenhouse Gas Inventory; Institute for Global Environmental Strategies: Hayama, Japan, 1997; Volume 2. [Google Scholar]
- Huang, S.H.; Jiang, W.W.; Lu, J.; Cao, J.M. Influence of nitrogen and phosphorus fertilizers on N2O emissions in rice fields. Zhongguo Huanjing Kexue/China Environ. Sci. 2005, 25, 540–543. [Google Scholar]
- Li, C.F.; Kou, Z.K.; Yang, J.H.; Cai, M.L.; Wang, J.P.; Cao, C.G. Soil CO2 fluxes from direct seeding rice fields under two tillage practices in central China. Atmos. Environ. 2010, 44, 2696–2704. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, B.; Liu, J.; Li, D.; Yang, Y.; Zhang, K.; Jiang, Y.; Hu, Y.; Zeng, Z. Azolla planting reduces methane emission and nitrogen fertilizer application in double rice cropping system in southern China. Agron. Sustain. Dev. 2017, 37, 29. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Yang, H.; Zhao, Q.G. Research progress of soil organic carbon reserves and its impacting factors. Soil 2000, 1, 11–17, (In Chinese, with English abstract). [Google Scholar]
- Sharma, L.K.; Sukhwinder, K.B. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2018, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Owens, J.; Beres, B.; Li, Y.; Hao, X. Nitrous oxide emissions with enhanced efficiency and conventional urea fertilizers in winter wheat. Nutr. Cycl. Agroecosyst. 2021, 119, 307–322. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Rochette, P.; Burton, D.L.; Price, M. Effect of fertilizer nitrogen management on N2O emissions in commercial corn fields. Can. J. Soil Sci. 2008, 88, 189–195. [Google Scholar] [CrossRef]
- Sogbedji, J.M.; Es, H.M.; Yang, C.L.; Geohring, L.D.; Magdoff, F.R. Nitrate leaching and nitrogen budget as affected by maize nitrogen rate and soil type. J. Environ. Qual. 2000, 29, 1813–1820. [Google Scholar] [CrossRef]
- Randall, G.W.; Mulla, D.J. Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. J. Environ. Qual. 2001, 30, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner-Riddle, C.; Thurtell, G.W. Nitrous oxide emissions from agricultural fields during winter and spring thaw as affected by management practices. Nutr. Cycl. Agroecosyst. 1998, 52, 151–163. [Google Scholar] [CrossRef]
- Ghosh, S.; Majumdar, D.; Jain, M.C. Methane and nitrous oxide emissions from an irrigated rice of North India. Chemosphere 2003, 51, 181–195. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Dell, C.J.; Han, K.; Bryant, R.B.; Schmidt, J.P. Nitrous oxide emissions with enhanced efficiency nitrogen fertilizers in a rainfed system. Agron. J. 2014, 106, 723–731. [Google Scholar] [CrossRef]
- Nkebiwe, M.; Weinmann, M.; Bartal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crop Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Breitenbeck, G.A.; Bremner, J.M. Effects of rate and depth of fertilizer application on emission of nitrous oxide from soil fertilized with anhydrous ammonia. Biol. Fertil. Soils 1986, 2, 201–204. [Google Scholar] [CrossRef]
- Sehy, U.; Ruser, R.; Munch, J.C. Nitrous oxide fluxes from maize fields: Relationship to yield, site-specific fertilization, and soil conditions. Agric. Ecosyst. Environ. 2003, 99, 97–111. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P.; Conant, R. N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ. Res. Lett. 2013, 8, 015013. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Singh, U.; Huda, A.; Islam, S.M.M.; Islam, M.R.; Biswas, J.C.; DeWald, J. Nitrogen use efficiency, crop productivity and environmental impacts of urea deep placement in lowland rice fields. In Proceedings of the 2016 International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016; pp. 1–4. Available online: https://www.researchgate.net/profile/Yam-Gaihre/publication/312196435.pdf (accessed on 22 May 2021).
- Chatterjee, D.; Mohanty, S.; Guru, P.K.; Swain, C.K.; Tripathi, R.; Shahid, M.; Kumar, U.; Kumar, A.; Bhattacharyya, P.; Gautam, P.; et al. Comparative assessment of urea briquette applicators on greenhouse gas emission, N loss and soil enzymatic activities in tropical lowland rice. Agric. Ecosyst. Environ. 2018, 252, 178–190. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2006, 13, 1–17. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Szara, E.; Skowro’nska, M.; Jadczyszyn, T. Soil N2O emissions under conventional and reduced tillage methods and maize cultivation. Plant Soil Environ. 2017, 63, 242–347. [Google Scholar]
- Adviento-Borbe, M.A.A.; Linquist, B. Assessing fertilizer N placement on CH4 and N2O emissions in irrigated rice systems. Geoderma 2016, 266, 40–45. [Google Scholar] [CrossRef]
- Linquist, B.; Van Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Van Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Haigh, B.M. The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O emission, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical vertosols. Soil Res. 2016, 54, 604–618. [Google Scholar] [CrossRef] [Green Version]
- Grave, R.A.; Nicoloso, R.D.S.; Cassol, P.C.; da Silva, M.L.B.; Mezzari, M.P.; Aita, C.; Wuaden, C.R. Determining the effects of tillage and nitrogen sources on soil N2O emission. Soil Tillage Res. 2018, 175, 1–12. [Google Scholar] [CrossRef]
- Bordoloi, N.; Baruah, K.K.; Bhattacharyya, P. Emission estimation of nitrous oxide (N2O) from a wheat cropping system under varying tillage practices and different levels of nitrogen fertiliser. Soil Res. 2016, 54, 767–776. [Google Scholar] [CrossRef]
- Lebender, U.; Senbayram, M.; Lammel, J.; Kuhlmann, H. Impact of mineral N fertilizer application rates on N2O emissions from arable soils under winter wheat. Nutr. Cycl. Agroecosyst. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycl. 2002, 16, 1058. [Google Scholar] [CrossRef]
- Venterea, R.T.; Burger, M.; Spokas, K.A. Nitrogen oxide and methane emissions under varying tillage and fertilizer management. J. Environ. Qual. 2005, 34, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Rees, R.M.; Skiba, U.M.; Ball, B.C. Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agric. Ecosyst. Environ. 2007, 121, 74–83. [Google Scholar] [CrossRef]
