The Interaction of Seasons and Biogeochemical Properties of Water Regulate the Air–Water CO2 Exchanges in Two Major Tropical Estuaries, Bay of Bengal (India)
Abstract
1. Introduction
2. Materials and Methods
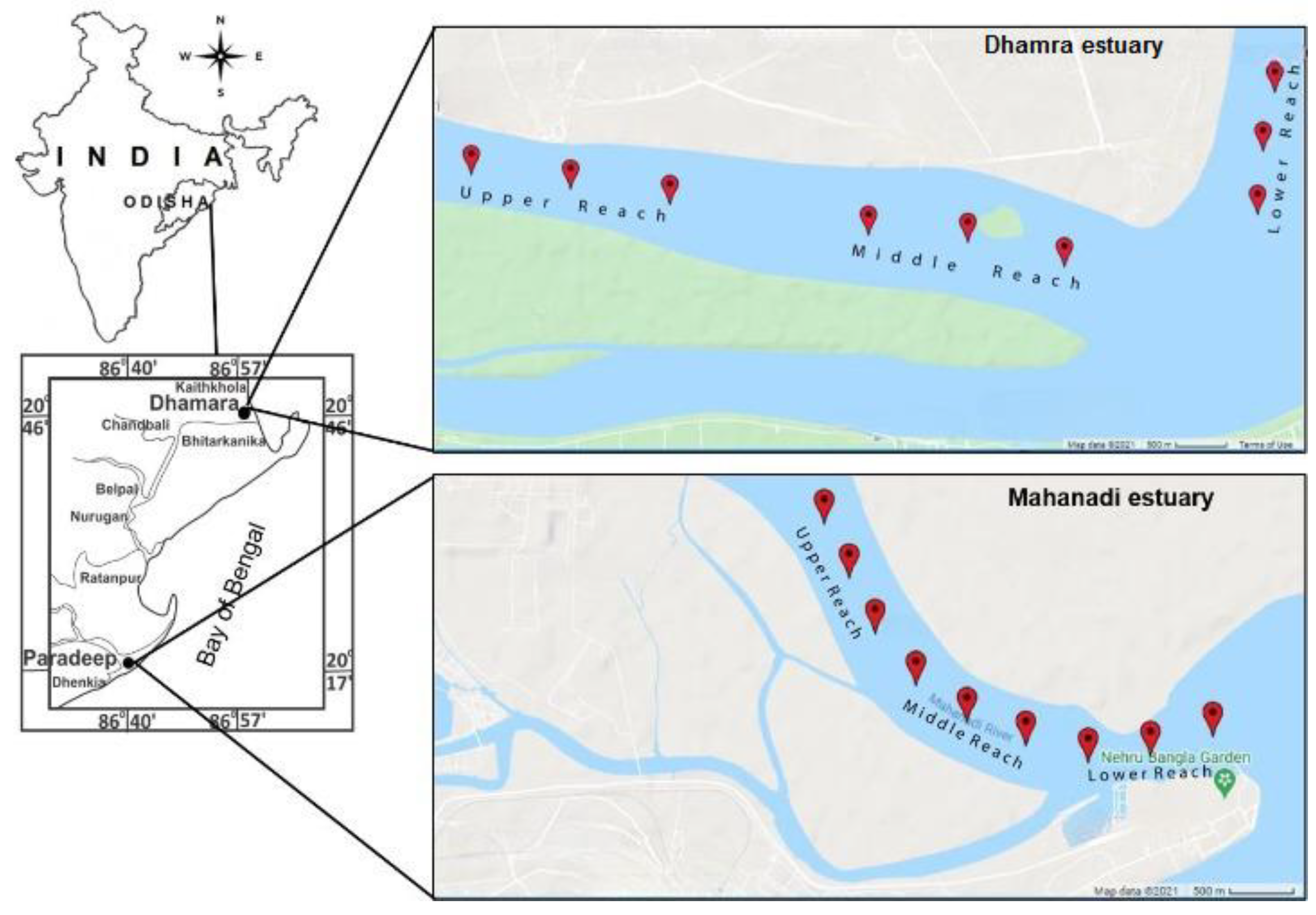
2.1. Study Area
2.2. Sampling Strategy
2.3. Biogeochemical Properties
2.3.1. Physicochemical Properties
2.3.2. Biological Properties
2.3.3. Air–Water CO2 Flux
2.4. Statistical Analysis
3. Results
3.1. Biogeochemical Properties of Estuaries
3.1.1. Biological Properties of Water in Estuaries
3.1.2. Physicochemical Properties of Water in Estuaries
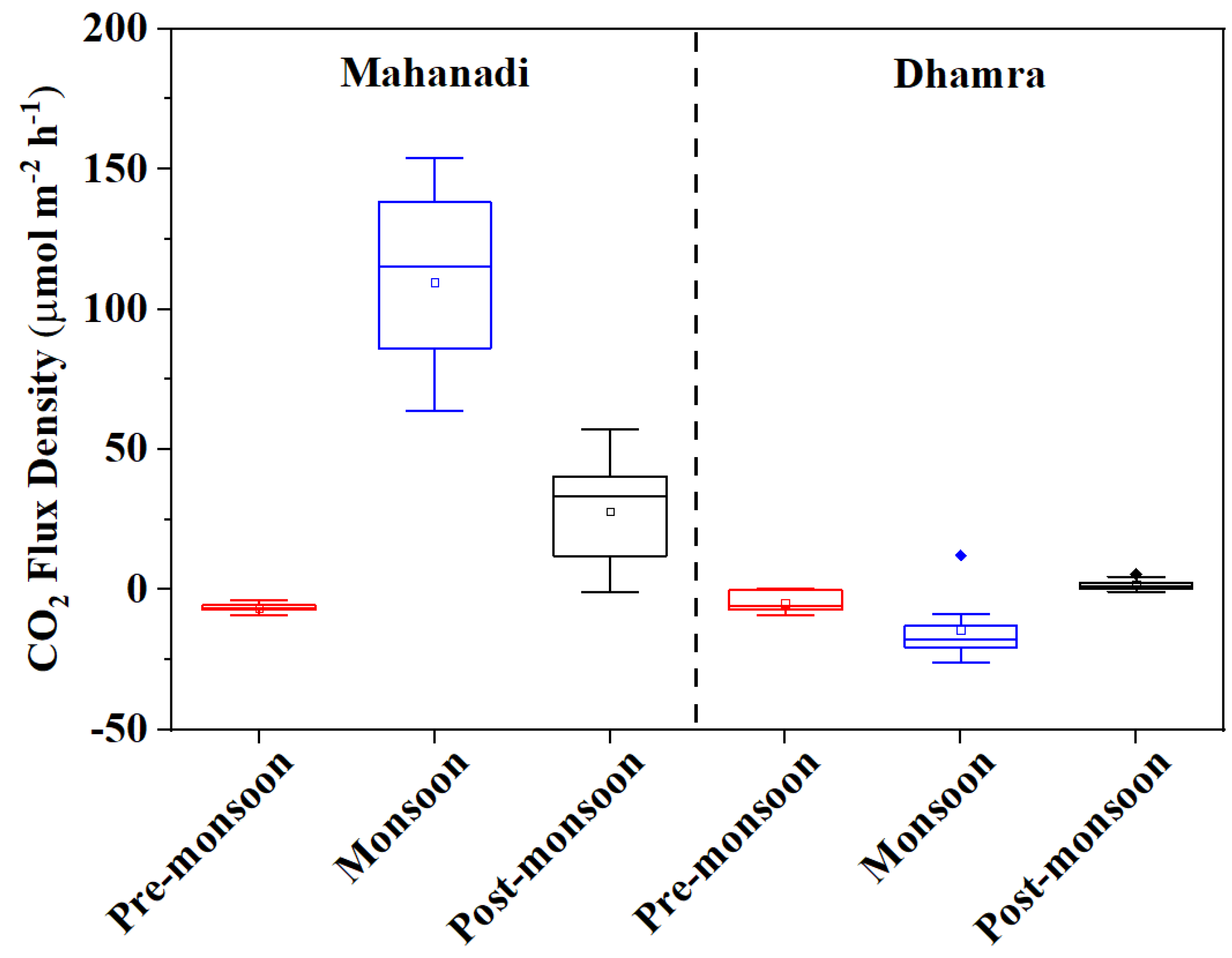
3.2. Air–Water CO2 Fluxes in Estuaries and Their Drivers
3.3. Correlation among Physicochemical and Biological Parameters of Estuaries
4. Discussion
4.1. Biological Bloom and Behavior in Estuaries
4.2. Temporal and Spatial Relationship of Physicochemical Properties of Estuaries
4.3. Drivers of Variations of Air–Water CO2 Exchanges and Their Prediction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borges, A.V.; Delille, B.; Frankignoulle, M. Budgeting Sinks and Sources of CO2 in the Coastal Ocean: Diversity of Ecosystems Counts. Geophys. Res. Lett. 2005, 32, 14. [Google Scholar] [CrossRef]
- Testa, J.M.; Kemp, W.M.; Hopkinson, C.S.; Smith, S.V. Ecosystem Metabolism. In Estuarine Ecology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 381–416. [Google Scholar]
- Laruelle, G.G.; Dürr, H.H.; Lauerwald, R.; Hartmann, J.; Slomp, C.P.; Goossens, N.; Regnier, P.A.G. Global Multi-Scale Segmentation of Continental and Coastal Waters from the Watersheds to the Continental Margins. Hydrol. Earth Syst. Sci. 2013, 17, 2029–2051. [Google Scholar] [CrossRef]
- Eyre, B.D.; Ferguson, A.J.P.; Webb, A.; Maher, D.; Oakes, J.M. Metabolism of Different Benthic Habitats and Their Contribution to the Carbon Budget of a Shallow Oligotrophic Sub-Tropical Coastal System (Southern Moreton Bay, Australia). Biogeochemistry 2011, 102, 87–110. [Google Scholar] [CrossRef]
