Diversity Analysis of Leaf Nutrient Endophytes and Metabolites in Dioecious Idesia polycarpa Maxim Leaves during Reproductive Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Acquisition
2.3. Determination of Leaf Nutrients and Analysis
2.4. Determination of Endophytic Bacteria in Leaves
2.4.1. Total DNA Extraction, PCR Amplification, and Sequencing
2.4.2. Diversity Analysis of Endophytic Bacteria
2.5. Determination of Metabolites in Leaves
2.5.1. Metabolite Extraction
2.5.2. The UPLC–MS/MS Analysis
2.6. Data Analysis
3. Results
3.1. Leaf Nutrient Characteristics in Different Periods
3.2. Differences of Endophytes in Leaves at Different Stages
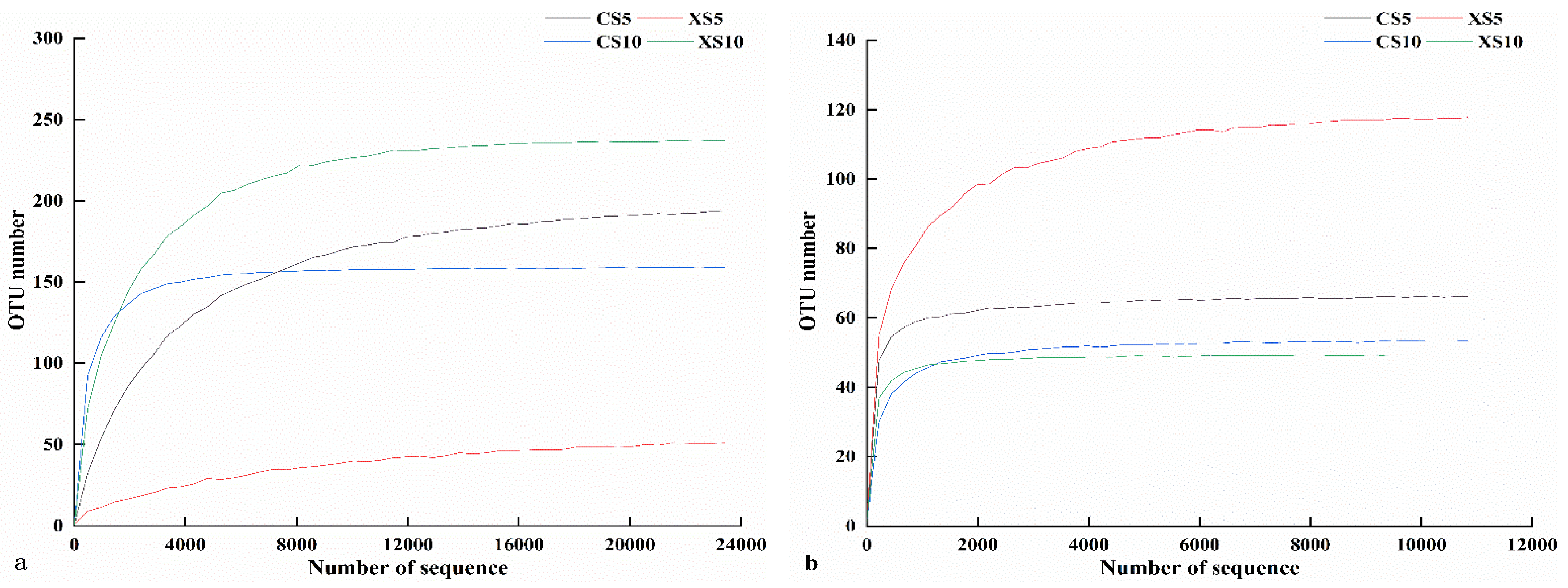