- Watson, C.; Laughlin, R.; McGeough, K. Modification of nitrogen fertilisers using inhibitors: Opportunities and potentials for improving nitrogen use efficiency. Proc. Int. Fertil. Soc. 2009, 658, 1–40. [Google Scholar]
- Nayak, D.; Saetnan, E.; Cheng, K.; Wang, W.; Koslowski, F.; Cheng, Y.F.; Zhu, W.Y.; Wang, J.K.; Liu, J.X.; Moran, D.; et al. Management opportunities to mitigate greenhouse gas emissions from Chinese agriculture. Agric. Ecosyst. Environ. 2015, 209, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Kelliher, F.M.; Cox, N.; Van Der Weerden, T.J.; De Klein, C.A.M.; Luo, J.; Cameron, K.C.; Di, H.J.; Giltrap, D.; Rys, G. Statistical analysis of nitrous oxide emission factors from pastoral agriculture field trials conducted in New Zealand. Environ. Pollut. 2014, 186, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, S.L.; Pu, C.; Zhang, X.Q.; Xue, J.F.; Zhang, R.; Wang, Y.Q.; Lal, R.; Zhang, H.L.; Chen, F. Methane and nitrous oxide emissions under no-till farming in China: A meta-analysis. Glob. Chang. Biol. 2016, 22, 1372–1384. [Google Scholar] [CrossRef]
- Shakoor, A.; Xu, Y.; Wang, Q.; Chen, N.; He, F.; Zuo, H.; Yin, H.; Yan, X.; Ma, Y.; Yang, S. Effects of fertilizer application schemes and soil environmental factors on nitrous oxide emission fluxes in a rice-wheat cropping system, east China. PLoS ONE 2018, 13, e0202016. [Google Scholar] [CrossRef]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.M.; et al. Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils-a global meta-analysis. J. Clean. Prod. 2021, 278, 124019. [Google Scholar] [CrossRef]
- Parn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Jearveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Prentice, I.C.; Spahni, R.; Niu, H.S. Modelling terrestrial nitrous oxide emissions and implications for climate feedback. New Phytol. 2012, 196, 472–488. [Google Scholar]
- Müller, C.; Kammann, C.; Ottow, J.C.G.; Jeager, H.J. Nitrous oxide emission from frozen grassland soil and during thawing periods. J. Plant Nutr. Soil Sci. 2003, 166, 46–53. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Wu, F.; Jia, Z.; Wang, S.; Chang, S.X.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—The role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Gupta, D.K.; Gupta, C.K.; Dubey, R.; Fagodiya, R.K.; Sharma, G. Role of biochar in carbon sequestration and greenhouse gas mitigation. In Biochar Applications in Agriculture and Environment Management; Springer: Cham, Switzerland, 2020; pp. 141–165. [Google Scholar]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- He, L.; Xu, Y.; Li, J.; Zhang, Y.; Liu, Y.; Lyu, H.; Wang, Y.; Tang, X.; Wang, S.; Zhao, X.; et al. Biochar mitigated more N-related global warming potential in rice season than that in wheat season: An investigation from ten-year biochar-amended rice-wheat cropping system of China. Sci. Total Environ. 2022, 821, 153344. [Google Scholar] [CrossRef]
- Cornelissen, G.; Rutherford, D.W.; Arp, H.P.H.; Dörsch, P.; Kelly, C.N.; Rostad, C.E. Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ. Sci. Technol. 2013, 47, 7704–7712. [Google Scholar] [CrossRef]
- Bakken, L.R.; Bergaust, L.; Liu, B.; Frostegård, Å. Regulation of denitrification at the cellular level: A clue to the understanding of N2O emissions from soils. Philos. Trans. R. Soc. B 2012, 367, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of soil pH increase by biochar on NO, N2O and N2 production during denitrification in acid soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Downie, A.; Berger, E.; Rust, J.; Scheer, C. Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust. J. Soil Res. 2010, 48, 555–568. [Google Scholar] [CrossRef]
- Oo, A.Z.; Sudo, S.; Akiyama, H.; Win, K.T.; Shibata, A.; Yamamoto, A.; Sano, T.; Hirono, Y. Effect of dolomite and biochar addition on N2O and CO2 emissions from acidic tea field soil. PLoS ONE 2018, 13, e0192235. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Wang, H.; Xu, N.; Liu, H.; Zhai, L. Biochar amendment with fertilizers increases peanut N uptake, alleviates soil N2O emissions without affecting NH3 volatilization in field experiments. Environ. Sci. Pollut. Res. 2018, 25, 8817–8826. [Google Scholar] [CrossRef]
- Cheng, Q.; Cheng, H.; Wu, Z.; Pu, X.; Lu, L.; Wang, J.; Zhao, J.; Zheng, A. Biochar amendment and Calamagrostis angustifolia planting affect sources and production pathways of N2O in agricultural ditch systems. Environ. Sci. Process. Impacts 2019, 21, 727–737. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Cai, Y.; Fu, S.; Luo, Y.; Wang, H.; Liang, C.; Lin, Z.; Hu, S.; Li, Y.; et al. Biochar decreases soil N2O emissions in Moso bamboo plantations through decreasing labile N concentrations, N-cycling enzyme activities and nitrification/denitrification rates. Geoderma 2019, 348, 135–145. [Google Scholar] [CrossRef]
- Aamer, M.; Shaaban, M.; Hassan, M.U.; Guoqin, H.; Ying, L.; Hai Ying, T.; Rasul, F.; Qiaoying, M.; Zhuanling, L.; Rasheed, A.; et al. Biochar mitigates the N2O emissions from acidic soil by increasing the nosZ and nirK gene abundance and soil pH. J. Environ. Manag. 2020, 255, 109891. [Google Scholar] [CrossRef]
- Aamer, M.; Shaaban, M.; Hassan, M.U.; Ying, L.; Haiying, T.; Qiaoying, M.; Munir, H.; Rasheed, A.; Xinmei, L.; Ping, L. N2O Emissions mitigation in acidic soil following biochar application under different moisture regimes. J. Soil Sci. Plant Nutr. 2020, 20, 2454–2464. [Google Scholar] [CrossRef]
