Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deep Learning Auto-Segmentation
2.2. Patients’ Selection
2.3. Technical Setup
2.4. Contour Methods
2.5. Contouring Time
2.6. Geometrical Analysis
2.7. Dosimetric Analysis
3. Results
3.1. Contouring Time
3.2. Geometrical Analysis
3.3. Dosimetric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, J.; Fong, A.; McVicar, N.; Smith, S.; Giambattista, J.; Wells, D.; Kolbeck, C.; Giambattista, J.; Gondara, L.; Alexander, A. Comparing deep learning-based auto-segmentation of organs at risk and clinical target volumes to expert inter-observer variability in radiotherapy planning. Radiother. Oncol. 2020, 144, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, T.; van Soest, J.; Gooding, M.; Peressutti, D.; Aljabar, P.; van der Stoep, J.; van Elmpt, W.; Dekker, A. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother. Oncol. 2018, 126, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipaanboord, B.; Boukerroui, D.; Peressutti, D.; van Soest, J.; Lustberg, T.; Dekker, A.; van Elmpt, W.; Gooding, M.J. An Evaluation of Atlas Selection Methods for Atlas-Based Automatic Segmentation in Radiotherapy Treatment Planning. IEEE Trans. Med. Imaging 2019, 38, 2654–2664. [Google Scholar] [CrossRef] [Green Version]
- Ibragimov, B.; Xing, L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med. Phys. 2017, 44, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Men, K.; Dai, J.; Li, Y. Automatic segmentation of the clinical target volume and organs at risk in the planning CT for rectal cancer using deep dilated convolutional neural networks. Med. Phys. 2017, 44, 6377–6389. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and operating a public information repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [Green Version]
- Zuley, M.L.; Jarosz, R.; Kirk, S.; Lee, Y.; Colen, R.; Garcia, K.; Delbeke, D.; Pham, M.; Nagy, P.; Sevinc, G.; et al. Radiology data from the cancer genome atlas head-neck squamous cell carcinoma [TCGA-HNSC] collection. Cancer Imaging Arch. 2016, 10. [Google Scholar] [CrossRef]
- Grossberg, A.; Mohamed, A.; Elhalawani, H.; Bennett, W.; Smith, K.; Nolan, T.; Chamchod, S.; Kantor, M.; Browne, T.; Hutcheson, K.; et al. Data from head and neck cancer CT atlas. Cancer Imaging Arch. 2017, 10, K9. [Google Scholar] [CrossRef]
- Zuley, M.L.; Jarosz, R.; Drake, B.F.; Rancilio, D.; Klim, A.; Rieger-Christ, K.; Lemmerman, J. Radiology data from the cancer genome atlas prostate adenocarcinoma [tcga-prad] collection. Cancer Imaging Arch. 2016, 9. [Google Scholar] [CrossRef]