- Maher, D.T.; Eyre, B.D. Carbon Budgets for Three Autotrophic Australian Estuaries: Implications for Global Estimates of the Coastal Air-Water CO 2 Flux. Glob. Biogeochem. Cycles 2012, 26, 1. [Google Scholar] [CrossRef]
- Cai, W.-J. Estuarine and Coastal Ocean Carbon Paradox: CO 2 Sinks or Sites of Terrestrial Carbon Incineration? Ann. Rev. Mar. Sci. 2011, 3, 123–145. [Google Scholar] [CrossRef]
- Van Dam, B.R.; Crosswell, J.R.; Anderson, I.C.; Paerl, H.W. Watershed-Scale Drivers of Air-Water CO 2 Exchanges in Two Lagoonal North Carolina (USA) Estuaries. J. Geophys. Res. Biogeosci. 2018, 123, 271–287. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Cai, W.-J.; Lehrter, J.; Chen, B.; Ouyang, Z.; Le, C.; Roberts, B.J.; Hussain, N.; Scaboo, M.K.; Zhang, J.; et al. Spring net community production and its coupling with the CO2 dynamics in the surface water of the northern Gulf of Mexico. Biogeosciences 2019, 16, 3507–3525. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Dürr, H.H.; Slomp, C.P.; Borges, A.V. Evaluation of Sinks and Sources of CO 2 in the Global Coastal Ocean Using a Spatially-Explicit Typology of Estuaries and Continental Shelves. Geophys. Res. Lett. 2010, 37, 15. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, M. On the Seasonal Variation of Air—Sea CO2 Fluxes in the Outer Changjiang (Yangtze River) Estuary, East China Sea. Mar. Chem. 2009, 117, 2–10. [Google Scholar] [CrossRef]
- Sarma, V.V.S.S.; Viswanadham, R.; Rao, G.D.; Prasad, V.R.; Kumar, B.S.K.; Naidu, S.A.; Kumar, N.A.; Rao, D.B.; Sridevi, T.; Krishna, M.S.; et al. Carbon Dioxide Emissions from Indian Monsoonal Estuaries. Geophys. Res. Lett. 2012, 39, 3. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Manna, S.; Das, S.; Hazra, S.; Roy, R.; Choudhury, S.B.; Rao, K.H.; Dadhwal, V.K.; Chakraborty, K.; et al. A Comparison of CO2 Dynamics and Air-Water Fluxes in a River-Dominated Estuary and a Mangrove-Dominated Marine Estuary. Geophys. Res. Lett. 2016, 43, 11726–11735. [Google Scholar] [CrossRef]
