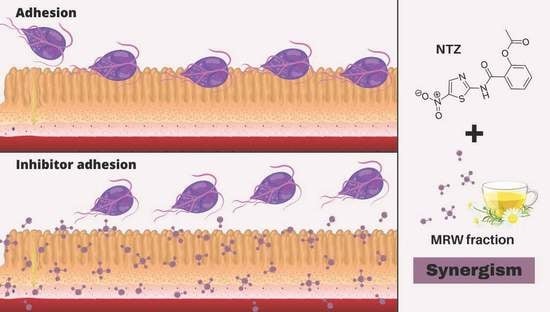
Synergistic Effect of Polysaccharides from Chamomile Tea with Nitazoxanide Increases Treatment Efficacy against Giardia intestinalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chamomile Tea Polysaccharides
2.2. G. intestinalis Isolates and Caco-2 Cell Culture
2.3. Trophozoites Growth Curves
2.4. Cell Viability
2.5. In Vitro Cytotoxic Effect
2.6. Adhesion Assay of Parasite in the Presence of MRW Fraction
2.7. Synergism between Antiprotozoal Drugs and MRW Fraction
2.8. G. intestinalis Trophozoite Recovery Assay
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argüello-García, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar] [PubMed]
- Lalle, M.; Hanevik, K. Infection and Drug Resistance Dovepress Treatment-refractory giardiasis: Challenges and solutions. Infect. Drug Resist. 2018, 11, 1921–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, T.B.; Hill, D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001, 14, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Solaymani-Mohammadi, S.; Singer, S.M. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J. Immunol. 2011, 187, 3769–3775. [Google Scholar] [CrossRef] [Green Version]
- Leitsch, D. Drug Resistance in the Microaerophilic Parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Upcroft, P.; Upcroft, J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef] [Green Version]
- Nash, T.E. Treatment of Giardia lamblia infections. Pediatr. Infect. Dis. 2001, 20, 193–195. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Eckmann, L. Drug Development against the Major Diarrhea-Causing Parasites of the Small Intestine, Cryptosporidium and Giardia. Front. Microbiol. 2015, 6, 1208. [Google Scholar] [CrossRef] [Green Version]
- White, A.C., Jr. Nitazoxanide: A new broad spectrum antiparasitic agent. Expert Rev. Anti-Infect. Ther. 2004, 2, 43–49. [Google Scholar] [CrossRef]
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-rivera, R.; Chavez, B.; Gonzalez-Robles, A.; Tapia, A.; Yepez-Mulia, L. In Vitro Effect of Nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis Trophozoites. J. Eukaryot. Microbiol. 2022, 49, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hashan, M.R.; Elhusseiny, K.M.; Huu-Hoai, L.; Tieu, T.M.; Low, S.K.; Minh, L.H.N.; Nghia, T.L.B.; Loc, L.Q.; Y, M.N.; Eid, P.S.; et al. Effect of nitazoxanide on diarrhea: A systematic review and network meta-analysis of randomized controlled trials. Acta Trop. 2020, 210, 105603. [Google Scholar] [CrossRef]
- Velázquez-Olvera, S.; Salgado-Zamora, H.; Jiménez-Cardoso, E.; Campos-Aldrete, M.E.; Pérez-González, C.; Bem Hadda, T. In vitro anti-Giardia lamblia activity of 2-aryl-3-hydroxymethyl imidazo [1,2-a] pyridines and -pyrimidines, individually and in combination with albendazole. Acta Trop. 2016, 155, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Hocayen, P.A.S.; Dallazen, J.L.; de Paula Werner, M.F.; Iacomini, M.; Andreatini, R.; Cordeiro, L.M.C. Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects. Int. J. Biol. Macromol. 2020, 164, 1675–1682. [Google Scholar] [CrossRef]
- Li, L.J.; Li, M.Y.; Li, Y.T.; Feng, J.J.; Hao, F.Q.; Lun, Z. Adjuvant activity of Sargassum pallidum polysaccharides against combined Newcastle disease, infectious bronchitis and avian influenza inactivated vaccines. Mar. Drugs 2012, 10, 2648–2660. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, L.; Fang, J.C.; Wong, J.H.; Ng, T.B.; Jiang, Y.; Wang, C.R.; Zhang, N.Y.; Wen, T.Y.; Qu, L.Y.; et al. Isolation and identification of a novel polysaccharide-peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci. Rep. 2012, 32, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Tamiello, C.S.; Adami, E.R.; de Oliveira, N.M.T.; Acco, A.; Iacomini, M.; Cordeiro, L.M.C. Structural features of polysaccharides from edible jambo (Syzygium jambos) fruits and antitumor activity of extracted pectins. Int. J. Biol. Macromol. 2018, 118, 1414–1421. [Google Scholar] [CrossRef]
- Lo, W.H.; Deng, F.S.; Chang, C.J.; Lin, C.H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Ganan, M.; Lorentzen, S.B.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antibiotic saving effect of combination therapy through synergistic interactions between well-characterized chito-oligosaccharides and commercial antifungals against medically relevant yeasts. PLoS ONE 2019, 14, e0227098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, P.F.P.; Iacomini, M.; Cordeiro, L.M.C. Chemical characterization of fructooligosaccharides, inulin and structurally diverse polysaccharides from chamomile tea. Carbohydr. Polym. 2019, 214, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; de A Matos, F.J. Plantas Medicinais Do Brasil Nativas E Exóticas, 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Sousa, M.P.; Matos, M.E.O.; Matos, F.J.A.; Machado, M.I.L.; Craveiro, A.A. Constituintes Químicos Ativos de Plantas Medicinais brasileiras; Laboratório de Produtos Naturais: Barueri, Brazil, 1991. [Google Scholar]
