Effects of Biofuel Crop Switchgrass (Panicum virgatum) Cultivation on Soil Carbon Sequestration and Greenhouse Gas Emissions: A Review
Abstract
1. Introduction
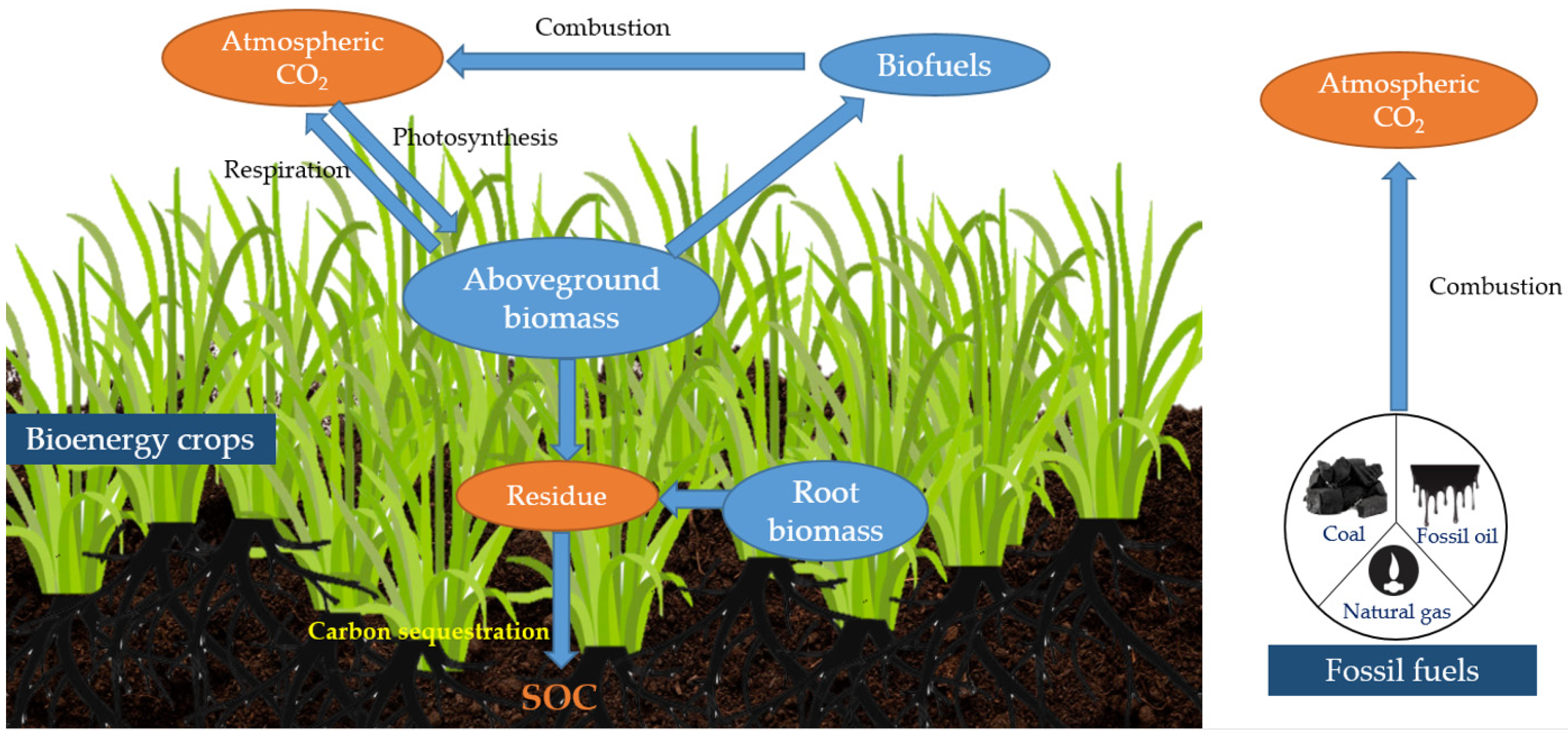
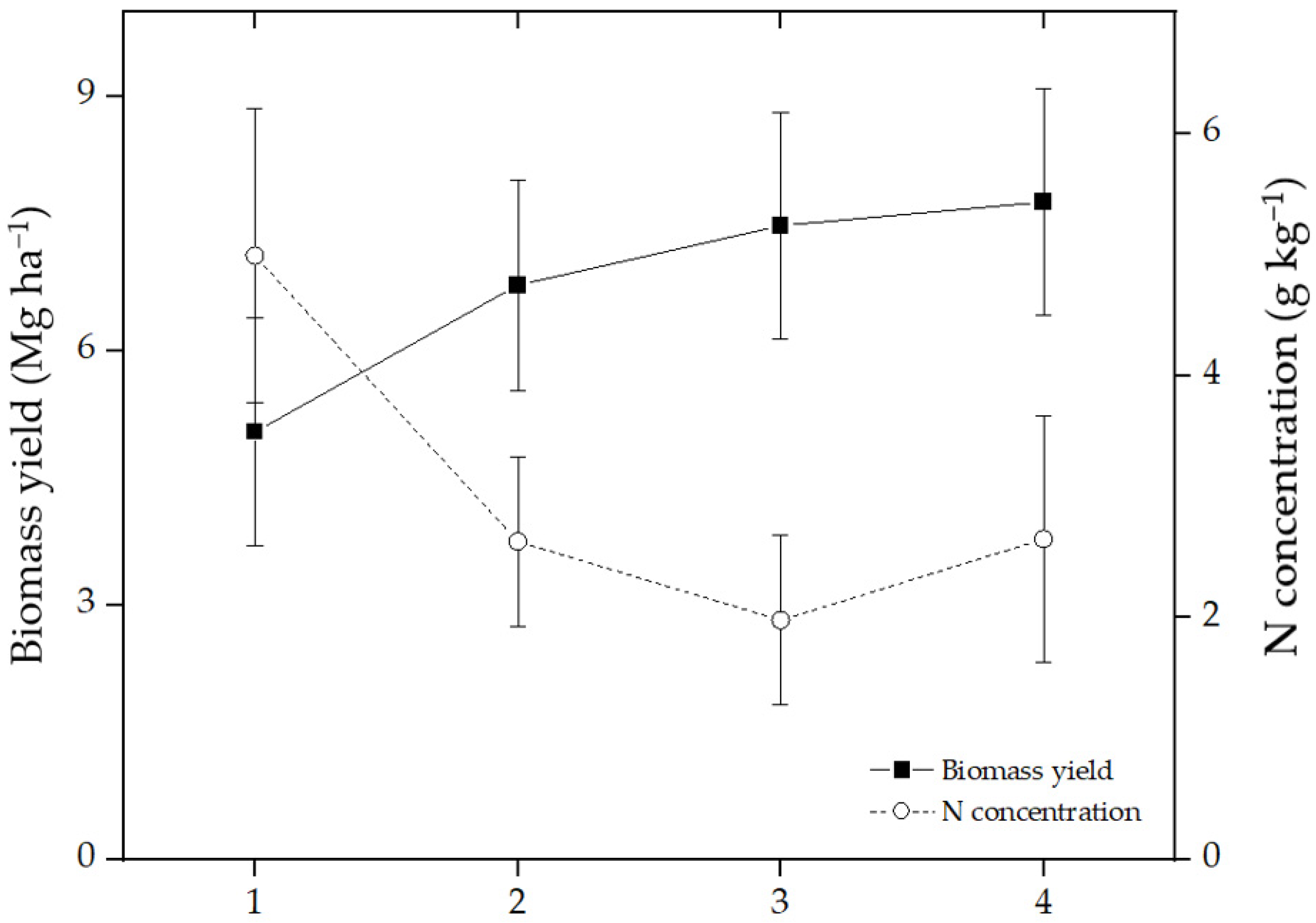
2. Carbon Sequestration by Switchgrass
3. Net Ecosystem CO2 Exchange of Switchgrass
4. CH4 Flux as Affected by Switchgrass Cultivation
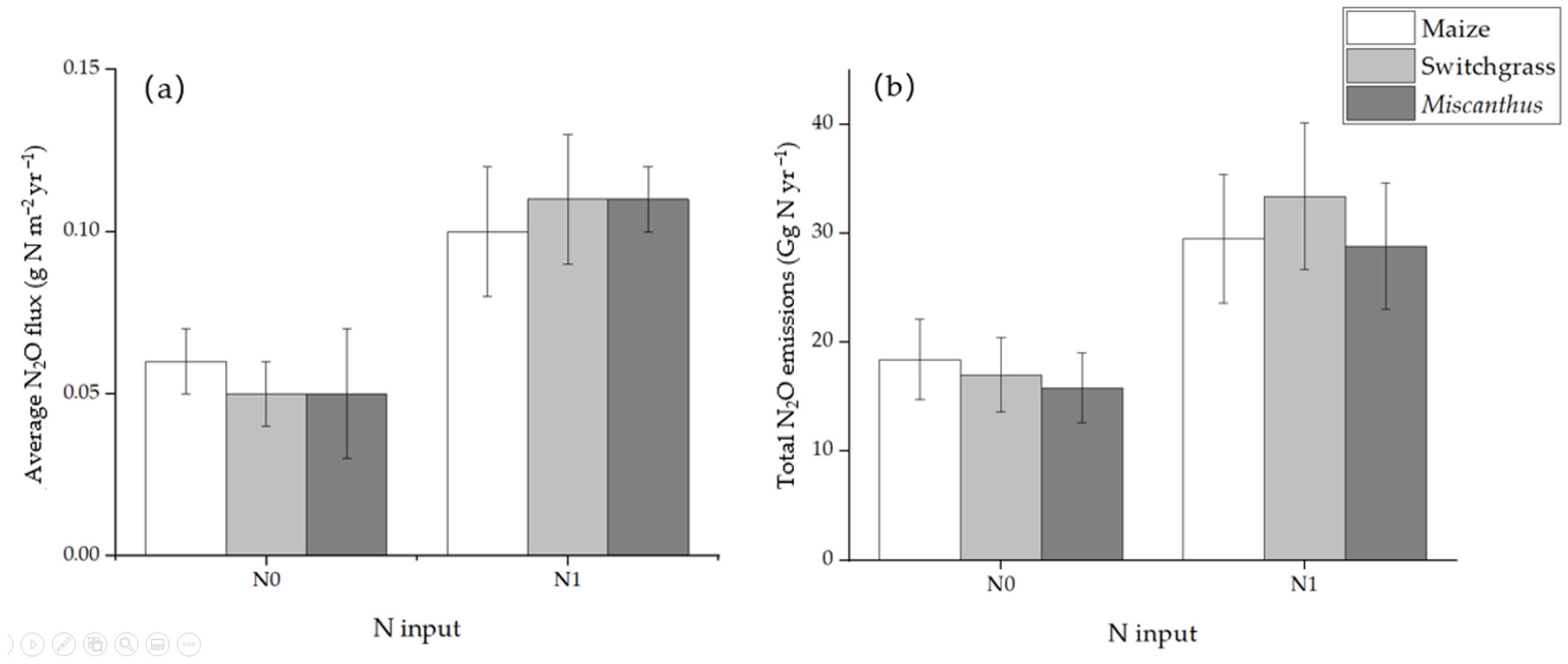
5. N2O Emission from Switchgrass Soil
5.1. N2O Emission of Switchgrass Soil with N Addition
5.2. Microbial Mechanism of N2O Emission from Switchgrass Field
5.3. Environmental Factors Affecting Soil N2O Emissions with Switchgrass
6. Application of Switchgrass Cultivation in Degraded Land
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynas, M.; Houlton, B.Z.; Perry, S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ. Res. Lett. 2021, 16, 114005. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Wang, H.; Singh, B.P.; Hu, S.; Luo, Y.; Li, J.; Xiao, Y.; Cai, X.; Li, Y. Responses of soil greenhouse gas emissions to different application rates of biochar in a subtropical Chinese chestnut plantation. Agric. For. Meteorol. 2019, 271, 168–179. [Google Scholar] [CrossRef]
- Forster, P.; Storelvmo, T. Chapter 7: The Earths energy budget, climate feedbacks, and climate sensitivity. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Eds.; Cambridge University Press: Cambridge, Britain, 2021; pp. 923–1025. Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter07.pdf (accessed on 8 July 2022).