- Rezaei, M.; Mohammadbeigi, A.; Khoshgard, K.; Haghparast, A. CT images and radiotherapy treatment planning of patients with breast cancer: A dataset. Data Brief 2017, 13, 390–395. [Google Scholar] [CrossRef]
- Brouwer, C.L.; Steenbakkers, R.J.; van den Heuvel, E.; Duppen, J.C.; Navran, A.; Bijl, H.P.; Chouvalova, O.; Burlage, F.R.; Meertens, H.; Langendijk, J.A.; et al. 3D Variation in delineation of head and neck organs at risk. Radiat. Oncol. 2012, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grégoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.L.; Luo, W.; Lee, A.W.; Wee, J.T.; Lee, N.; Zhou, G.Q.; Tang, L.L.; Tao, C.J.; Guo, R.; et al. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother. Oncol. 2014, 110, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Gay, H.A.; Barthold, H.J.; O’Meara, E.; Bosch, W.R.; El Naqa, I.; Al-Lozi, R.; Rosenthal, S.A.; Lawton, C.; Lee, W.R.; Sandler, H.; et al. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e353–e362, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, C.L.; Steenbakkers, R.J.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.; Huang, V.; Giambattista, J.A.; Teke, T.; Kolbeck, C.; Giambattista, J.; Atrchian, S. Training and Validation of Deep Learning-Based Auto-Segmentation Models for Lung Stereotactic Ablative Radiotherapy Using Retrospective Radiotherapy Planning Contours. Front. Oncol. 2021, 11, 626499. [Google Scholar] [CrossRef]
- Wong, J.; Huang, V.; Wells, D.; Giambattista, J.; Giambattista, J.; Kolbeck, C.; Otto, K.; Saibishkumar, E.P.; Alexander, A. Implementation of deep learning-based auto-segmentation for radiotherapy planning structures: A workflow study at two cancer centers. Radiat. Oncol. 2021, 16, 101. [Google Scholar] [CrossRef]
- Zabel, W.J.; Conway, J.L.; Gladwish, A.; Skliarenko, J.; Didiodato, G.; Goorts-Matthews, L.; Michalak, A.; Reistetter, S.; King, J.; Nakonechny, K.; et al. Clinical Evaluation of Deep Learning and Atlas-Based Auto-Contouring of Bladder and Rectum for Prostate Radiation Therapy. Pract. Radiat. Oncol. 2021, 11, e80–e89. [Google Scholar] [CrossRef]
- Cefaro, G.A.; Genovesi, D.; Perez, C.A. Delineating Organs at Risk in Radiation Therapy; Springer: London, UK, 2013. [Google Scholar]
- Nielsen, M.H.; Berg, M.; Pedersen, A.N.; Andersen, K.; Glavicic, V.; Jakobsen, E.H.; Jensen, I.; Josipovic, M.; Lorenzen, E.L.; Nielsen, H.M.; et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: National guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013, 52, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Gambacorta, M.A.; Chiloiro, G.; Valentini, V. Should We Tailor the Delineation of Pelvic Structures According to Tumor Presentation? In Multidisciplinary Management of Rectal Cancer; Springer: Cham, Switzerland, 2018; pp. 165–179. [Google Scholar]