- Keister, D.B. Axenic Culture of Giardia lamblia in TYI-S-33 Medium Supplemented with Bile. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Evans-Osses, I.; Mojoli, A.; Monguió-Tortajada, M.; Marcilla, A.; Aran, V.; Amorim, M.; Inal, J.; Borràs, F.E.; Ramirez, M.I. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur. J. Cell Biol. 2017, 96, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Lü, Z.; Li, X.; Li, K.; Wang, C.; Du, T.; Huang, W.; Ji, M.; Li, C.; Xu, F.; Xu, P.; et al. Structure-Activity Study of Nitazoxanide Derivatives as Novel STAT3 Pathway Inhibitors. ACS Med. Chem. Lett. 2021, 12, 696–703. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Aminoguanidines: New leads for treatment of Giardia duodenalis infection. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 38–44. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharm. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Shukla, G.; Bhatia, R.; Sharma, A. Prebiotic inulin supplementation modulates the immune response and restores gut morphology in Giardia duodenalis-infected malnourished mice. Parasitol. Res. 2016, 115, 4189–4198. [Google Scholar] [CrossRef]
- Shaaban, Y.; Hassan, Z.; Hussein, R.; Hassan, A.; Salama, D. Evaluation of the role of combined prebiotic and probiotic supplements as prophylactic and therapeutic agents against experimental giardiasis. Parasitol. United J. 2021, 14, 193–203. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F. Prebiotics for Gastrointestinal Infections and Acute Diarrhea. In Dietary Interventions in Gastrointestinal Diseases; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 179–191. [Google Scholar]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Shoaf-Sweeney, K.D.; Hutkins, R.W. Chapter 2 Adherence, Anti-Adherence, and Oligosaccharides: Preventing Pathogens from Sticking to the Host. Adv. Food Nutr. Res. 2008, 55, 101–161. [Google Scholar]
- Sousa, M.C.; Gonçalves, C.A.; Bairos, V.A.; Poiares-Da-Silva, J. Adherence of Giardia lamblia trophozoites to Int-407 human intestinal cells. Clin. Diagn. Lab. Immunol. 2001, 8, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Lauwaet, T.; Bliss, L.A.; Reed, S.L.; Gillin, F.D.; Bode, L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br. J. Nutr. 2012, 108, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, J.; Manderson, K.; Wells, A.; Hotchkiss, A.T., Jr.; Gibson, G.R.; Formentin, K.; Beer, M.; Rastall, R.A. Oligosaccharide-mediated inhibition of the adhesion of pathogenic Escherichia coli strains to human gut epithelial cells in vitro. J. Food Prot. 2008, 71, 2272–2277. [Google Scholar] [CrossRef]
- Guggenbichler, J.P.; De Bettignies-Dutz, A.; Meissner, P.; Schellmoser, S.; Jurenitsch, J. Acidic oligosaccharides from natural sources block adherence of Escherichia coli on uroepithelial cells. Pharm. Pharm. Lett. 1997, 7, 35–38. [Google Scholar]
- Hotchkiss, A.; Buddington, R. Intestinal Infections and Prebiotics: The Role of Oligosaccharides in Promoting Health. Funct. Food Rev. 2011, 3, 119–134. [Google Scholar]
- Ebersbach, T.; Andersen, J.B.; Bergström, A.; Hutkins, R.W.; Licht, T.R. Xylo-oligosaccharides inhibit pathogen adhesion to enterocytes in vitro. Res. Microbiol. 2012, 163, 22–27. [Google Scholar] [CrossRef]
- Licht, T.R.; Ebersbach, T.; Frøkiær, H. Prebiotics for prevention of gut infections. Trends Food Sci. Technol. 2012, 23, 70–82. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatke, B.; Chaves, P.F.P.; Cordeiro, L.M.C.; Ramirez, M.I. Synergistic Effect of Polysaccharides from Chamomile Tea with Nitazoxanide Increases Treatment Efficacy against Giardia intestinalis. Life 2022, 12, 2091. https://doi.org/10.3390/life12122091
Sabatke B, Chaves PFP, Cordeiro LMC, Ramirez MI. Synergistic Effect of Polysaccharides from Chamomile Tea with Nitazoxanide Increases Treatment Efficacy against Giardia intestinalis. Life. 2022; 12(12):2091. https://doi.org/10.3390/life12122091
Chicago/Turabian StyleSabatke, Bruna, Pedro Felipe P. Chaves, Lucimara M. C. Cordeiro, and Marcel I. Ramirez. 2022. "Synergistic Effect of Polysaccharides from Chamomile Tea with Nitazoxanide Increases Treatment Efficacy against Giardia intestinalis" Life 12, no. 12: 2091. https://doi.org/10.3390/life12122091
APA StyleSabatke, B., Chaves, P. F. P., Cordeiro, L. M. C., & Ramirez, M. I. (2022). Synergistic Effect of Polysaccharides from Chamomile Tea with Nitazoxanide Increases Treatment Efficacy against Giardia intestinalis. Life, 12(12), 2091. https://doi.org/10.3390/life12122091