3.2.1. OTU Distribution and Alpha Diversity
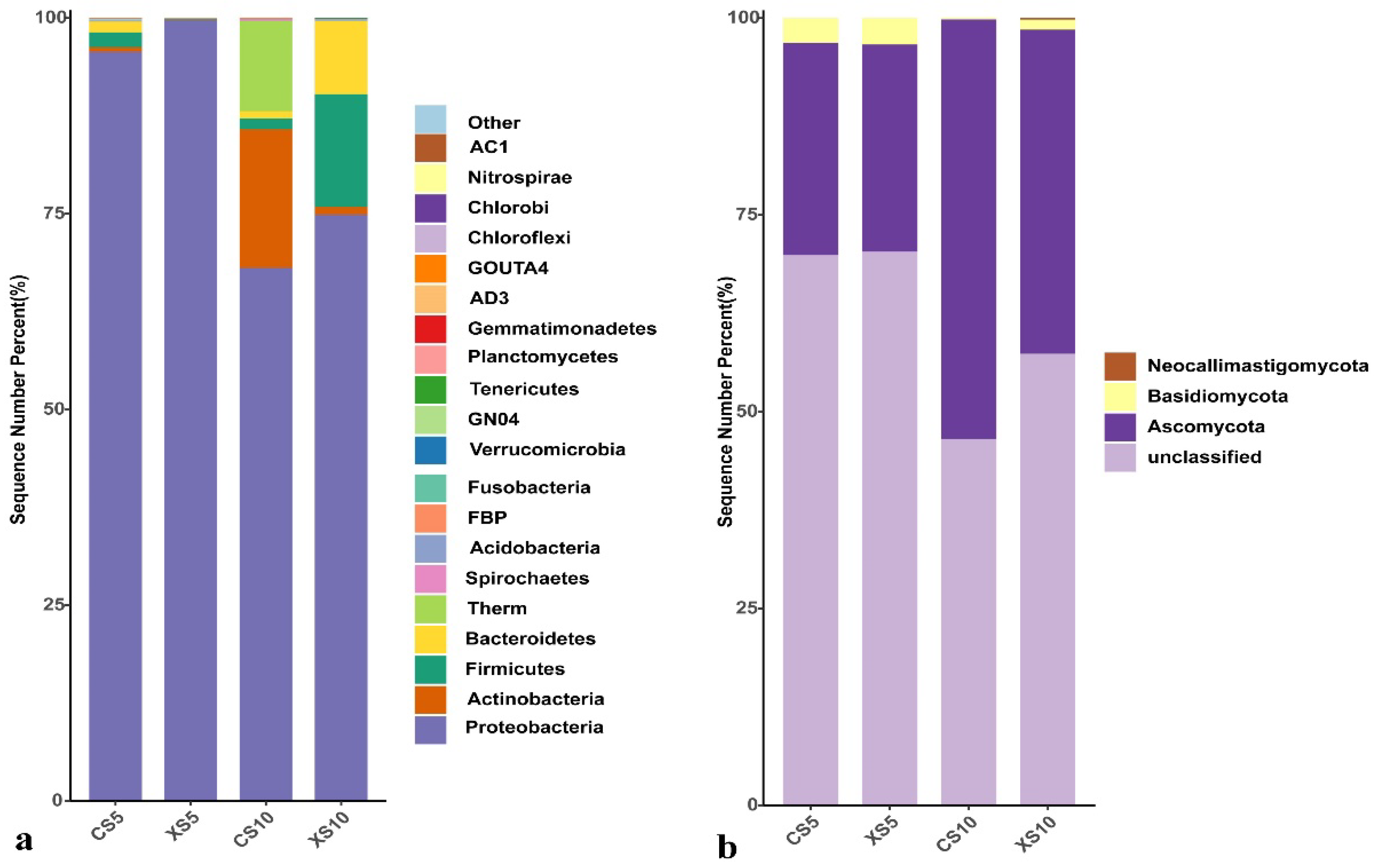
3.2.2. Phylum Level Analysis of Leaf Endophytic Bacteria Community
3.2.3. Genus Level Analysis of Leaf Endophytic Bacteria Community
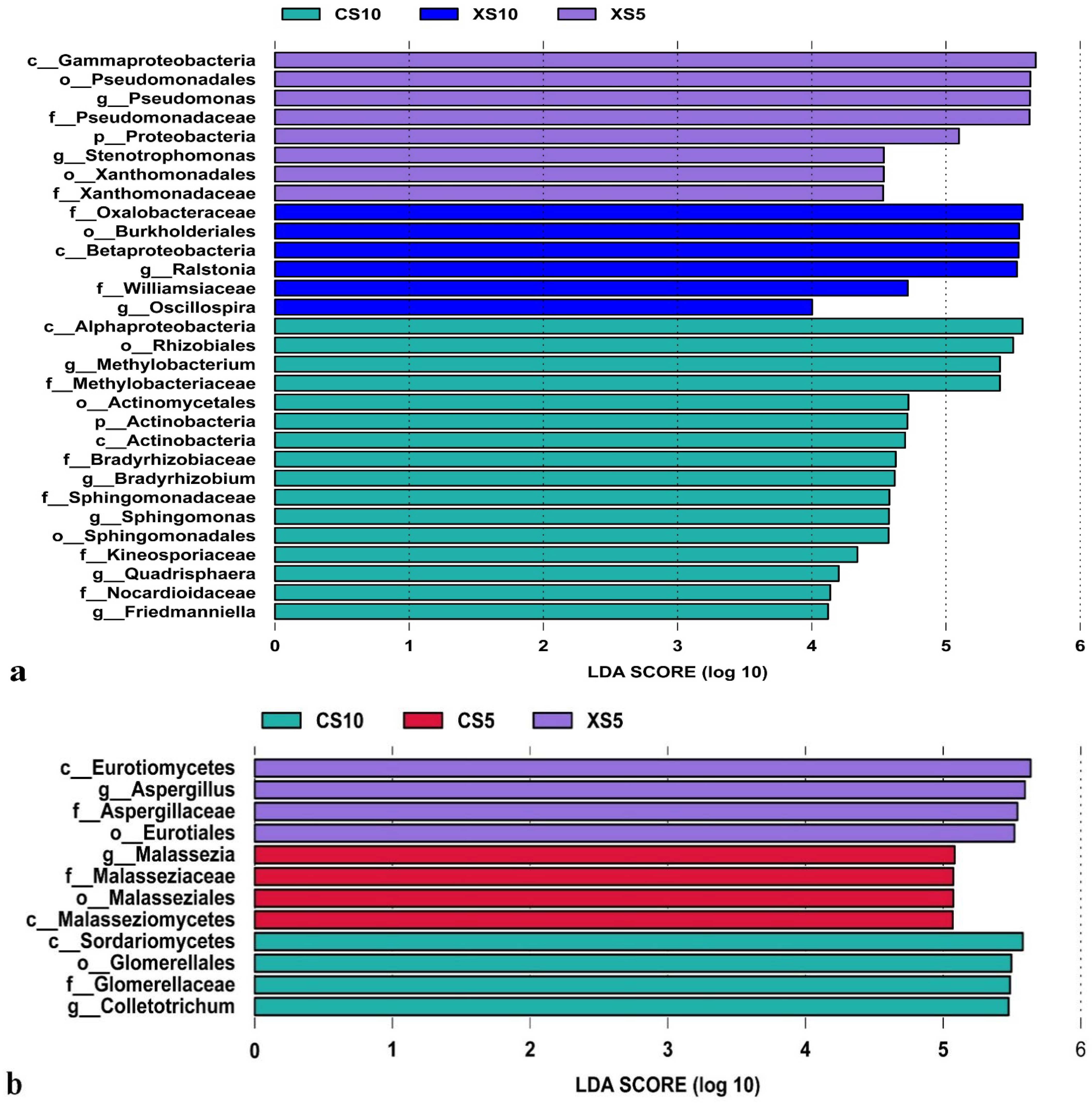
3.2.4. Difference Analysis of Endophytes in Leaves
3.3. Leaf Metabolite Difference
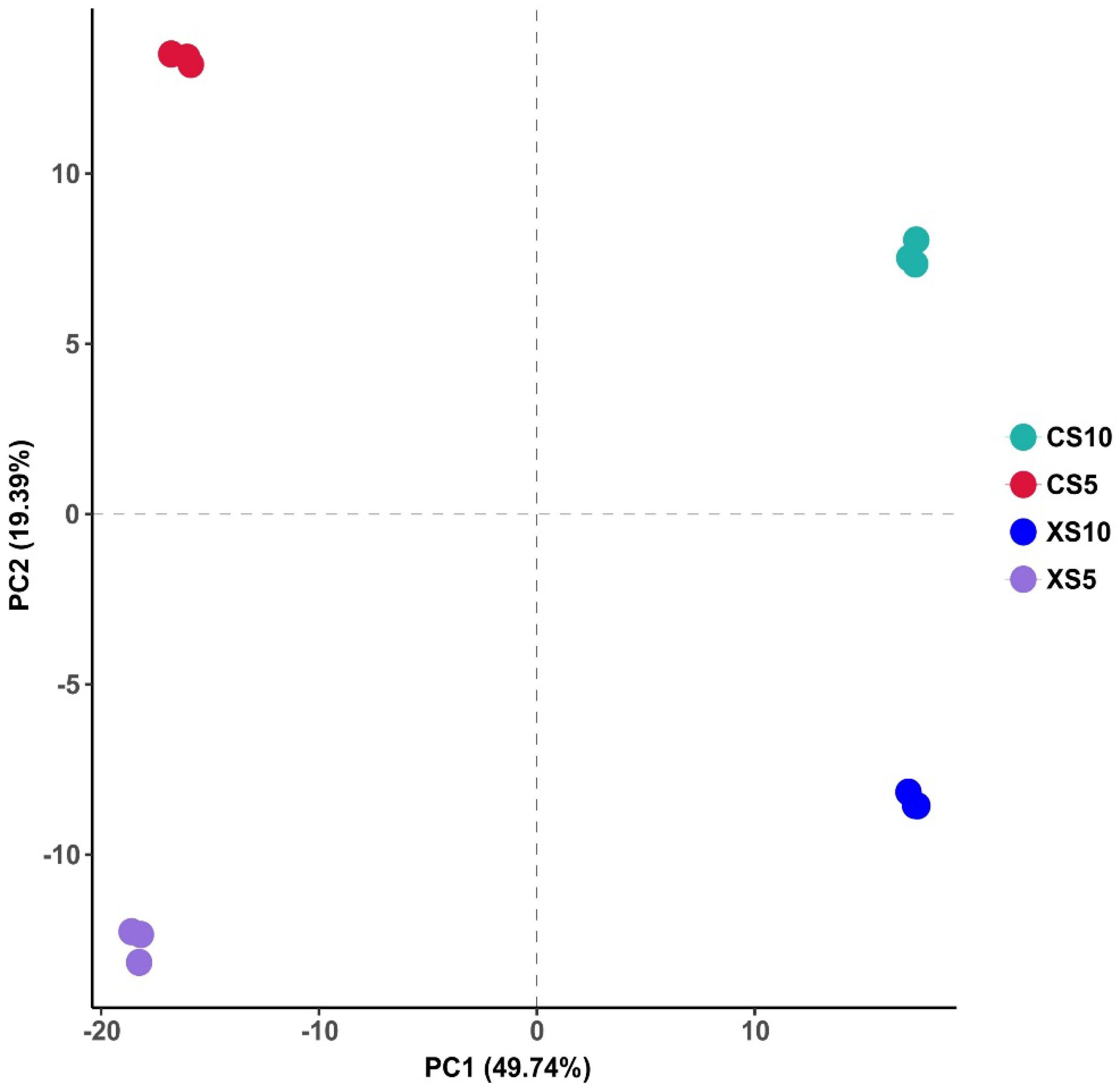
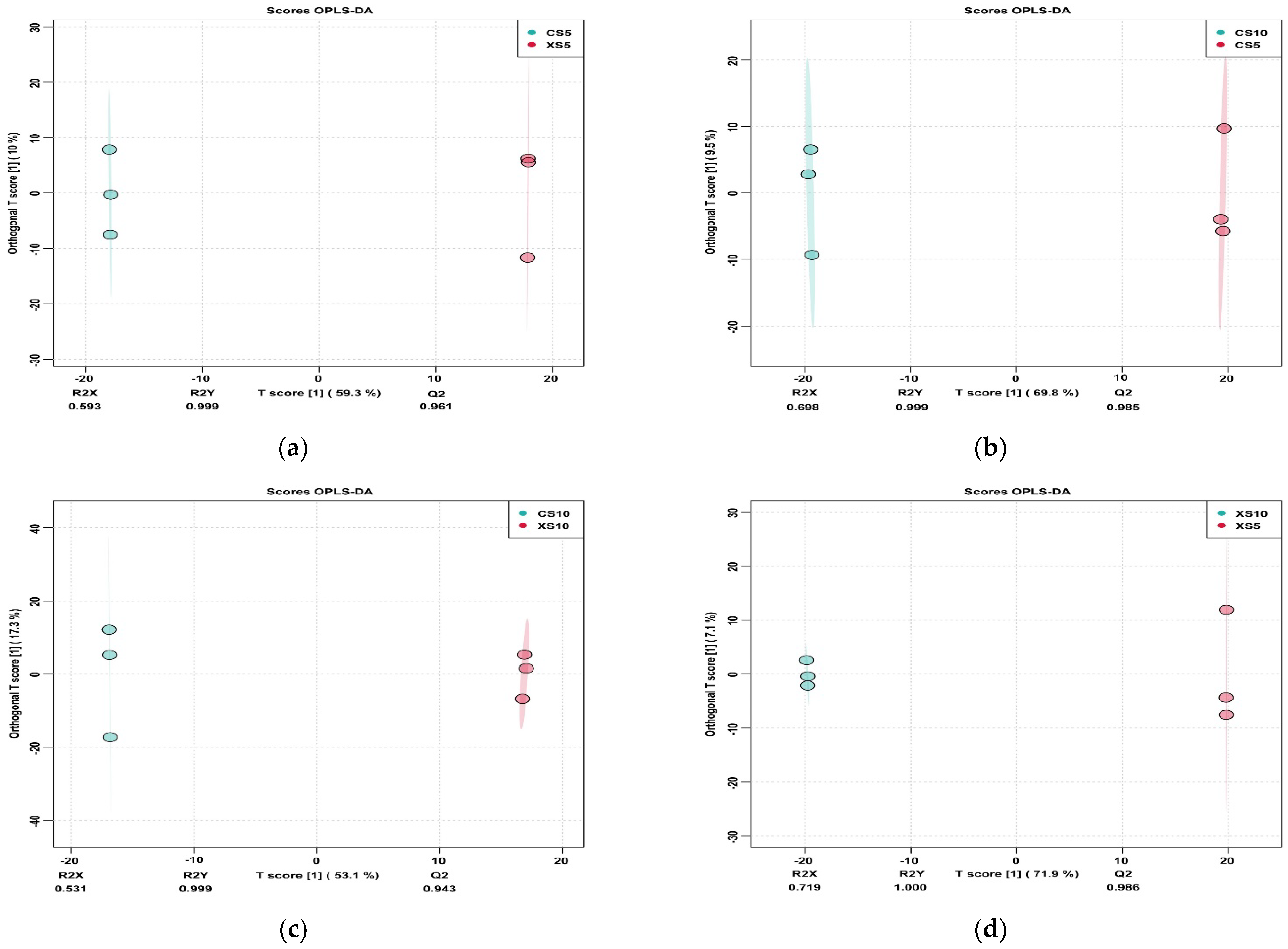
3.3.1. Sample Principal Component Analysis
3.3.2. Screening of Differential Metabolites in Leaves
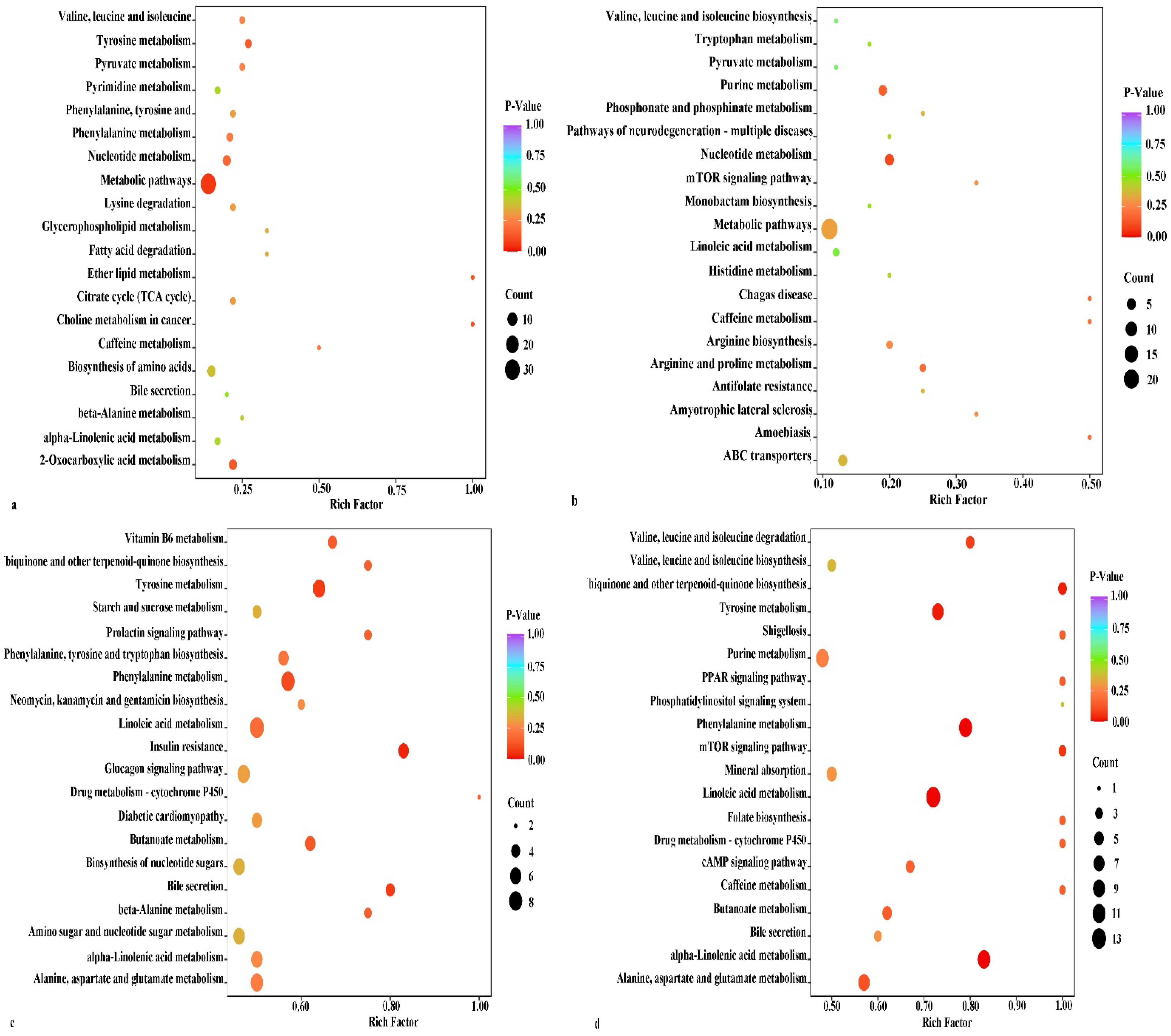
3.3.3. Metabolic Pathway Analysis
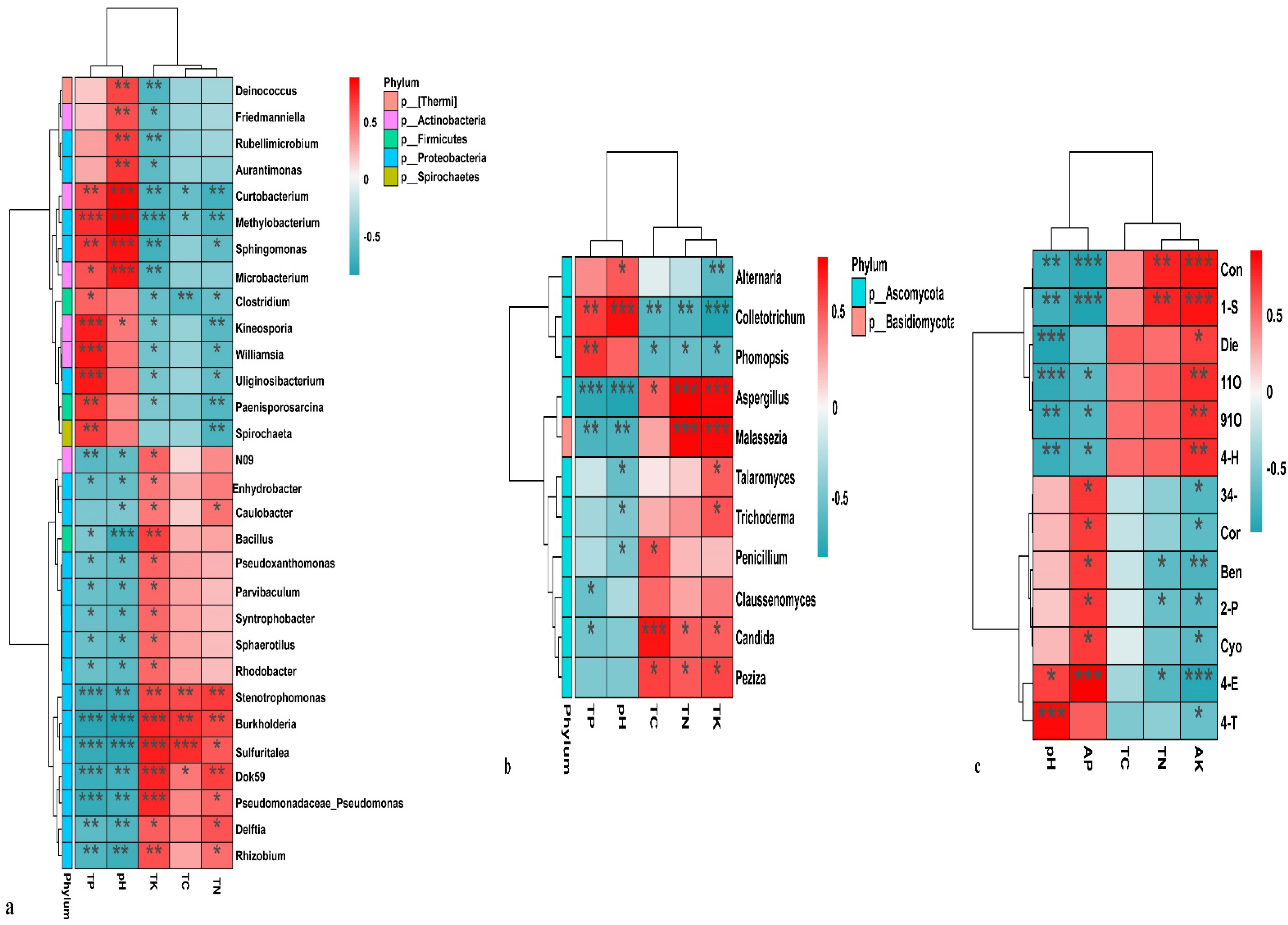
3.4. Correlation Analysis of Leaf Nutrients with the Microbial Community and Differential Metabolites