- Barracosa, P.; Cardoso, I.; Marques, F.; Pinto, A.; Oliveira, J.; Trindade, H.; Rodrigues, P.; Pereira, J.L.S. Effect of biochar on emission of greenhouse gases and productivity of cardoon crop (Cynara cardunculus L.). J. Soil Sci. Plant Nutr. 2020, 20, 1524–1531. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Zhang, A.; Rahaman, M.A.; Yang, Z. Inhibited effect of biochar application on N2O emissions is amount and time-dependent by regulating denitrification in a wheat-maize rotation system in North China. Sci. Total Environ. 2020, 721, 137636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, Y.; Hu, R.; Xu, X.; Xian, J.; Yang, Y.; Liu, L.; Cheng, Z. Effect of rice straw and swine manure biochar on N2O emission from paddy soil. Sci. Rep. 2020, 10, 10843. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.S.; Carranca, C.; Coutinho, J.; Trindade, H. The effect of soil type on gaseous emissions from flooded rice fields in Portugal. J. Soil Sci. Plant Nutr. 2020, 20, 1732–1740. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fertil. Soils 2013, 50, 321–332. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.; Hu, R.; Lin, S.; Wu, Y.; Ullah, B.; Zhao, J.; Liu, S.; Li, Y. Dissolved organic carbon and nitrogen mineralization strongly affect CO2 emissions following lime application to acidic soil. J. Chem. Soc. Pak. 2015, 36, 875–879. [Google Scholar]
- Feng, K.; Yan, F.; Hütsch, B.W.; Schubert, S. Nitrous oxide emission as affected by liming an acidic mineral soil used for arable agriculture. Nutr. Cycl. Agroecosyst. 2003, 13, 289–298. [Google Scholar] [CrossRef]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, F.; Altaf, M.M.; Ahmed, W.; Tufail, M.A.; Ashraf, M. Does biochar accelerate the mitigation of greenhouse gaseous emissions from agricultural soil?—A global meta-analysis. Environ. Res. 2021, 202, 111789. [Google Scholar] [CrossRef]
- Yamulki, S.; Harrison, R.M.; Goulding, K.W.T.; Webster, C.P. N2O, NO and NO2 fluxes from a grassland: Effect of soil pH. Soil Biol. Biochem. 1997, 29, 1199–1208. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.; Almøy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, M.; Peng, Q.; Lin, S.; Wu, Y.; Zhao, J.; Hu, R. Nitrous Oxide emission from two acidic soils as affected by dolomite application. Soil Res. 2014, 52, 841–848. [Google Scholar] [CrossRef]
- Senbayram, M.; Budai, A.; Bol, R.; Chadwick, D.; Marton, L.; Gündogan, R.; Wu, D. Soil NO3− level and O2 availability are key factors in controlling N2O reduction to N2 following long-term liming of an acidic sandy soil. Soil Biol. Biochem. 2019, 132, 165–173. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Wu, L.; Hu, R.; Younas, A.; Nunez-Delgado, A.; Xu, P.; Sun, Z.; Lin, S.; Xu, X.; et al. The effects of pH change through liming on soil N2O emissions. Processes 2020, 8, 702. [Google Scholar] [CrossRef]
- Hussain, S.; Peng, S.; Fahad, S.; Khaliq, A.; Huang, J.; Cui, K.; Nie, L. Rice management interventions to mitigate greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 3342–3360. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Zhang, H.; Han, Y.; Deng, O.; Tang, X.; Luo, L.; Zeng, J.; Chen, G.; Wang, C.; Gao, X. Regulating CH4, N2O, and NO emissions from an alkaline paddy field under rice–wheat rotation with controlled release N fertilizer. Environ. Sci. Pollut. Res. 2021, 28, 18246–18259. [Google Scholar] [CrossRef]
- Huérfano, X.; Fuertes-Mendizábal, T.; Fernández-Diez, K.; Estavillo, J.M.; González-Murua, C.; Menéndez, S. The new nitrification inhibitor 3,4-dimethylpyrazole succinic (DMPSA) as an alternative to DMPP for reducing N2O emissions from wheat crops under humid Mediterranean conditions. Eur. J. Agron. 2016, 80, 78–87. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdenas, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Tenuta, M.; Beauchamp, E.G. Nitrous oxide production from granular nitrogen fertilizers applied to a silt loam soil. Can. J. Soil Sci. 2003, 83, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, J.; et al. Climate Change 2007: Changes in Atmospheric Constituents and in Radiative Forcing: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ar4-wg1-chapter2-1.pdf (accessed on 11 May 2021).
- Zebarth, B.J.; Snowdon, E.; Burton, D.L.; Goyer, C.; Dowbenko, R. Controlled release fertilizer product effects on potato crop response and nitrous oxide emissions under rain-fed production on a medium-textured soil. Can. J. Soil Sci. 2012, 92, 759–769. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: Summary of available data. Soil Sci. Plant Nutr. 2006, 52, 774–787. [Google Scholar] [CrossRef]
- Zekri, M.; Koo, R.C.J. Evaluation of controlled-release fertilizers for young citrus trees. J. Am. Soc. Hortic. Sci. 1991, 116, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Liu, G.; Ma, J.; Xu, H.; Yagi, K. Effect of controlled-release fertilizer on nitrous oxide emission from a winter wheat field. Nutr. Cycl. Agroecosyst. 2012, 94, 111–122. [Google Scholar] [CrossRef]
- Jarosiewicz, A.; Tomaszewska, M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003, 51, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, G.; Ma, J.; Zhang, G.; Xu, H.; Yagi, K. Effect of controlled-release fertilizer on mitigation of N2O emission from paddy field in South China: A multi-year field observation. Plant Soil 2013, 371, 473–486. [Google Scholar] [CrossRef]
- Nawaz, M.; Chattha, M.U.; Chattha, M.B.; Ahmad, R.; Munir, H.; Usman, M.; Hassan, M.U.; Khan, S.; Kharal, M. Assessment of compost as nutrient supplement for spring planted sugarcane (Saccharum officinarum L.). J. Anim. Plant Sci. 2017, 27, 283–293. [Google Scholar]
- Nawaz, M.; Khan, S.; Ali, H.; Ijaz, M.; Chattha, M.U.; Hassan, M.U.; Irshad, S.; Hussain, S.; Khan, S. Assessment of Environment-friendly Usage of Spent Wash and its Nutritional Potential for Sugarcane Production. Commun. Soil Sci. Plant Anal. 2019, 50, 1239–1249. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Feng, H.; Liu, J.; Si, B.; Zhang, A.; Chen, J.; Cheng, G.; Sun, B.; Pi, X.; et al. Effects of straw and plastic film mulching on greenhouse gas emissions in Loess Plateau, China: A field study of 2 consecutive wheat-maize rotation cycles. Sci. Total Environ. 2017, 579, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Fan, F.; Yin, C.; Song, A.; Huang, P.; Tang, Y.; Zhu, P.; Peng, C.; Li, T.; Wakelin, S.A.; et al. Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol. Biochem. 2016, 93, 131–141. [Google Scholar] [CrossRef]