- Merlotti, A.; Alterio, D.; Vigna-Taglianti, R.; Muraglia, A.; Lastrucci, L.; Manzo, R.; Gambaro, G.; Caspiani, O.; Miccichè, F.; Deodato, F.; et al. Technical guidelines for head and neck cancer IMRT on behalf of the Italian association of radiation oncology—Head and neck working group. Radiat. Oncol. 2014, 9, 264. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- D’Aviero, A.; Re, A.; Catucci, F.; Piccari, D.; Votta, C.; Piro, D.; Piras, A.; Di Dio, C.; Iezzi, M.; Preziosi, F.; et al. Clinical Validation of a Deep-Learning Segmentation Software in Head and Neck: An Early Analysis in a Developing Radiation Oncology Center. Int. J. Environ. Res. Public Health 2022, 19, 9057. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Chakraborty, S.; Nygren, I.; Sinha, R. Small bowel dose parameters predicting grade ≥ 3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: An independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gong, Y.; Yang, Y.; Guo, Q.; Qian, J.; Tian, Y. Evaluation of small bowel motion and feasibility of using the peritoneal space to replace bowel loops for dose constraints during intensity-modulated radiotherapy for rectal cancer. Radiat. Oncol. 2020, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, Y.; Fang, Y.; Wang, J.; Hu, W. A Preliminary Experience of Implementing Deep-Learning Based Auto-Segmentation in Head and Neck Cancer: A Study on Real-World Clinical Cases. Front. Oncol. 2021, 11, 638197. [Google Scholar] [CrossRef]
- Hall, W.H.; Guiou, M.; Lee, N.Y.; Dublin, A.; Narayan, S.; Vijayakumar, S.; Purdy, J.A.; Chen, A.M. Development and validation of a standardized method for contouring the brachial plexus: Preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Nelms, B.E.; Tomé, W.A.; Robinson, G.; Wheeler, J. Variations in the contouring of organs at risk: Test case from a patient with oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 368–378. [Google Scholar] [CrossRef]





| Treatment Site | OAR | DVH Metric | MC Mean ± SD | LC Mean ± SD |
|---|---|---|---|---|
| H&N | Oral cavity | Mean dose | 42.8 ± 4.8 | 47.9 ± 3.6 |
| Esophagus | D1cc | 44.6 ± 3.4 | 45.7 ± 5.2 | |
| Larynx | D1cc | 49.2 ± 2.4 | 49.3 ± 1.4 | |
| Larynx | Mean dose | 34.8 ± 3.6 | 35.9 ± 2.1 | |
| Mandible | D1 cc | 63.3 ± 6.6 | 62.9 ± 7 | |
| Spinal Cord | D0.03 cc | 28.6 ± 1.7 | 28.4 ± 1.8 | |
| Spinal Cord | Dmax | 29.5 ± 1.3 | 29.1 ± 1.5 | |
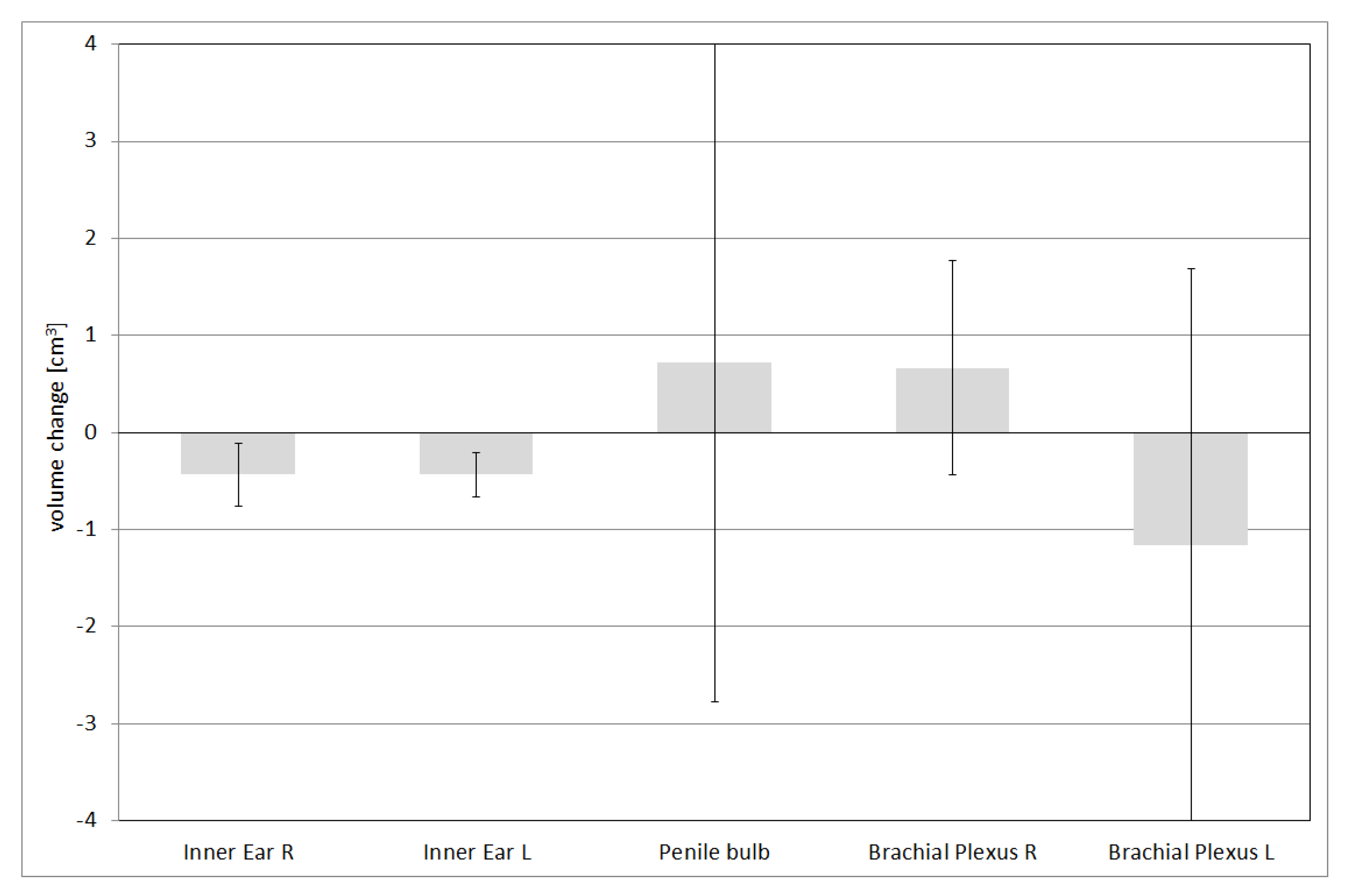
| Inner Ear R | Mean dose | 11.3 ± 6.8 | 10.2 ± 6.2 | |
| Inner Ear L | Mean dose | 10.5 ± 6.1 | 9.3 ± 5.5 | |
| Parotid R | Mean dose | 32.4 ± 7.7 | 36.9 ± 8.7 | |