- Robertson, G.P.; Grace, P.R. Greenhouse gas fluxes in tropical and temperate agriculture: The need for a full-cost accounting of global warming potentials. In Tropical Agriculture in Transition—Opportunities for Mitigating Greenhouse Gas Emissions? Springer: Dordrecht, The Netherlands, 2004; pp. 51–63. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Canziani, O.F.; Leary, N.A.; Dokken, D.J.; White, K.S. Climate Change 2001: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; pp. 75–913. [Google Scholar]
- Frank, S.; Havlík, P.; Stehfest, E.; van Meijl, H.; Witzke, P.; Pérez-Domínguez, I.; van Dijk, M.; Doelman, J.C.; Fellmann, T.; Koopman, J.F. Agricultural non-CO2 emission reduction potential in the context of the 1.5 °C target. Nat. Clim. Chang. 2019, 9, 66–72. [Google Scholar] [CrossRef]
- Kole, C.; Joshi, C.P.; Shonnard, D.R. Handbook of Bioenergy Crop Plants; CRC Press: Boca Raton, FL, USA, 2012; pp. 3–119. [Google Scholar]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. 2006. Available online: https://www.ipcc-nggip.iges.or.jp/meeting/pdfiles/Washington_Report.pdf (accessed on 1 September 2022).
- Porter, C.L., Jr. An analysis of variation between upland and lowland switchgrass, Panicum virgatum L., in central Oklahoma. Ecology 1966, 47, 980–992. [Google Scholar] [CrossRef]
- Gonulal, E.; Soylu, S.; Sahin, M. Effects of different water stress levels on biomass yield and agronomic traits of switchgrass (Panicum virgatum L.) cultivars under arid and semi-arid conditions. Turkish J. Field Crop. 2021, 26, 25–34. [Google Scholar] [CrossRef]
- Bransby, D.I.; McLaughlin, S.B.; Parrish, D.J. A review of carbon and nitrogen balances in switchgrass grown for energy. Biomass Bioenergy 1998, 14, 379–384. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 2005, 28, 515–535. [Google Scholar] [CrossRef]
- Lemus, R.; Lal, R. Bioenergy crops and carbon sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Kucharik, C.J.; Brye, K.R.; Norman, J.M.; Foley, J.A.; Gower, S.T.; Bundy, L.G. Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern Wisconsin: Potential for SOC sequestration during the next 50 years. Ecosystems 2001, 4, 237–258. [Google Scholar] [CrossRef]
- Ussiri, D.A.; Lal, R. Long-term tillage effects on soil carbon storage and carbon dioxide emissions in continuous corn cropping system from an alfisol in Ohio. Soil Tillage Res. 2009, 104, 39–47. [Google Scholar] [CrossRef]
- Ma, Z.; Wood, C.W.; Bransby, D.I. Soil management impacts on soil carbon sequestration by switchgrass. Biomass Bioenergy 2000, 18, 469–477. [Google Scholar] [CrossRef]
- Liebig, M.A.; Johnson, H.A.; Hanson, J.D.; Frank, A.B. Soil carbon under switchgrass stands and cultivated cropland. Biomass Bioenergy 2005, 28, 347–354. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Facini, O.; Nocentini, A.; Nardino, M.; Rossi, F.; Monti, A. Four-year measurement of net ecosystem gas exchange of switchgrass in a Mediterranean climate after long-term arable land use. Glob. Chang. Biol. Bioenergy 2019, 11, 466–482. [Google Scholar] [CrossRef]
- Anderson Teixeira, K.J.; Davis, S.C.; Masters, M.D.; Delucia, E.H. Changes in soil organic carbon under biofuel crops. Glob. Chang. Biol. Bioenergy 2009, 1, 75–96. [Google Scholar] [CrossRef]
- Hong, C.O.; Owens, V.N.; Bransby, D.; Farris, R.; Fike, J.; Heaton, E.; Kim, S.; Mayton, H.; Mitchell, R.; Viands, D. Switchgrass response to nitrogen fertilizer across diverse environments in the USA: A regional feedstock partnership report. Bioenergy Res. 2014, 7, 777–788. [Google Scholar] [CrossRef]