- Pattanaik, S.; Acharya, D.; Sahoo, R.K.; Satapathy, D.R.; Panda, C.R.; Choudhury, S.B.; Nagamani, P.V.; Roy, R. Short-Term Variability of Physico-Chemical Properties and PCO2 Fluxes off Dhamra Estuary from North-Eastern India. J. Indian Soc. Remote Sens. 2019, 47, 1197–1208. [Google Scholar] [CrossRef]
- Pattanaik, S.; Chanda, A.; Sahoo, R.K.; Swain, S.; Satapathy, D.R.; Panda, C.R.; Choudhury, S.B.; Mohapatra, P.K. Contrasting Intra-Annual Inorganic Carbon Dynamics and Air–Water CO2 Exchange in Dhamra and Mahanadi Estuaries of Northern Bay of Bengal, India. Limnology 2020, 21, 129–138. [Google Scholar] [CrossRef]
- Migné, A.; Gévaert, F.; Créach, A.; Spilmont, N.; Chevalier, E.; Davoult, D. Photosynthetic Activity of Intertidal Microphytobenthic Communities during Emersion: In Situ Measurements of Chlorophyll Fluorescence (PAM) and CO 2 Flux (IRGA) 1. J. Phycol. 2007, 43, 864–873. [Google Scholar] [CrossRef]
- Bennington, V.; McKinley, G.A.; Dutkiewicz, S.; Ullman, D. What Does Chlorophyll Variability Tell Us about Export and Air-Sea CO 2 Flux Variability in the North Atlantic? Glob. Biogeochem. Cycles 2009, 23, 3. [Google Scholar] [CrossRef]
- Serôdio, J.; Cruz, S.; Vieira, S.; Brotas, V. Non-Photochemical Quenching of Chlorophyll Fluorescence and Operation of the Xanthophyll Cycle in Estuarine Microphytobenthos. J. Exp. Mar. Biol. Ecol. 2005, 326, 157–169. [Google Scholar] [CrossRef]
- Kromkamp, J.C.; Morris, E.P.; Forster, R.M.; Honeywill, C.; Hagerthey, S.; Paterson, D.M. Relationship of Intertidal Surface Sediment Chlorophyll Concentration to Hyperspectral Reflectance and Chlorophyll Fluorescence. Estuaries Coasts 2006, 29, 183–196. [Google Scholar] [CrossRef]