| Parotid R | V30Gy | 48.8 ± 10.9 | 56.2 ± 11 | |
| Parotid L | Mean dose | 29 ± 6.1 | 33.3 ± 6.6 | |
| Parotid L | V30Gy | 43.6 ± 9.8 | 53 ± 12.4 | |
| Lung R | D30% | 5.6 ± 8 | 5.5 ± 7.9 | |
| Lung L | D30% | 4.6 ± 6.4 | 4.5 ± 6.2 | |
| Thyroid | V45Gy | 77.7 ± 25.4 | 77.7 ± 25.4 | |
| Brain Stem | D0.03cc | 35.1 ± 2.9 | 34.6 ± 2.5 | |
| Brachial Plexus R | D0.03 cc | 56.6 ± 1.8 | 58.7 ± 4.6 | |
| Brachial Plexus L | D0.03 cc | 56.8 ± 1.8 | 58.8 ± 3.7 | |
| Left Breast | Lung R | V5Gy | 0 ± 0 | 0 ± 0 |
| Lung L | V10Gy | 12.1 ± 2.3 | 12.1 ± 2.3 | |
| Lung L | V20Gy | 8.5 ± 1.8 | 8.5 ± 1.8 | |
| Lung L | V5Gy | 19.9 ± 3.3 | 19.9 ± 3.4 | |
| Heart | V25Gy | 1.2 ± 1 | 1 ± 0.9 | |
| Breast | D1cc | 0.7 ± 0.1 | 1.4 ± 0.2 | |
| Prostate | Penile bulb | Dmean | 14.8 ± 3.2 | 22.2 ± 15 |
| Femoral Head R | Dmax | 35.3 ± 3 | 34.9 ± 3 | |
| Femoral Head L | Dmax | 37.5 ± 6.1 | 37.4 ± 5.8 | |
| Rectum | V50Gy | 17.4 ± 4.3 | 19.7 ± 3.6 | |
| Rectum | V60Gy | 7.2 ± 2 | 8.4 ± 1.9 | |
| Rectum | V65Gy | 3.8 ± 0.8 | 4.6 ± 1.7 | |
| Rectum | V68Gy | 2 ± 0.7 | 2.7 ± 1.6 | |
| Bladder | V60Gy | 14 ± 2.3 | 16.6 ± 4.5 | |
| Rectum | Femoral Head R | V30Gy | 27.6 ± 4.8 | 27.6 ± 4.5 |
| Femoral Head R | V40Gy | 1.1 ± 0.8 | 0.8 ± 0.8 | |
| Femoral Head R | V45Gy | 0 ± 0 | 0 ± 0 | |
| Femoral Head L | V30Gy | 24.3 ± 9.7 | 22.7 ± 11.7 | |
| Femoral Head L | V40Gy | 0.8 ± 1.3 | 0.5 ± 0.9 | |
| Femoral Head L | V45Gy | 0 ± 0 | 0 ± 0 | |
| Bladder | V35Gy | 30.1 ± 22 | 32.7 ± 24.8 | |
| Bladder | V40Gy | 19.5 ± 16.8 | 20.1 ± 17.3 | |
| Bladder | V50Gy | 0.6 ± 1.1 | 1.3 ± 1.1 | |
| Bowel | V45Gy | 10.4 ± 15.7 | 289.4 ± 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radici, L.; Ferrario, S.; Borca, V.C.; Cante, D.; Paolini, M.; Piva, C.; Baratto, L.; Franco, P.; La Porta, M.R. Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow. Life 2022, 12, 2088. https://doi.org/10.3390/life12122088
Radici L, Ferrario S, Borca VC, Cante D, Paolini M, Piva C, Baratto L, Franco P, La Porta MR. Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow. Life. 2022; 12(12):2088. https://doi.org/10.3390/life12122088
Chicago/Turabian StyleRadici, Lorenzo, Silvia Ferrario, Valeria Casanova Borca, Domenico Cante, Marina Paolini, Cristina Piva, Laura Baratto, Pierfrancesco Franco, and Maria Rosa La Porta. 2022. "Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow" Life 12, no. 12: 2088. https://doi.org/10.3390/life12122088
APA StyleRadici, L., Ferrario, S., Borca, V. C., Cante, D., Paolini, M., Piva, C., Baratto, L., Franco, P., & La Porta, M. R. (2022). Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow. Life, 12(12), 2088. https://doi.org/10.3390/life12122088