- Omonode, R.A.; Vyn, T.J. Vertical distribution of soil organic carbon and nitrogen under warm-season native grasses relative to croplands in west-central Indiana, USA. Agr. Ecosyst. Environ. 2006, 117, 159–170. [Google Scholar] [CrossRef]
- Follett, R.F.; Vogel, K.P.; Varvel, G.E.; Mitchell, R.B.; Kimble, J. Soil carbon sequestration by switchgrass and no-till maize grown for bioenergy. Bioenergy Res. 2012, 5, 866–875. [Google Scholar] [CrossRef]
- Lee, D.K.; Owens, V.N.; Doolittle, J.J. Switchgrass and soil carbon sequestration response to ammonium nitrate, manure, and harvest frequency on conservation reserve program land. Agron. J. 2007, 99, 462–468. [Google Scholar] [CrossRef]
- Mulkey, V.R.; Owens, V.N.; Lee, D.K. Management of switchgrass-dominated conservation reserve program lands for biomass production in South Dakota. Crop Sci. 2006, 46, 712–720. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Grote, J.B. Cropping systems effects on improving soil carbon stocks of exposed subsoil. Soil Sci. Soc. Am. J. 2007, 71, 1381–1388. [Google Scholar] [CrossRef]
- Zan, C.S.; Fyles, J.W.; Girouard, P.; Samson, R.A. Carbon sequestration in perennial bioenergy, annual corn and uncultivated systems in southern Quebec. Agr. Ecosyst. Environ. 2001, 86, 135–144. [Google Scholar] [CrossRef]
- Tulbure, M.G.; Wimberly, M.C.; Boe, A.; Owens, V.N. Climatic and genetic controls of yields of switchgrass, a model bioenergy species. Agr. Ecosyst. Environ. 2012, 146, 121–129. [Google Scholar] [CrossRef]
- Bates, C.T.; Escalas, A.; Kuang, J.; Hale, L.; Wang, Y.; Herman, D.; Nuccio, E.E.; Wan, X.; Bhattacharyya, A.; Fu, Y. Conversion of marginal land into switchgrass conditionally accrues soil carbon but reduces methane consumption. ISME J. 2022, 16, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Cescatti, A.; Wohlfahrt, G.; Buchmann, N.; Zhu, J.; Chen, G.; Moyano, F.; Pumpanen, J.; Hirano, T. Effect of climate warming on the annual terrestrial net ecosystem CO2 exchange globally in the boreal and temperate regions. Sci. Rep. 2017, 7, 3108. [Google Scholar] [CrossRef]
- Zeri, M.; Anderson-Teixeira, K.; Hickman, G.; Masters, M.; DeLucia, E.; Bernacchi, C.J. Carbon exchange by establishing biofuel crops in Central Illinois. Agr. Ecosyst. Environ. 2011, 144, 319–329. [Google Scholar] [CrossRef]
- Eichelmann, E.; Wagner-Riddle, C.; Warland, J.; Deen, B.; Voroney, P. Comparison of carbon budget, evapotranspiration, and albedo effect between the biofuel crops switchgrass and corn. Agr. Ecosyst. Environ. 2016, 231, 271–282. [Google Scholar] [CrossRef]
- Wagle, P.; Kakani, V.G. Seasonal variability in net ecosystem carbon dioxide exchange over a young switchgrass stand. Glob. Chang. Biol. Bioenergy 2014, 6, 339–350. [Google Scholar] [CrossRef]
- Wagle, P.; Kakani, V.G.; Huhnke, R.L. Net ecosystem carbon dioxide exchange of dedicated bioenergy feedstocks: Switchgrass and high biomass sorghum. Agric. For. Meteorol. 2015, 207, 107–116. [Google Scholar] [CrossRef]
- Wagle, P.; Kakani, V.G.; Huhnke, R.L. Evapotranspiration and ecosystem water use efficiency of switchgrass and high biomass sorghum. Agron. J. 2016, 108, 1007–1019. [Google Scholar] [CrossRef]
- Eichelmann, E.; Wagner Riddle, C.; Warland, J.; Deen, B.; Voroney, P. Carbon dioxide exchange dynamics over a mature switchgrass stand. Glob. Chang. Biol. Bioenergy 2016, 8, 428–442. [Google Scholar] [CrossRef]
- Skinner, R.H.; Adler, P.R. Carbon dioxide and water fluxes from switchgrass managed for bioenergy production. Agr. Ecosyst. Environ. 2010, 138, 257–264. [Google Scholar] [CrossRef]