- Maricle, B.R.; Lee, R.W.; Hellquist, C.E.; Kiirats, O.; Edwards, G.E. Effects of salinity on chlorophyll fluorescence and CO2 fixation in C4 estuarine grasses. Photosynthetica 2007, 45, 433–440. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical Speciation and Risk Assessment of Heavy Metals in the River Estuarine Sediments—A Case Study: Mahanadi Basin, India. J. Hazard Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- CPCB. Basin Sub-Basin Inventory of Water Pollution: The Mahanadi Basin, ADSORBS/23/1999–2000; Central Pollution Control Board: New Delhi, India, 2000. [Google Scholar]
- Woodroffe, C. Mangrove Sediments and Geomorphology. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; Volume 41, pp. 7–41. [Google Scholar]
- Kumar, M.; Mohapatra, S.; Karim, A.A.; Dhal, N.K. Heavy Metal Fractions in Rhizosphere Sediment Vis-à-Vis Accumulation in Phoenix Paludosa (Roxb.) Mangrove Plants at Dhamra Estuary of India: Assessing Phytoremediation Potential. Chem. Ecol. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Friess, D.A.; Yando, E.S.; Alemu, J.B.; Wong, L.W.; Soto, S.D.; Bhatia, N. Ecosystem Services and Disservices of Mangrove Forests and Salt Marshes. In Oceanography and Marine Biology: An Annual Review; Hawkins, S.J., Allcock, A.L., Bates, A.E., Evans, A.J., Firth, L.B., McQuaid, C.D., Russell, B.D., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; Taylor & Francis: Baco Raton, FL, USA; London, UK; New York, NY, USA, 2020; Volume 58, pp. 107–141. [Google Scholar]
- Patnaik, M.R.; Purohit, K.L.; Patra, A.K. Mangrove Swamps of Bhitarkanika, Orissa, India—A Great Eco Habitat for Wildlife. Cheetal 1995, 34, 1–9. [Google Scholar]
- APHA; AWWA. Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) Methods of Seawater Analysis, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Und Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Strasserf, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace DW, R.; Allison, L.J. Program Developed for CO2 System Calculations; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998.