4. Discussion
4.1. Nutrient Contents in Idesia Polycarpa Leaves
4.2. Endophytes in Idesia Polycarpa Leaves
4.3. Metabolites in Idesia polycarpa Leaves
4.4. Leaf Nutrients with the Microbial Community and Differential Metabolites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slate, M.L.; Rosenstiel, T.N.; Eppley, S.M. Sex-Specific Morphological and Physiological Differences in the Moss Ceratodon purpureus (Dicranales). Ann. Bot. 2017, 120, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wen, G.; Zhang, M.; Zhou, Z. Study on the Differences of Eco-Physiological Characteristics of Male and Female Rhus typhina. For. Res. 2013, 26, 263–268. [Google Scholar]
- Rana, S.; Liu, Z. Study on the Pattern of Vegetative Growth in Young Dioecious Trees of Idesia polycarpa Maxim. Trees—Struct. Funct. 2021, 35, 69–80. [Google Scholar] [CrossRef]
- Kader, A.; Sinha, S.N. Sex-Related Differences of Excoecaria agallocha L. with a View to Defence and Growth. Trop. Life Sci. Res. 2022, 33, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Low, J.Z.; Gudimella, R.; Tammi, M.T.; Harikrishna, J.A. Expression Patterns of Inflorescence- and Sex-Specific Transcripts in Male and Female Inflorescences of African Oil Palm (Elaeis guineensis). Ann. Appl. Biol. 2016, 168, 274–289. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Guo, Q.Q.; Luo, S.Q.; Pan, J.W.; Yao, S.; Guo, Y.Y. Stoichiometry Characteristics of Leaves and Soil of Four Shrubs under Pinus massoniana Plantations. Cent. South Univ. For. Technol. 2022, 42, 129–137. [Google Scholar]
- Ma, Y.; Zhang, S.; Wu, Z.; Sun, W. Metabolic Variations in Brown Rice Fertilised with Different Levels of Nitrogen. Foods 2022, 11, 3539. [Google Scholar] [CrossRef]
- Sun, X.W.; Wang, X.C.; Quan, X.K.; Yang, Q.J.; Sun, H.Z. Photosynthetic Characteristics of Dioecious Populus davidiana, Fraxinus mandshurica and Taxus cuspidata. J. Nanjing For. Univ. 2022, 1–12. Available online: http://kns.cnki.net/kcms/detail/32.1161.S.20220409.1715.002.html (accessed on 30 November 2022).