- Zenone, T.; Gelfand, I.; Chen, J.; Hamilton, S.K.; Robertson, G.P. From set-aside grassland to annual and perennial cellulosic biofuel crops: Effects of land use change on carbon balance. Agric. For. Meteorol. 2013, 182, 1–12. [Google Scholar] [CrossRef]
- Tlustos, P.; Willison, T.W.; Baker, J.C.; Murphy, D.V.; Pavlikova, D.; Goulding, K.; Powlson, D.S. Short-term effects of nitrogen on methane oxidation in soils. Biol. Fertil. Soils 1998, 28, 64–70. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Chan, A.; Parkin, T.B. Effect of land use on methane flux from soil. J. Environ. Qual. 2001, 30, 786–797. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Sutradhar, A.K.; Miller, E.C.; Arnall, D.B.; Dunn, B.L.; Girma, K.; Raun, W.R. Switchgrass forage yield and biofuel quality with no-tillage interseeded winter legumes in the southern Great Plains. J. Plant. Nutr. 2017, 40, 2382–2391. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Mosier, A.R.; Smith, K.A.; Winiwarter, W. N2O Release from Agro-Biofuel Production Negates Global Warming Reduction by Replacing Fossil Fuels; Springer: Berlin/Heidelberg, Germany, 2016; pp. 227–238. [Google Scholar] [CrossRef]
- Qin, Z.; Zhuang, Q.; Zhu, X. Carbon and nitrogen dynamics in bioenergy ecosystems: 2. Potential greenhouse gas emissions and global warming intensity in the conterminous United States. Glob. Chang. Biol. Bioenergy 2015, 7, 25–39. [Google Scholar] [CrossRef]
- Qin, Z.; Zhuang, Q.; Zhu, X. Carbon and nitrogen dynamics in bioenergy ecosystems: 1. Model development, validation and sensitivity analysis. Glob. Chang. Biol. Bioenergy 2014, 6, 740–755. [Google Scholar] [CrossRef]
- Wile, A.; Burton, D.L.; Sharifi, M.; Lynch, D.; Main, M.; Papadopoulos, Y.A. Effect of nitrogen fertilizer application rate on yield, methane and nitrous oxide emissions from switchgrass (Panicum virgatum L.) and reed canarygrass (Phalaris arundinacea L.). Can. J. Soil. Sci. 2014, 94, 129–137. [Google Scholar] [CrossRef]
- Nikièma, P.; Rothstein, D.E.; Min, D.; Kapp, C.J. Nitrogen fertilization of switchgrass increases biomass yield and improves net greenhouse gas balance in northern Michigan, USA. Biomass Bioenergy 2011, 35, 4356–4367. [Google Scholar] [CrossRef]
- Schmer, M.R.; Liebig, M.A.; Hendrickson, J.R.; Tanaka, D.L.; Phillips, R.L. Growing season greenhouse gas flux from switchgrass in the northern great plains. Biomass Bioenergy 2012, 45, 315–319. [Google Scholar] [CrossRef]
- Ruan, L.; Bhardwaj, A.K.; Hamilton, S.K.; Robertson, G.P. Nitrogen fertilization challenges the climate benefit of cellulosic biofuels. Environ. Res. Lett. 2016, 11, 64007. [Google Scholar] [CrossRef]
- McGowan, A.R.; Min, D.H.; Williams, J.R.; Rice, C.W. Impact of nitrogen application rate on switchgrass yield, production costs, and nitrous oxide emissions. J. Environ. Qual. 2018, 47, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.D.; Davis, E.B.; Borsuk, M.E.; Gunderson, C.A.; Lynd, L.R. Biomass production in switchgrass across the United States: Database description and determinants of yield. Agron. J. 2010, 102, 1158–1168. [Google Scholar] [CrossRef]
- Duran, B.E.; Duncan, D.S.; Oates, L.G.; Kucharik, C.J.; Jackson, R.D. Nitrogen fertilization effects on productivity and nitrogen loss in three grass-based perennial bioenergy cropping systems. PLoS ONE 2016, 11, e151919. [Google Scholar] [CrossRef]