- Peng, T.-H.; Takahashi, T.; Broecker, W.S.; Olafsson, J. Seasonal Variability of Carbon Dioxide, Nutrients and Oxygen in the Northern North Atlantic Surface Water: Observations and a Model. Tellus B 1987, 39 B, 439–458. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon Dioxide in Water and Seawater: The Solubility of a Non-Ideal Gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between Wind Speed and Gas Exchange over the Ocean. J. Geophys. Res. 1992, 97, 7373. [Google Scholar] [CrossRef]
- Liss, P.S.; Merlivat, L. Air Sea Gas Exchange Rates: Introduction and Synthesis. In The Role of Air Sea Exchange in Geochemical Cycling; Buat-Menard, P., Ed.; Springer: Dordrecht, The Netherlands, 1986; Volume 1986, pp. 113–119. [Google Scholar]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-Driven Trends in Contemporary Ocean Productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef]
- Schuster, U.; Watson, A.J. A Variable and Decreasing Sink for Atmospheric CO 2 in the North Atlantic. J. Geophys. Res. 2007, 112, C11006. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The Oceanic Sink for Anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; CRC Press: Boca Raton, FL, USA, 2014; p. 445. [Google Scholar]
- Prasil, O.; Suggett, D.J.; Cullen, J.J.; Babin, M. Aquafluo 2007: Chlorophyll Fluorescence in Aquatic Sciences, an International Conference Held in Nové Hrady. Photosynth. Res. 2007, 95, 111–115. [Google Scholar] [CrossRef]
- Eggert, A.; Häubner, N.; Klausch, S.; Karsten, U.; Schumann, R. Quantification of Algal Biofilms Colonising Building Materials: Chlorophyll a Measured by PAM-Fluorometry as a Biomass Parameter. Biofouling 2006, 22, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Mohapatra, P.K.; Schiewer, U. Influence of Dimethoate on the Structure and Function of the Natural Phytoplankton Assemblage of the Darss-Zingstbodden Chain Reared in a Laboratory. Pol. J. Environ. Stud. 1996, 2, 31–36. [Google Scholar]
- Wang, C.; Wang, Z.; Wang, P.; Zhang, S. Multiple Effects of Environmental Factors on Algal Growth and Nutrient Thresholds for Harmful Algal Blooms: Application of Response Surface Methodology. Environ. Modeling Assess. 2016, 21, 247–259. [Google Scholar] [CrossRef]
- Chakrapani, G.J.; Subramanian, V. Preliminary Studies on the Geochemistry of the Mahanadi River Basin, India. Chem. Geol. 1990, 81, 241–253. [Google Scholar] [CrossRef]
- Giannini, F.; Mendes, C.R.B.; Garcia, C.A.E.; Carvalho, A.C.O.; Ciotti, A.M. Phytoplankton Community and the Fluorescence-Derived Photo-Physiological Parameters in the South Atlantic Ocean. J. Mar. Syst. 2021, 218, 103538. [Google Scholar] [CrossRef]
| Parameters | Mahanadi | Dhamra | |||||
|---|---|---|---|---|---|---|---|