- Wei, D.; Xu-Ri; Wang, Y.; Wang, Y.; Liu, Y.; Yao, T. Responses of CO2, CH4 and N2O fluxes to livestock exclosure in an alpine steppe on the Tibetan Plateau, China. Plant Soil 2012, 359, 45–55. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Baggs, E.M. Do combined applications of crop residues and inorganic fertilizer lower emission of N2O from soil? Soil Use Manag. 2010, 26, 412–424. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Huygens, D.; Boeckx, P.; Kuyper, T.W.; Lubbers, I.M.; Rütting, T.; Groffman, P.M. The soil n cycle: New insights and key challenges. Soil 2015, 1, 235–256. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Arora, P.; Tomer, R.; Mishra, S.V.; Bhatia, A.; Pathak, H.; Chakraborty, D.; Kumar, V.; Dubey, D.S.; Harit, R.C.; et al. Greenhouse gases emission from soils under major crops in Northwest India. Sci. Total Environ. 2016, 542, 551–561. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, M.; Liu, J.; Hao, Y.; Zhou, Y.; Qu, D.; Yang, X. Trade-off between soil organic carbon sequestration and nitrous oxide emissions from winter wheat-summer maize rotations: Implications of a 25-year fertilization experiment in Northwestern China. Sci. Total Environ. 2017, 595, 371–379. [Google Scholar] [CrossRef]
- Wassmann, R.; Lantin, R.S.; Neue, H.U.; Buendia, L.V.; Corton, T.M.; Lu, Y. Characterization of methane emissions from rice fields in Asia. III. Mitigation options and future research needs. Nutr. Cycl. Agroecosyst. 2000, 58, 23–36. [Google Scholar] [CrossRef]
- Corton, T.M.; Bajita, J.B.; Grospe, F.S.; Pamplona, R.R.; Asis, C.A.; Wassmann, R.; Lantin, R.S.; Buendia, L.V. Methane emission from irrigated and intensively managed rice fields in Central Luzon (Philippines). Nutr. Cycl. Agroecosyst. 2000, 58, 37–53. [Google Scholar] [CrossRef]
- Wassmann, R.; Buendia, L.V.; Lantin, R.S.; Bueno, C.S.; Lubigan, L.A.; Umali, A.; Nocon, N.N.; Javellana, A.M.; Neue, H.U. Mechanisms of crop management impact on methane emissions from rice fields in Los Baños, Philippines. Nutr. Cycl. Agroecosyst. 2000, 58, 107–119. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Chattha, M.B.; Khan, I.; Usman, M.; Ali, A.; Nawaz, M. Composted sugarcane by-product press mud cake supports wheat growth and improves soil properties. Int. J. Plant Prod. 2019, 13, 241–249. [Google Scholar] [CrossRef]
- Grigatti, M.; Barbanti, L.; Hassan, M.U.; Ciavatta, C. Fertilizing potential and CO2 emissions following the utilization of fresh and composted food-waste anaerobic digestates. Sci. Total Environ. 2020, 698, 134198. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.; Ge, Y.; Fahmy, A.E. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.; Bouvier, C.; Bettarel, Y.; Bouvier, T.; Henry-des-Tureaux, T.; Janeau, J.L.; Lamballe, P.; Van Nguyen, B.; Jouquet, P. Influence of buffalo manure, compost, vermicompost and biochar amendments on bacterial and viral communities in soil and adjacent aquatic systems. Appl. Soil Ecol. 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-Des-Tureaux, T.; Rumpel, C.; Janeau, J.L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Rodriguez, V.; de los Angeles Valdez-Perez, M.; Luna-Guido, M.; Ceballos-Ramirez, J.M.; Franco-Hernández, O.; van Cleemput, O.; Marsch, R.; Thalasso, F.; Dendooven, L. Emission of nitrous oxide and carbon dioxide and dynamics of mineral N in wastewater sludge, vermicompost or inorganic fertilizer amended soil at different water contents: A laboratory study. Appl. Soil Ecol. 2011, 49, 263–267. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef] [Green Version]
- Nigussie, A.; Kuyper, T.W.; Bruun, S.; De-Neergaard, A. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J. Clean. Prod. 2016, 139, 429–439. [Google Scholar] [CrossRef]
- Di, W.U.; Yanfang, F.; Lihong, X.; Manqiang, L.I.U.; Bei, Y.; Feng, H.U.; Linzhang, Y. Biochar combined with vermicompost increases crop production while reducing ammonia and nitrous oxide emissions from a paddy soil. Pedosphere 2019, 29, 82–94. [Google Scholar]
- Kool, D.M.; Van Groenigen, J.W.; Wrage, N. Source determination of nitrous oxide based on nitrogen and oxygen isotope tracing, dealing with oxygen exchange. Methods Enzymol. 2011, 496, 139–160. [Google Scholar] [PubMed]
- Ostrom, N.E.; Ostrom, P.H. The isotopomers of nitrous oxide, analytical considerations and application to resolution of microbial production pathways. In Handbook of Environmental Isotope Geochemistry; Baskaran, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 453–476. [Google Scholar]
- Hino, T.; Matsumoto, Y.; Nagano, S.; Sugimoto, H.; Fukumori, Y.; Murata, T.; Iwata, S.; Shiro, Y. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 2010, 330, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhl, L.; van der Heijden, M.G.A. Arbuscular mycorrhizal fungal species differ in their effect on nutrient leaching. Soil Biol. Biochem. 2016, 94, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2014, 386, 1–19. [Google Scholar] [CrossRef]
- Storer, K.; Coggan, A.; Ineson, P.; Hodge, A. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol. 2018, 220, 1285–1295. [Google Scholar] [CrossRef] [Green Version]
- Bender, S.F.; Plantenga, F.; Neftel, A.; Jocher, M.; Oberholzer, H.R.; Köhl, L.; Giles, M.; Daniell, T.J.; Van Der Heijden, M.G.A. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J. 2014, 8, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Ma, F.; Shan, D. Effects of arbuscular mycorrhizal fungi on N2O emissions from rice paddies. Water Air Soil Pollut. 2015, 226, 222. [Google Scholar] [CrossRef]
- Thomas, B.W.; Hao, X.; Larney, F.J.; Goyer, C.; Chantigny, M.H.; Charles, A. Nonlegume cover crops can increase non-growing season nitrous oxide emissions. Soil Sci. Soc. Am. J. 2017, 81, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Dungan, R.S.; Leytem, A.B.; Tarkalson, D.D. Greenhouse gas emissions from an irrigated cropping rotation with dairy manure utilization in a semiarid climate. Agron. J. 2021, 113, 1222–1237. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant. Cell Environ. 2016, 39, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Sahi, S.T. Performance of sorghum cultivars for biomass quality and biomethane yield grown in semi-arid area of Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 12800–12807. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Barbanti, L.; Chattha, M.B.; Mahmood, A.; Khan, I.; Nawaz, M. Combined cultivar and harvest time to enhance biomass and methane yield in sorghum under warm dry conditions in Pakistan. Ind. Crops Prod. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Chattha, M.B.; Mahmood, A.; Sahi, S.T. Chemical composition and methane yield of sorghum as influenced by planting methods and cultivars. J. Anim. Plant Sci. 2019, 29, 251–259. [Google Scholar]