- Oates, L.G.; Duncan, D.S.; Gelfand, I.; Millar, N.; Robertson, G.P.; Jackson, R.D. Nitrous oxide emissions during establishment of eight alternative cellulosic bioenergy cropping systems in the North Central United States. Glob. Chang. Biol. Bioenergy 2016, 8, 539–549. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Nishizawa, T.; Zhu, L.; Shiratori, Y.; Ohte, N.; Koba, K.; Otsuka, S.; Senoo, K. Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J. 2015, 9, 1954–1965. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Pannu, M.W.; Meinhardt, K.A.; Bertagnolli, A.; Fransen, S.C.; Stahl, D.A.; Strand, S.E. Nitrous oxide emissions associated with ammonia-oxidizing bacteria abundance in fields of switchgrass with and without intercropped alfalfa. Environ. Microbiol. Rep. 2019, 11, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.; Meinhardt, K.A.; Orellana, L.H.; Hatt, J.K.; Pannu, M.W.; Stahl, D.A.; Konstantinidis, K.T. The influence of alfalfa-switchgrass intercropping on microbial community structure and function. Environ. Microbiol. 2021, 23, 6828–6843. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Deen, B.; Dunfield, K.E. Impacts of surface-applied residues on N-cycling soil microbial communities in miscanthus and switchgrass cropping systems. Appl. Soil. Ecol. 2018, 130, 79–83. [Google Scholar] [CrossRef]
- Casler, M.D.; Boe, A.R. Cultivar× environment interactions in switchgrass. Crop Sci. 2003, 43, 2226–2233. [Google Scholar] [CrossRef]
- Behrman, K.D.; Kiniry, J.R.; Winchell, M.; Juenger, T.E.; Keitt, T.H. Spatial forecasting of switchgrass productivity under current and future climate change scenarios. Ecol. Appl. 2013, 23, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.S.; Oates, L.G.; Gelfand, I.; Millar, N.; Robertson, G.P.; Jackson, R.D. Environmental factors function as constraints on soil nitrous oxide fluxes in bioenergy feedstock cropping systems. Glob. Chang. Biol. Bioenergy 2019, 11, 416–426. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a conceptual model of soil emissions of nitrous and nitric oxides: Using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. Bioscience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Nuccio, E.E.; McFarlane, K.J.; Ramon, C.E.; Saha, M.; Firestone, M.K.; Pett Ridge, J. Quantifying the effects of switchgrass (Panicum virgatum) on deep organic C stocks using natural abundance 14C in three marginal soils. Glob. Chang. Biol. Bioenergy 2020, 12, 834–847. [Google Scholar] [CrossRef]
- Zamulina, I.V.; Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Burachevskaya, M.V.; Bauer, T.V. Soil organic matter and biological activity under long-term contamination with copper. Environ. Geochem. Health 2022, 44, 387–398. [Google Scholar] [CrossRef]
- Raskin, I.; Salt, D.E.; Smith, R.D. Phytoremediation. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Cooney, C.M. News: Sunflowers remove radionuclides from water in ongoing phytoremediation field tests. Environ. Sci. Technol. 1996, 30, 194A. [Google Scholar] [CrossRef]
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of grasses for potential biofuel production and phytoremediation of heavy metal contaminated soils. Int. J. Phytoremediation 2015, 17, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Murphy, I.J.; Coats, J.R. The capacity of switchgrass (Panicum virgatum) to degrade atrazine in a phytoremediation setting. Environ. Toxicol. Chem. 2011, 30, 715–722. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, M.; Ma, X.; Zhang, H.; Wang, Y.; Cui, J.; Gao, W.; Gui, J. Model optimization of cadmium and accumulation in switchgrass (Panicum virgatum L.): Potential use for ecological phytoremediation in Cd-contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 16758–16771. [Google Scholar] [CrossRef]