- Song, A.Y.; Dong, L.S.; Chen, J.X.; Peng, L.; Liu, J.T.; Xia, J.B.; Chen, Y.P. Comparation of Seasonal Dynamics of Mineral Elements Contents in Different Organs of Male and Female Plants of Fraxinus velutina. Sci. Silvae Sin. 2019, 55, 162–170. [Google Scholar]
- Zuo, C.G.; Wang, J.Y.; Niu, X.X.; Liu, P.; Guan, L.H.; Dang, W.F.; Yang, H.M.; Chu, M.; Wang, N.; Lin, Q.; et al. Effects of Endophytes and Rhizosphere Bacteria on Cotton Growth Promotion and Disease Resistance Induction. Southwest China J. Agric. Sci. 2022, 35, 757–763. [Google Scholar]
- Abdul, L.K.; Boshra, A.H.; Ali, E.; Sajid, A.; Khadija, A.; Javid, H.; Ahmed, A.; Lee, I. Indole Acetic Acid and ACC Deaminase from Endophytic Bacteria Improves the Growth of Solanum lycopersicum. EJB Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar]
- Gou, Q.; Lv, Y.; Zhang, T.; Li, J.Y.; Zhao, H.J.; Liu, J.L. Dynamics and Influencing Factors of Endophytic Bacterial Community in Leaves of Lycium barbarum during Different Growth Periods. Chin. J. Ecol. 2020, 39, 2593–2601. [Google Scholar]
- Zhang, W.Y.; Yan, L.; Lai, X.J.; Duan, W.J.; Zhang, Y.Z. Analysis of Microbial Diversity and Metabolome of Flue-Cured Tobacco with Different Fermentation Time. Acta Tab. Sin. 2022, 28, 84–94. [Google Scholar]
- Xu, G.Q.; Liu, Y.X.; Cao, P.X.; Ji, Y.L.; Li, J.K.; Li, X.Y.; Liu, X. Endophytes Diversity of Oxytropis glacialis at Different Tissues Based on Illumina MiSeq Sequencing. Acta Ecol. Sin. 2021, 41, 4993–5003. [Google Scholar]
- Ren, T.Y.; Han, Y.; Zhen, P.; Li, Y.Q.; Wang, X.Y.; Yan, F.X. Study on the Endophytes of Different Varieties of Barley. Liquor-Mak. Sci. Technol. 2022, 4, 22–29. [Google Scholar]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-Aging and Brightening Effects of a Topical Treatment Containing Vitamin C, Vitamin E, and Raspberry Leaf Cell Culture Extract: A Split-Face, Randomized Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [Green Version]
- Rong, B.B.; Xu, G.Q.; Wang, L.H. Rapid Extraction of Active Components from Arctium lappa Leaves and Its Anti-Fungal Properties of Wood Rot Fungi. J. Beijing For. Univ. 2017, 39, 109–116. [Google Scholar]
- Wang, H.; Rana, S.; Li, Z.; Geng, X.; Wang, Y.; Cai, Q.; Li, S.; Sun, J.; Liu, Z. Morphological and Anatomical Changes during Floral Bud Development of the Trioecious Idesia polycarpa Maxim. Rev. Bras. Bot. 2022, 45, 679–688. [Google Scholar] [CrossRef]
- Wen, L.; Xiang, X.; Wang, Z.; Yang, Q.; Guo, Z.; Huang, P.; Mao, J.; An, X.; Kan, J. Evaluation of Cultivars Diversity and Lipid Composition Properties of Idesia polycarpa Var. vestita Diels. J. Food Sci. 2022, 87, 3841–3855. [Google Scholar] [CrossRef]
- Yang, F.X.; Su, Y.Q.; Li, X.H.; Zhang, Q.; Sun, R.C. Preparation of Biodiesel from Idesia polycarpa Var. vestita Fruit Oil. Ind. Crops Prod. 2009, 29, 622–628. [Google Scholar] [CrossRef]
- Rana, S.; Jemim, R.S.; Li, Z.; Geng, X.; Wang, Y.; Cai, Q.; Liu, Z. Study of the Pattern of Reproductive Allocation and Fruit Development in Young Dioecious Trees of Idesia polycarpa Maxim. S. Afr. J. Bot. 2022, 146, 472–480. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000; pp. 263–270. [Google Scholar]
- Niklas, K.J.; Cobb, E.D. N, P, and C Stoichiometry of Eranthis hyemalis (Ranunculaceae) and the Allometry of Plant Growth. Am. J. Bot. 2005, 92, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Chadha, K.L. Standardization of Time of Sampling for Leaf Nutrient Diagnosis on Kinnow Mandarin in North-West India. J. Plant Nutr. 2011, 34, 1820–1827. [Google Scholar] [CrossRef]
- Wang, M.; Murphy, M.T.; Moore, T.R. Nutrient Resorption of Two Evergreen Shrubs in Response to Long term Fertilization in a Bog. Oecologia 2014, 174, 365–377. [Google Scholar] [CrossRef]
- Fu, H.; Ren, Y.; Chen, Z.X.; Shi, Y.L.; Chen, C.; Sun, G.Q.; Zhou, Y.Q.; Li, Q. Nutrient Differences Between Male and Female Zanthoxylum Schinifolium Sieb. et Zucc. and Their Effects on Soil Nutrient. Non-Wood. For. Res. 2022, 40, 192–199. [Google Scholar]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Rahman Khan, M.A.; Mochida, K.; Tran, L.S.P. Potassium in Plant Physiological Adaptation to Abiotic Stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant Developmental Stage Drives the Differentiation in Ecological Role of the Maize Microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, H.Y.; Lin, Z.A.; Yuan, L.; Xu, J.K.; Zhang, S.Q.; Li, Y.T.; Zhao, B.Q.; Wen, Y.C. Long-Term Fertilisation Regimes Influence the Diversity and Community of Wheat Leaf Bacterial Endophytes. Ann. Appl. Biol. 2021, 179, 176–184. [Google Scholar] [CrossRef]