- Hassan, M.U.; Chattha, M.U.; Barbanti, L.; Mahmood, A.; Chattha, M.B.; Khan, I.; Mirza, S.; Aziz, S.A.; Nawaz, M.; Aamer, M. Cultivar and seeding time role in sorghum to optimize biomass and methane yield under warm dry climate. Ind. Crops Prod. 2020, 145, 111983. [Google Scholar] [CrossRef]
- Lou, Y.; Inubushi, K.; Mizuno, T.; Hasegawa, T.; Lin, Y.; Sakai, H.; Cheng, W.; Kobayashi, K. CH4 emission with differences in atmospheric CO2 enrichment and rice cultivars in a Japanese paddy soil. Glob. Chang. Biol. 2008, 14, 2678–2687. [Google Scholar] [CrossRef]
- Abalos, D.; van Groenigen, J.W.; De Deyn, G.B. What plant functional traits can reduce nitrous oxide emissions from intensively managed grasslands? Glob. Chang. Biol. 2018, 24, 248–258. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Han, S.; Wang, Y.; Chen, G. N2O emissions by trees under natural conditions. Environ. Sci. 2001, 22, 7–11. [Google Scholar]
- Ferch, N.J.; Römheld, V. Release of water-dissolved nitrous oxide by plants: Does the transpiration water flow contribute to the emission of dissolved N2O by sunflower? In Proceedings of the 14th International Plant Nutrition Colloqium, Beijing, China, 14–17 September 2001; pp. 228–229. [Google Scholar]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Nùñez, J.; Arenas, L.; Rao, I.; Trujillo, C.; Alvarez, C.; Arango, J.; Rasche, F.; Chirinda, N. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 2017, 107, 156–163. [Google Scholar] [CrossRef]
- Pappa, V.A.; Rees, R.M.; Walker, R.L.; Baddeley, J.A.; Watson, C.A. Nitrous oxide emissions and nitrate leaching in an arable rotation resulting from the presence of an intercrop. Agric. Ecosyst. Environ. 2011, 141, 153–161. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Sun, W.; Zheng, X.; Wang, Y. Contribution of plants to N2O emissions in soil-winter wheat ecosystem: Pot and field experiments. Plant Soil 2005, 269, 205–211. [Google Scholar] [CrossRef]
- Hou, A.X.; Chen, G.X.; Wang, Z.P.; Van Cleemput, O.; Patrick, W.H. Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Sci. Soc. Am. J. 2000, 64, 2180–2186. [Google Scholar] [CrossRef]
- Pathak, H.; Chakrabarti, B.; Bhatia, A.; Jain, N.; Aggarwal, P.K. Potential and cost of low carbon technologies in rice and wheat systems: A case study for the Indo-Gangetic Plains. In Low Carbon Technologies for Agriculture: A Study on Rice and Wheat Systems in the Indo-Gangetic Plains; Pathak, H., Aggarwal, P.K., Eds.; Indian Agricultural Research Institute: New Delhi, India, 2012; pp. 12–40. [Google Scholar]
- Wegner, B.R.; Chalise, K.S.; Singh, S.; Lai, L.; Abagandura, G.O.; Kumar, S.; Osborne, S.L.; Lehman, R.M.; Jagadamma, S. Response of soil surface greenhouse gas fluxes to crop residue removal and cover crops under a corn–soybean rotation. J. Environ. Qual. 2018, 47, 1146–1154. [Google Scholar] [CrossRef]
- Lehman, R.M.; Osborne, S.L.; Duke, S.E. Diversified no-till crop rotation reduces nitrous oxide emissions, increases soybean yields, and promotes soil carbon accrual. Soil Sci. Soc. Am. J. 2017, 81, 76–83. [Google Scholar] [CrossRef]
- Behnke, G.D.; Zuber, S.M.; Pittelkow, C.M.; Nafziger, E.D.; Villamil, M.B. Long-term crop rotation and tillage effects on soil greenhouse gas emissions and crop production in Illinois, USA. Agric. Ecosyst. Environ. 2018, 261, 62–70. [Google Scholar] [CrossRef]
- Omonode, R.A.; Smith, D.R.; Gál, A.; Vyn, T.J. Soil nitrous oxide emissions in corn following three decades of tillage and rotation treatments. Soil Sci. Soc. Am. J. 2011, 75, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Barton, L.; Murphy, D.V.; Butterbach-Bahl, K. Influence of crop rotation and liming on greenhouse gas emissions from a semi-arid soil. Agric. Ecosyst. Environ. 2013, 167, 23–32. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.M.; Reynolds, W.D.; McLaughlin, N.B. Nitrous oxide and carbon dioxide emissions from monoculture and rotational cropping of corn, soybean and winter wheat. Can. J. Soil Sci. 2008, 88, 163–174. [Google Scholar] [CrossRef]
- Graham, R.F.; Wortman, S.E.; Pittelkow, C.M. Comparison of organic and integrated nutrient management strategies for reducing soil N2O emissions. Sustainability 2017, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Luo, J.; Li, J.; Yu, H.; Fan, J.; Liu, D. Effect of long-term compost and inorganic fertilizer application on background N2O and fertilizer-induced N2O emissions from an intensively cultivated soil. Sci. Total Environ. 2013, 465, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ding, W.; Luo, J. Nitrous oxide emissions from Chinese maize-wheat rotation systems: A 3-year field measurement. Atmos. Environ. 2013, 65, 112–122. [Google Scholar] [CrossRef]
- He, W.; Dutta, B.; Grant, B.B.; Chantigny, M.H.; Hunt, D.; Bittman, S.; Tenuta, M.; Worth, D.; VanderZaag, A.; Desjardins, R.L.; et al. Assessing the effects of manure application rate and timing on nitrous oxide emissions from managed grasslands under contrasting climate in Canada. Sci. Total Environ. 2020, 716, 135374. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Wang, R.; Liu, C.; Lin, S.; Butterbach-Bahl, K. Benefits of integrated nutrient management on N2O and NO mitigations in water-saving ground cover rice production systems. Sci. Total Environ. 2019, 646, 1155–1163. [Google Scholar]
- Dittert, K.; Lampe, C.; Gasche, R.; Butterbach-Bahl, K.; Wachendorf, M.; Papen, H.; Sattelmacher, B.; Taube, F. Short-term effects of single or combined application of mineral N fertilizer and cattle slurry on the fluxes of radiatively active trace gases from grassland soil. Soil Biol. Biochem. 2005, 37, 1665–1674. [Google Scholar] [CrossRef]