- Greipsson, S.; McElroy, T.; Koether, M. Effects of supplementary nutrients (soil-nitrogen or foliar-iron) on switchgrass (Panicum virgatum L.) grown in Pb-contaminated soil. J. Plant. Nutr. 2022, 45, 2919–2930. [Google Scholar] [CrossRef]
- Hart, G.; Koether, M.; McElroy, T.; Greipsson, S. Evaluation of chelating agents used in phytoextraction by switchgrass of lead contaminated soil. Plants 2022, 11, 1012. [Google Scholar] [CrossRef]
- David, K.; Ragauskas, A.J. Switchgrass as an energy crop for biofuel production: A review of its ligno-cellulosic chemical properties. Energ. Environ. Sci. 2010, 3, 1182–1190. [Google Scholar] [CrossRef]
| Net Change in SOC (Mg ha−1 per 30 cm) | |||
|---|---|---|---|
| Ages (Year) | Switchgrass | Sugarcane | Miscanthus |
| 5 | 2.66 | −34.21 | 2.31 |
| 10 | 4.64 | −31.57 | 2.97 |
| 15 | 6.49 | −28.93 | 3.63 |
| Depth (cm) | Clay Loam | Sandy Loam |
|---|---|---|
| Root Biomass (kg m−2) | ||
| 0–20 | 7.28 ± 0.44 | 7.44 ± 0.39 |
| 20–40 | 2.66 ± 0.10 | 1.97 ± 0.43 |
| 40–60 | 1.75 ± 0.07 | 1.84 ± 0.33 |
| 60–80 | 1.25 ± 0.08 | 3.23 ± 0.31 |
| 80–100 | 1.16 ± 0.07 | 2.26 ± 0.25 |
| Location | Year | Crop | NEE (g C m−2 yr−1) | Citation |
|---|---|---|---|---|
| Urbana, IL, USA | 2009 | Switchgrass | −453 ± 20 | [30] |
| Miscanthus | −281 ± 30 | |||
| Corn | −307 ± 40 | |||
| 2010 | Switchgrass | −485 ± 20 | ||
| Guelph, ON, Canada | 2014 | Switchgrass | −336 ± 40 | [31] |
| Corn | 64 ± 41 | |||
| Chickasha, OK, USA | 2012 | Switchgrass | −490 ± 59 | [33] |
| Sorghum | −261 ± 48 | |||
| 2013 | Switchgrass | −406 ± 24 | ||
| Sorghum | −330 ± 45 | |||
| Cadriano, Italy | 2014–2016 | Switchgrass | −733 | [18] |
| Guelph, ON, Canada | 2012 | Switchgrass | −380 ± 25 | [35] |
| 2013 | −430 ± 30 | |||
| Ligonier, PA, USA | 2005–2006 | Switchgrass | −118 | [36] |
| 2006–2007 | −248 | |||
| 2007–2008 | −189 |
| Location | Year | N Source | N Treatment (kg N ha−1 yr−1) | Yield (t ha−1 yr−1) | N2O Emissions (g N ha−1 yr−1) | Citation |
|---|---|---|---|---|---|---|
| Truro, NS, Canada | 2009 | NH4NO3 | 0 | 7.1 | 463 | [46] |
| 40 | 6.6 | 345 | ||||
| 120 | 7 | 933 | ||||
| Mandan, ND, USA | 2010 | Urea | 0 | 3.67 | 58.94 | [48] |
| 67 | 4.47 | 184.29 | ||||
| MI, USA | 2009–2011 | Urea | 0 | 5.95 | 374.32 | [49] |
| 28 | 6.91 | 512.34 | ||||
| 56 | 7.85 | 698.45 | ||||
| 84 | 7.62 | 964.03 | ||||
| 112 | 7.72 | 1321.62 | ||||
| 140 | 8.26 | 1806.78 | ||||
| 168 | 7.82 | 2486.41 | ||||
| 196 | 8.03 | 2867 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Luo, L.; Li, A.; Lai, X.; Zhang, X.; Yu, Y.; Wang, H.; Wu, N.; Zhang, L. Effects of Biofuel Crop Switchgrass (Panicum virgatum) Cultivation on Soil Carbon Sequestration and Greenhouse Gas Emissions: A Review. Life 2022, 12, 2105. https://doi.org/10.3390/life12122105
Bai J, Luo L, Li A, Lai X, Zhang X, Yu Y, Wang H, Wu N, Zhang L. Effects of Biofuel Crop Switchgrass (Panicum virgatum) Cultivation on Soil Carbon Sequestration and Greenhouse Gas Emissions: A Review. Life. 2022; 12(12):2105. https://doi.org/10.3390/life12122105
Chicago/Turabian StyleBai, Jian, Laicong Luo, Aixin Li, Xiaoqin Lai, Xi Zhang, Yadi Yu, Hao Wang, Nansheng Wu, and Ling Zhang. 2022. "Effects of Biofuel Crop Switchgrass (Panicum virgatum) Cultivation on Soil Carbon Sequestration and Greenhouse Gas Emissions: A Review" Life 12, no. 12: 2105. https://doi.org/10.3390/life12122105
APA StyleBai, J., Luo, L., Li, A., Lai, X., Zhang, X., Yu, Y., Wang, H., Wu, N., & Zhang, L. (2022). Effects of Biofuel Crop Switchgrass (Panicum virgatum) Cultivation on Soil Carbon Sequestration and Greenhouse Gas Emissions: A Review. Life, 12(12), 2105. https://doi.org/10.3390/life12122105