- Liu, R.; Yu, W.K.; Hu, S.X.; Shang, S. Microbial Community Structure in Leaf Space of Female and Male Fraxinus Chinensis in Yellow River Delta. J. Anhui Sci. Technol. Univ. 2022, 36, 35–39. [Google Scholar]
- Shi, H.; Xu, P.W.; Yu, W.; Cheng, Y.Z.; Ding, A.M.; Wang, W.F.; Wu, S.X.; Sun, Y.H. Metabolomic and Transcriptomic Analysis of Roots of Tobacco Varieties Resistant and Susceptible to Bacterial Wilt. Genomics 2022, 114, 110471. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, Z.; Alemu, T.; Desta, F.A.; Assefa, F. Plant Growth-Promoting Rhizobacterial Inoculation to Improve Growth, Yield, and Grain Nutrient Uptake of Teff Varieties. Front. Microbiol. 2022, 13, 896770. [Google Scholar] [CrossRef] [PubMed]
- Vanacore, A.; Forgione, M.C.; Cavasso, D.; Nguyen, H.N.A.; Molinaro, A.; Saenz, J.P.; D’Errico, G.; Paduano, L.; Marchetti, R.; Silipo, A. Role of EPS in Mitigation of Plant Abiotic Stress: The Case of Methylobacterium extorquens PA1. Carbohydr. Polym. 2022, 295, 119863. [Google Scholar] [CrossRef] [PubMed]
- Agapov, A.A.; Kulbachinskiy, A.V. Mechanisms of Stress Resistance and Gene Regulation in the Radioresistant Bacterium Deinococcus radiodurans. Biochemistry 2015, 80, 1201–1216. [Google Scholar] [CrossRef]
- Zaidi, S.S.E.A.; Mahfouz, M.M.; Mansoor, S. CRISPR-Cpf1: A New Tool for Plant Genome Editing. Trends Plant Sci. 2017, 22, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, S.; Wu, K.X.; Mu, L.Q. Differences in Leaf Metabolism of Wild Rosa acicularis and Rosa acicularis ‘Luhe’ Based on GC-MS. Bull. Bot. Res. 2022, 42, 1070–1078. [Google Scholar]
- Rabska, M.; Pers-Kamczyc, E.; Żytkowiak, R.; Adamczyk, D.; Iszkuło, G. Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions. Int. J. Mol. Sci. 2020, 21, 8094. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Liu, Q.; He, H.; Xu, X.; Dong, T. Sexual differences in growth and defence of Populus yunnanensis under drought stress. Can. J. Res. 2019, 49, 491–499. [Google Scholar] [CrossRef]
- Aouadi, M.; Escribano-Bailón, M.T.; Guenni, K.; Salhi Hannachi, A.; Dueñas, M. Qualitative and Quantitative Analyses of Phenolic Compounds by HPLC–DAD–ESI/MS in Tunisian Pistacia vera L. Leaves Unveiled a Rich Source of Phenolic Compounds with a Significant Antioxidant Potential. J. Food Meas. Charact. 2019, 13, 2448–2460. [Google Scholar] [CrossRef]
- Zhen, J.B.; Song, S.J.; Liu, L.L.; Liu, D.; Ouyang, Y.F.; Chi, J.N. Comparative Analysis of Differential Metabolites in Flowers of Gossypium hirsutum L. and Aurea helianthus. J. China Agric. Univ. 2021, 26, 20–33. [Google Scholar]
- Nasslahsen, B.; Prin, Y.; Ferhout, H.; Smouni, A.; Duponnois, R. Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices. J. Fungi 2022, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.G.L.; Kang, P.; Hu, J.P.; Pan, Y.Q.; Zhou, Y.; Ma, R.; Peng, Y.Y.; Liu, J.Y. Difference in Endophyte Community Structure Between Young and Mature Branches of Artemisia ordosica. Microbiol. China 2022, 49, 569–582. [Google Scholar]
- Chen, J.; Wu, Y.; Zhuang, X.; Guo, J.; Hu, X.; Xiao, J. Diversity Analysis of Leaf Endophytic Fungi and Rhizosphere Soil Fungi of Korean Epimedium at Different Growth Stages. Environ. Microbiomes 2022, 17, 1–14. [Google Scholar]
- Zhang, J.H.; Feng, B.B.; Xu, X.Y. Effect of Combined Fertilization of Nitrogen, Phosphorus and Potassium on Intrinsic Quality of Chrysanthemi indici Flos. J. Henan Agric. Sci. 2013, 42, 92–97. [Google Scholar]
- Gao, J.N.; Zhang, D.; Yang, M.N.; Chen, Y.L. Effects of Combined Application of Nitrogen, Phosphorus and Potassium on Biomass and Quality Components Content of Tobacco Leaves. Southwest China J. Agric. Sci. 2021, 34, 1269–1276. [Google Scholar]


| Period | Sexuality | pH | Total Carbon g·kg−1 | Total Nitrogen g·kg−1 | Total Phosphorus g·kg−1 | Total Potassium g·kg−1 |
|---|---|---|---|---|---|---|
| May | CS | 5.70 ± 0.48 a | 551.17 ± 43.57 a | 34.15 ± 0.63 a | 4.60 ± 0.17 a | 10.78 ± 1.10 a |