- Meng, L.; Ding, W.; Cai, Z. Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol. Biochem. 2005, 37, 2037–2045. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Wuta, M.; Nyamangara, J.; Smith, J.L.; Rees, R.M. Nitrous oxide and methane emissions from cultivated seasonal wetland (dambo) soils with inorganic, organic and integrated nutrient management. Nutr. Cycl. Agroecosyst. 2014, 100, 161–175. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Shi, Y.; Chirinda, N.; Olesen, J.E.; Mapanda, F.; Wuta, M.; Wu, W.; Meng, F.; Oelofse, M.; De Neergaard, A.; et al. Combining organic and inorganic nitrogen fertilisation reduces N2O emissions from cereal crops: A comparative analysis of China and Zimbabwe. Mitig. Adap. Strateg. Glob. Chang. 2017, 22, 233–245. [Google Scholar]
- Dhadli, H.S.; Brar, B.S.; Black, T.A. N2O emissions in a long-term soil fertility experiment under maize–wheat cropping system in Northern India. Geoderma Reg. 2016, 7, 102–109. [Google Scholar] [CrossRef]
- Das, S.; Adhya, T.K. Effect of combine application of organic manure and inorganic fertilizer on methane and nitrous oxide emissions from a tropical flooded soil planted to rice. Geoderma 2014, 213, 185–192. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Budai, A.; Bakken, L.; Dittert, K. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012, 147, 4–12. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSwiney, C.P.; Robertson, G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob. Chang. Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]
- Sarkodie-Addo, J.; Lee, H.C.; Baggs, E.M. Nitrous oxide emissions after application of inorganic fertilizer and incorporation of green manure residues. Soil Use Manag. 2006, 19, 331–339. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, X.; Zhang, Q.; Wei, Q.; Liu, X.; Deng, W.; Wang, J.; Yang, W.; Deng, B.; Zhang, L. Biochar derived from spent mushroom substrate reduced N2O emissions with lower water content but increased CH4 emissions under flooded condition from fertilized soils in Camellia oleifera plantations. Chemosphere 2022, 287, 132110. [Google Scholar] [CrossRef]
- Deng, B.; Yuan, X.; Siemann, E.; Wang, S.; Fang, H.; Wang, B.; Gao, Y.; Shad, N.; Liu, X.; Zhang, W.; et al. Feedstock particle size and pyrolysis temperature regulate effects of biochar on soil nitrous oxide and carbon dioxide emissions. Waste Manag. 2021, 120, 33–40. [Google Scholar] [CrossRef]
- Xu, X.; He, C.; Yuan, X.; Zhang, Q.; Wang, S.; Wang, B.; Guo, X.; Zhang, L. Rice straw biochar mitigated more N2O emissions from fertilized paddy soil with higher water content than that derived from ex situ biowaste. Environ. Poll. 2020, 263, 114477. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Shi, Y.; Zhang, L.; Fang, H.; Gao, Y.; Luo, L.; Feng, W.; Hu, X.; Wan, S.; Huang, W.; et al. Effects of spent mushroom substrate-derived biochar on soil CO2 and N2O emissions depend on pyrolysis temperature. Chemosphere 2020, 246, 125608. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Fang, H.; Jiang, N.; Feng, W.; Luo, L.; Wang, J.; Wang, H.; Hu, D.; Guo, X.; Zhang, L. Biochar is comparable to dicyandiamide in the mitigation of nitrous oxide emissions from Camellia oleifera Abel. fields. Forests 2019, 10, 1076. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.L.; Wang, S.L.; Xu, X.T.; Wang, H.; Hu, D.N.; Guo, X.M.; Shi, Q.H.; Siemann, E.; Zhang, L. Effects of biochar and dicyandiamide combination on nitrous oxide emissions from Camellia oleifera field soil. Environ. Sci. Poll. Res. 2019, 26, 4070–4077. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.W. A Primer on Environmental Policy Design; Routledge: London, UK, 2001; pp. 1–35. [Google Scholar]
- Sterner, T. Policy Instruments for Environmental and Natural Resource Management; Resources for the Future Press: Washington, DC, USA, 2003. [Google Scholar]
- UNFCCC. The Kyoto Protocol to the United Nations Framework Convention on Climate Change; done at COP3 on 11 December 1997, at Kyoto; UNFCCC: Kyoto, Japan, 1997. [Google Scholar]
- Oreggioni, G.D.; Ferraio, F.M.; Crippa, M.; Muntean, M.; Schaaf, E.; Guizzardi, D.; Solazzo, E.; Duerr, M.; Perry, M.; Vignati, E. Climate change in a changing world: Socio-economic and technological transitions, regulatory frameworks and trends on global greenhouse gas emissions from EDGAR v. 5.0. Glob. Environ. Chang. 2021, 70, 102350. [Google Scholar] [CrossRef]
- EC (European Commission). Communication from the Commission to the European Parliament, the European Council, the Council, the Council, the European Economic and Social Committee and the Committee of the Regions: Stepping up Europe’s 2030 Climate Ambition. Investing in a Climate-Neutral Future for the Benefit of Our People. 2020. Available online: https://ec.europa.eu/clima/policies/eu-climate-action/2030_ctp_en (accessed on 3 March 2022).
- House of Commons. The Climate Change Act 2008 (2050 Target Amendment) Order 2019. 2019. Available online: https://www.legislation.gov.uk/ukdsi/2019/9780111187654/pdfs/ukdsi_9780111187654_en.pdf (accessed on 3 March 2022).
- WB. Data of Industry, Value Added (% of GDP). 2020. Available online: https://data.worldbank.org/indicator/nv.ind.totl.zs (accessed on 3 March 2022).
- UNDP. Population Statistics, World Population Prospects (WPP), the 2019 Revision Report United Nations; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- BP. British Petroleum Statistics of World Energy. 2019. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 3 March 2022).
- Lapillonne, B.; Sudries, L. Energy Efficiency Trends in the EU: Have We Got off Track? Presentation Given at the Odyssee-Mure Webinar Series on Energy Efficiency Organised by Leonardo ENERGY June 25th 2020. 2020. Available online: https://www.odyssee-mure.eu/events/webinar/energy-efficiency-trends-webinar-june-2020.pdf (accessed on 3 March 2022).
- UNFCCC. Doha Amendment to the Kyoto Protocol. 2012. Available online: https://treaties.un.org/doc/Publication/CN/2012/CN.718.2012-Eng.pdf (accessed on 24 January 2020).