| XS | 5.73 ± 0.53 a | 521.20 ± 13.67 a | 29.32 ± 1.89 b | 5.13 ± 0.1 a | 8.80 ± 1.34 a | |
| July | CS | 5.67 ± 0.04 a | 446.13 ± 3.53 a | 18.40 ± 1.98 a | 4.37 ± 0.41 a | 9.90 ± 2.98 a |
| XS | 5.72 ± 0.09 a | 439.39 ± 4.55 a | 17.62 ± 2.14 a | 3.17 ± 0.33 a | 3.08 ± 0.58 b | |
| October | CS | 6.15 ± 0.03 a | 489.20 ± 12.53 a | 24.84 ± 2.57 a | 6.82 ± 0.42 a | 5.24 ± 0.03 a |
| XS | 5.97 ± 0.05 b | 492.86 ± 2.89 a | 23.83 ± 2.21 a | 7.96 ± 0.54 a | 4.56 ± 0.39 a |
| Samples | Goods Coverage | Shannon | Simpson | ACE Richness |
|---|---|---|---|---|
| CS5 | 1.00 ± 0.00 a | 1.44 ± 0.6 bc | 0.30 ± 0.06 c | 197.38 ± 144.84 a |
| XS5 | 1.00 ± 0.00 a | 0.93 ± 0.06 c | 0.27 ± 0.01 c | 55 ± 13.05 a |
| CS10 | 1.00 ± 0.00 a | 5.18 ± 0.17 a | 0.92 ± 0.01 a | 157 ± 6.03 a |
| XS10 | 1.00 ± 0.00 a | 3.51 ± 1.23 ab | 0.69 ± 0.12 b | 236.67 ± 78.56 a |
| Samples | Goods Coverage | Shannon | Simpson | ACE Richness |
|---|---|---|---|---|
| CS5 | 1.00 ± 0.00 a | 5.12 ± 0.25 a | 0.95 ± 0.01 a | 67.33 ± 1.45 ab |
| XS5 | 1.00 ± 0.00 a | 5.14 ± 0.57 a | 0.95 ± 0.02 a | 105.33 ± 25.67 a |
| CS10 | 1.00 ± 0.00 a | 3.85 ± 0.59 a | 0.82 ± 0.09 a | 67 ± 13.53 ab |
| XS10 | 1.00 ± 0.00 a | 4.53 ± 0.14 a | 0.92 ± 0.02 a | 49.33 ± 3.33 b |
| Comparison Group | Total Number of Differential Metabolites | VIP Value Top Five Metabolites | Log2 FC | p-Value | VIP | Metabolite Types |
|---|---|---|---|---|---|---|
| CS5 vs. XS5 | 108 | Cyclo (l-phe-l-pro) | 9.671 | 0.000 | 1.288 | Amino acids and derivatives |
| 2-Phenylethyl beta-d-glucopyranoside | 13.186 | 0.001 | 1.288 | Phenolic acids | ||
| Salireposide | 10.385 | 0.002 | 1.288 | Phenolic acids | ||
| 4-O-(6′-O-glucosyl-p-coumaroyl)-4-hydroxybenzyl alcohol | 3.356 | 0.000 | 1.288 | Phenolic acids | ||
| 4-Ethoxyphenol | 11.342 | 0.005 | 1.288 | Phenolic acids | ||
| CS10 vs. XS10 | 87 | 3,4-Dimethoxyphenol | 10.558 | 0.004 | 1.346 | Phenolic acids |
| 8-Hydroxyguanosine | 11.931 | 0.004 | 1.346 | Nucleotides and derivatives | ||
| Salireposide | −10.336 | 0.005 | 1.346 | Phenolic acids | ||
| Diethyl phosphate | 9.431 | 0.004 | 1.346 | Organic acids | ||
| Cordycepin (3′-Deoxyadenosine) | 10.013 | 0.005 | 1.346 | Nucleotides and derivatives | ||
| CS5 vs. CS10 | 245 | 1-Stearidonoyl-glycerol | −15.663 | 0.000 | 1.175 | Lipids |
| Cyclo(l-phe-l-pro) | 8.051 | 0.000 | 1.175 | Amino acids and derivatives | ||
| 4-Ethoxyphenol | 12.138 | 0.001 | 1.175 | Phenolic acids | ||
| Coniferaldehyde | −12.447 | 0.001 | 1.175 | Phenolic acids | ||
| Benzoic acid | 11.595 | 0.001 | 1.175 | Phenolic acids | ||
| XS5 vs. XS10 | 254 | 1-Stearidonoyl-glycerol | −12.498 | 0.000 | 1.166 | Lipids |
| 4-(3,4,5-Trihydroxybenzoxy) benzoic acid | 3.737 | 0.000 | 1.166 | Phenolic acids | ||
| 1,18-Octadecanediol | −10.018 | 0.001 | 1.166 | Lipids | ||
| 9,12-Octadecadien-6-ynoic acid | −11.759 | 0.002 | 1.166 | Lipids | ||
| 4-Hydroxy-3-methoxymandelate | −14.586 | 0.003 | 1.166 | Organic acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Rana, S.; Liu, Z.; Wang, Y.; Cai, Q.; Geng, X.; Zhou, H.; Zhang, T.; Wang, S.; Xue, X.; et al. Diversity Analysis of Leaf Nutrient Endophytes and Metabolites in Dioecious Idesia polycarpa Maxim Leaves during Reproductive Stages. Life 2022, 12, 2041. https://doi.org/10.3390/life12122041
Feng J, Rana S, Liu Z, Wang Y, Cai Q, Geng X, Zhou H, Zhang T, Wang S, Xue X, et al. Diversity Analysis of Leaf Nutrient Endophytes and Metabolites in Dioecious Idesia polycarpa Maxim Leaves during Reproductive Stages. Life. 2022; 12(12):2041. https://doi.org/10.3390/life12122041
Chicago/Turabian StyleFeng, Jian, Sohel Rana, Zhen Liu, Yanmei Wang, Qifei Cai, Xiaodong Geng, Huina Zhou, Tao Zhang, Shasha Wang, Xiaoyan Xue, and et al. 2022. "Diversity Analysis of Leaf Nutrient Endophytes and Metabolites in Dioecious Idesia polycarpa Maxim Leaves during Reproductive Stages" Life 12, no. 12: 2041. https://doi.org/10.3390/life12122041
APA StyleFeng, J., Rana, S., Liu, Z., Wang, Y., Cai, Q., Geng, X., Zhou, H., Zhang, T., Wang, S., Xue, X., Li, M., Jemim, R. S., & Li, Z. (2022). Diversity Analysis of Leaf Nutrient Endophytes and Metabolites in Dioecious Idesia polycarpa Maxim Leaves during Reproductive Stages. Life, 12(12), 2041. https://doi.org/10.3390/life12122041