- Zelazna, A.; Bojar, M.; Bojar, E. Corporate Social Responsibility towards the Environment in Lublin Region, Poland: A comparative study of 2009 and 2019. Sustainability 2020, 12, 4463. [Google Scholar] [CrossRef]
- Tran, K.T.; Nguyen, P.V. Corporate Social Responsibility: Findings from the Vietnamese Paint Industry. Sustainability 2020, 12, 1044. [Google Scholar] [CrossRef] [Green Version]
- Novelli, V.; Geatti, P.; Bianco, F.; Ceccon, L.; Del Frate, S.; Badin, P. The EMAS registration of the livenza furniture district in the province of Pordenone (Italy). Sustainability 2020, 12, 898. [Google Scholar] [CrossRef] [Green Version]
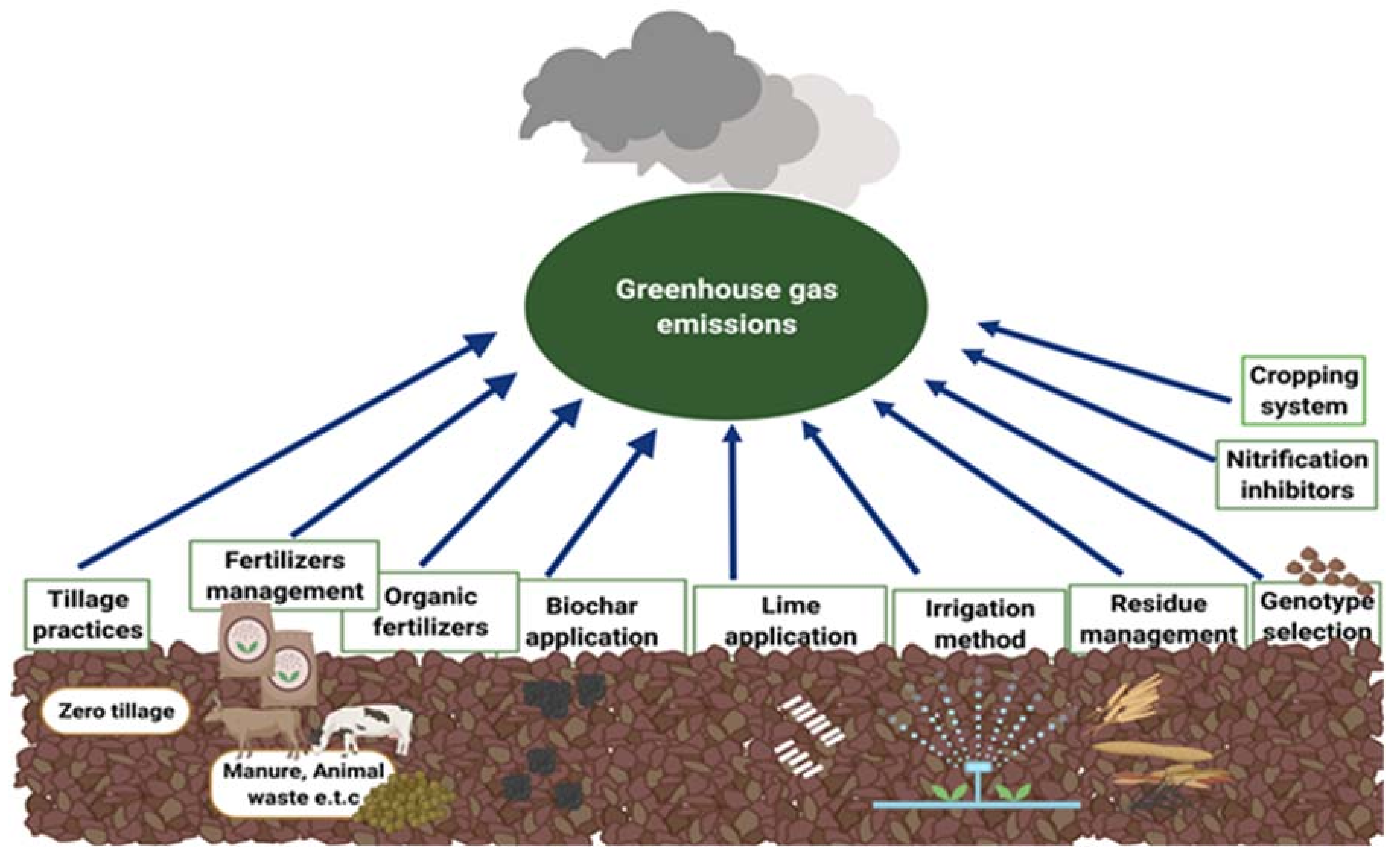
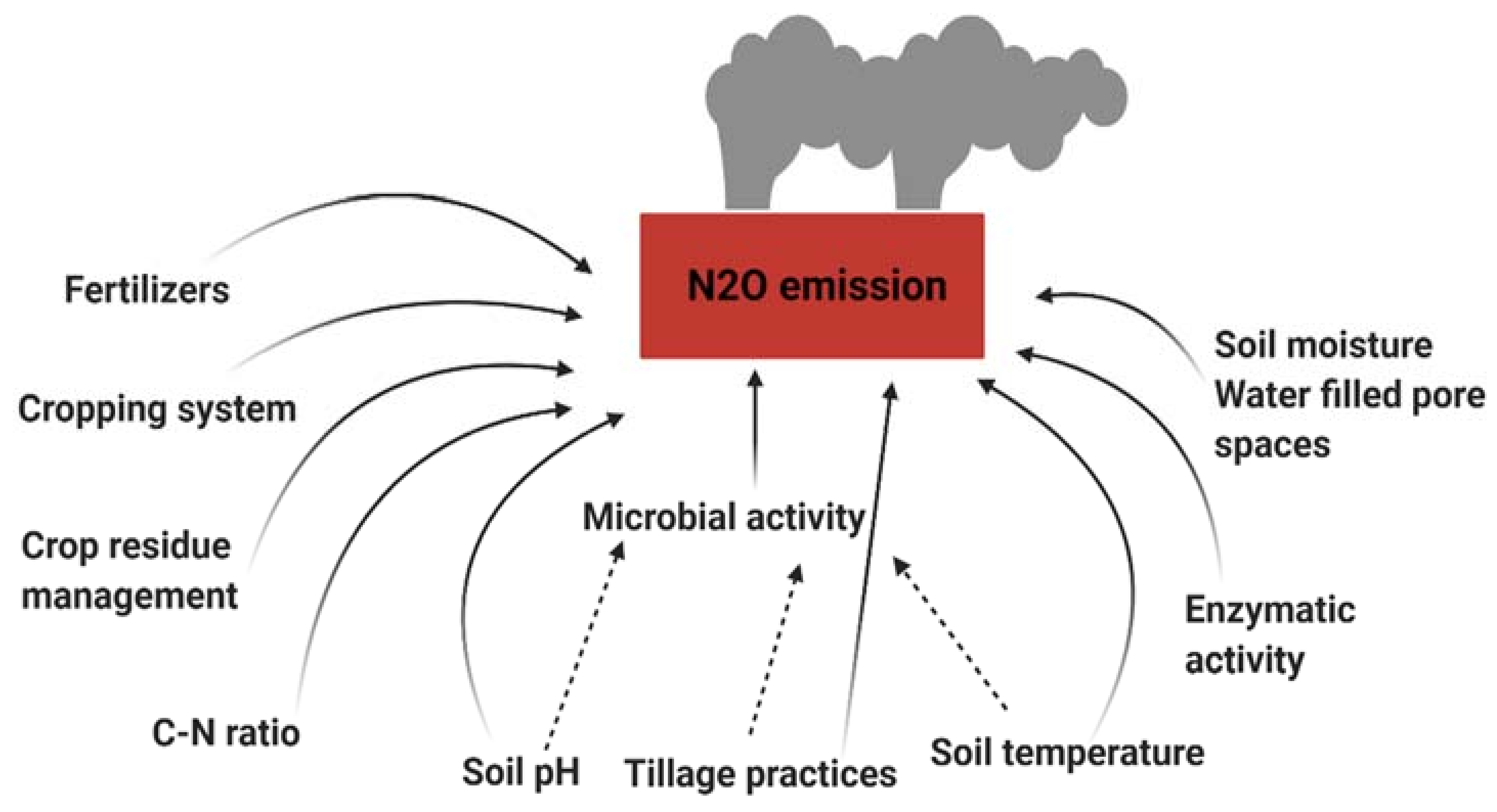
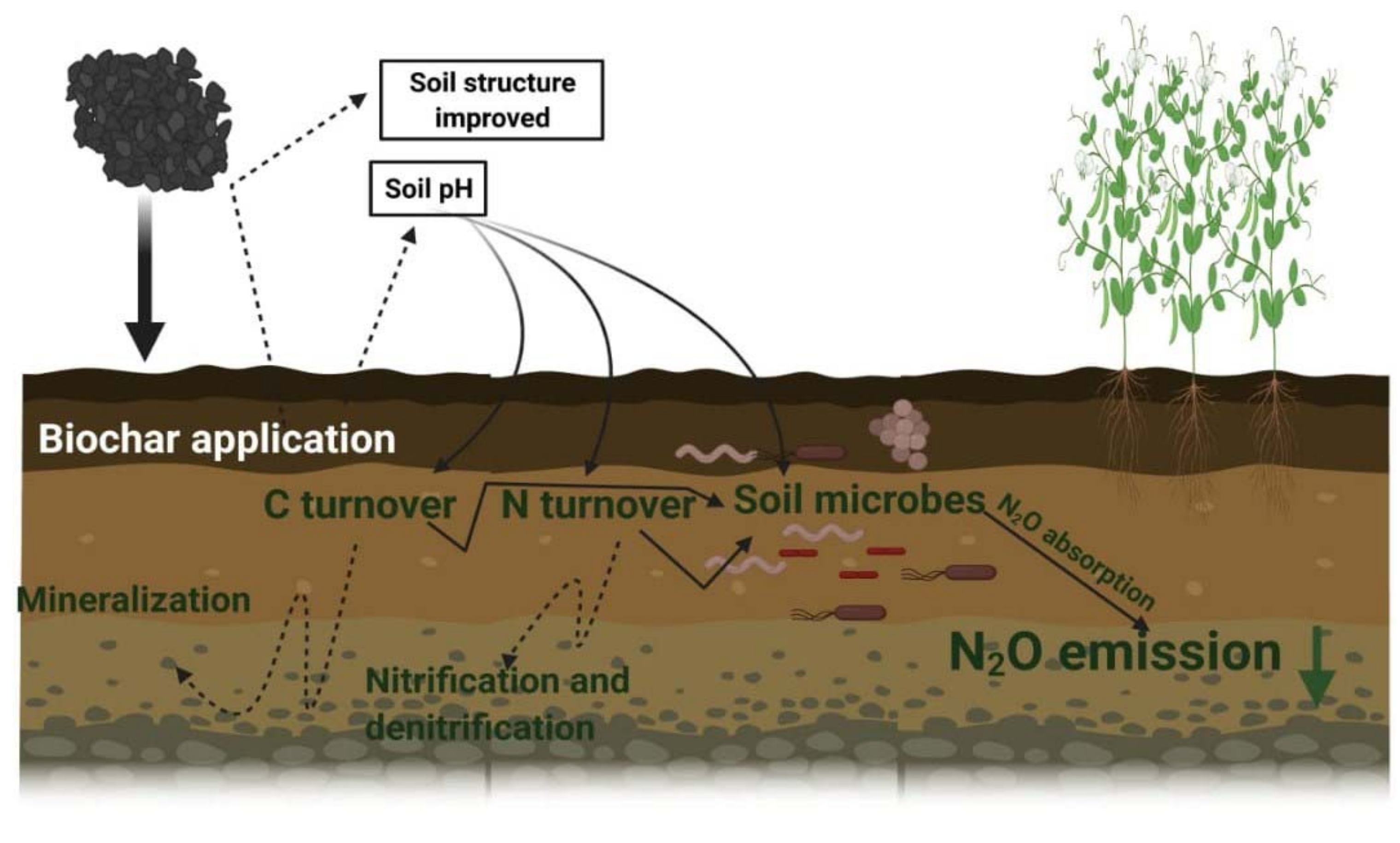
| Crop Rotation | Total Rate of N (kg ha−1) | N2O Emission Trend | References |
|---|---|---|---|
| Maize–wheat | Organic: 150 composted manure (CM), Inorganic: 150 urea, INM: 75 CM + 75 Urea | No significant difference was recorded | [300] |
| Maize–wheat | Organic: 150 CM, Inorganic: urea, INM: 75 CM + 75 urea | No significant difference was recorded | [301] |
| Maize–wheat | Organic: 150 CM, Inorganic: urea, INM: 75 CM + 75 urea | INM, Organic < Inorganic | [302] |
| Rapeseed | Organic: 97.5 cattle manure, Inorganic: ammonium nitrate (AN) 120, INM: 65 cattle manure + 60 AN | INM < Organic, Inorganic | [303] |
| Maize–wheat | Organic: 120 cattle manure, Inorganic: AN 120, INM: 60 CM + 60 AN | Organic < INM < Inorganic | [304] |
| Maize–wheat | Inorganic: 100% NPK, INM: 100% NPK + FYM | Inorganic < INM | [305] |
| Rice | Inorganic: 120 kg urea, INM: Compost (30 kg/ha + urea 90 kg/ha | INM < Inorganic | [306] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management Strategies to Mitigate N2O Emissions in Agriculture. Life 2022, 12, 439. https://doi.org/10.3390/life12030439
Hassan MU, Aamer M, Mahmood A, Awan MI, Barbanti L, Seleiman MF, Bakhsh G, Alkharabsheh HM, Babur E, Shao J, et al. Management Strategies to Mitigate N2O Emissions in Agriculture. Life. 2022; 12(3):439. https://doi.org/10.3390/life12030439
Chicago/Turabian StyleHassan, Muhammad Umair, Muhammad Aamer, Athar Mahmood, Masood Iqbal Awan, Lorenzo Barbanti, Mahmoud F. Seleiman, Ghous Bakhsh, Hiba M. Alkharabsheh, Emre Babur, Jinhua Shao, and et al. 2022. "Management Strategies to Mitigate N2O Emissions in Agriculture" Life 12, no. 3: 439. https://doi.org/10.3390/life12030439
APA StyleHassan, M. U., Aamer, M., Mahmood, A., Awan, M. I., Barbanti, L., Seleiman, M. F., Bakhsh, G., Alkharabsheh, H. M., Babur, E., Shao, J., Rasheed, A., & Huang, G. (2022). Management Strategies to Mitigate N2O Emissions in Agriculture. Life, 12(3), 439. https://doi.org/10.3390/life12030439