| Pre-Monsoon | Monsoon | Post-Monsoon | Pre-Monsoon | Monsoon | Post-Monsoon | ||
| Chl a (mg m−3) | Mean | 3.41 ± 1.36 1.71–6.28 | 3.16 ± 0.77 2.26–4.68 | 6.40 ± 1.23 4.51–8.84 | 6.83 ± 2.13 3.68–9.20 | 4.23 ± 1.69 2.40–6.92 | 7.15 ± 2.70 2.46–10.27 |
| Range | |||||||
| Fluorescence minimum | Mean | 44.73 ± 8.01 34.27–59.48 | 41.10 ± 7.36 34.24–56.34 | 67.86 ± 9.18 52.45–82.67 | 69.21 ± 15.64 45.78–87.95 | 50.66 ± 13.93 34.53–71.69 | 72.41 ± 18.95 37.23–92.45 |
| Range | |||||||
| Fluorescence maximum | Mean | 70.88 ± 16.5 51.78–103.86 | 64.86 ± 15.12 50.35–97.44 | 119.34 ± 20.07 88.67–153.75 | 125.8 ± 34.57 73.95–171.83 | 84.82 ± 29.91 51.68–130.79 | 134.08 ± 45.68 53.24–184.62 |
| Range | |||||||
| Yield | Mean | 0.36 ± 0.03 0.32–0.43 | 0.36 ± 0.03 0.32–0.42 | 0.43 ± 0.02 0.40–0.46 | 0.44 ± 0.03 0.38–0.49 | 0.39 ± 0.05 0.33–0.45 | 0.44 ± 0.07 0.30–0.51 |
| Range | |||||||
| Phycobilisome fluorescence | Mean | 17.44 ± 6.93 8.72–32.17 | 26.42 ± 6.25 18.34–38.94 | 39.27 ± 7.83 27.61–54.77 | 35.7 ± 10.94 19.32–48.21 | 37.37 ± 14.28 22.35–60.54 | 46.07 ± 17.42 16.04–66.27 |
| Range | |||||||
| PSII fluorescence | Mean | 3.45 ± 1.38 1.71–6.42 | 3.47 ± 0.79 2.53–4.98 | 6.60 ± 1.28 4.62–9.17 | 6.49 ± 1.98 3.53–8.65 | 3.94 ± 1.49 2.43–6.31 | 6.98 ± 2.52 2.54–9.94 |
| Range | |||||||
| PBS/PSII | Mean | 5.07 ± 0.11 4.89–5.20 | 7.61 ± 0.25 7.24–7.96 | 5.94 ± 0.08 5.74–6.03 | 5.50 ± 0.10 5.34–5.64 | 9.51 ± 0.30 8.88–9.83 | 6.55 ± 0.21 6.31–6.97 |
| Range | |||||||
| Parameters | Mahanadi | Dhamra | |||||
|---|---|---|---|---|---|---|---|
| Pre-Monsoon | Monsoon | Post-Monsoon | Pre-Monsoon | Monsoon | Post-Monsoon | ||
| CO2 flux (µmol m−2 h−1) | Mean | −6.5 ± 1.6 −9.2–−3.5 | 109.5 ± 31.5 63.8–154.1 | 28 ± 20.1 −0.7–57.1 | −4.7 ± 3.7 −9.2–0.3 | −14.2 ± 11.1 −25.8–12.2 | 1.8 ± 2.2 −0.7–5.6 |
| Range | |||||||
| fCO2 (water) (µatm) | Mean | 270.3 ± 17.4 240.5–289.6 | 759.4 ± 109.9 600.3–910.9 | 840.0 ± 294.2 417.6–1268.0 | 300.2 ± 31.4 270.5–365.2 | 258.8 ± 38.5 208.8–327.3 | 432.2 ± 31.2 395.4–485.6 |
| Range | |||||||
| fCO2 (air) (µatm) | Mean | 381.7 ± 2.724 379.4–388.2 | 379.7 ± 2.681 378.2–386.8 | 421.7 ± 7.397 414.1–432.7 | 382.2 ± 2.118 378.8–384.9 | 386.6 ± 3.9 382.2–394.90 | 406.3 ± 0.993 405.4–408.7 |
| Range | |||||||
| TA (µmol kg−1) | Mean | 1564.0 ± 54.63 1476.9–1669.5 | 1240.8 ± 105.8 1123.8–1457.9 | 1284.4 ± 250.2 1012.4–1669.3 | 1873.4 ± 213.1 1513.0–2151 | 1022.0 ± 52.3 919.8–1079.7 | 1831.9 ± 29.1 1769.2–1876.2 |
| Range | |||||||
| DIC (µmol kg−1) | Mean | 1415.9 ± 49.0 1346.8–1502.2 | 1234.6 ± 105.6 1112.5–1448.9 | 1270.1 ± 249.5 998.1–1648.7 | 1606.4 ± 140.9 1329.3–1774.6 | 934.2 ± 46.4 853.5–998.0 | 1683.5 ± 28.8 1622.3–1731.1 |
| Range | |||||||
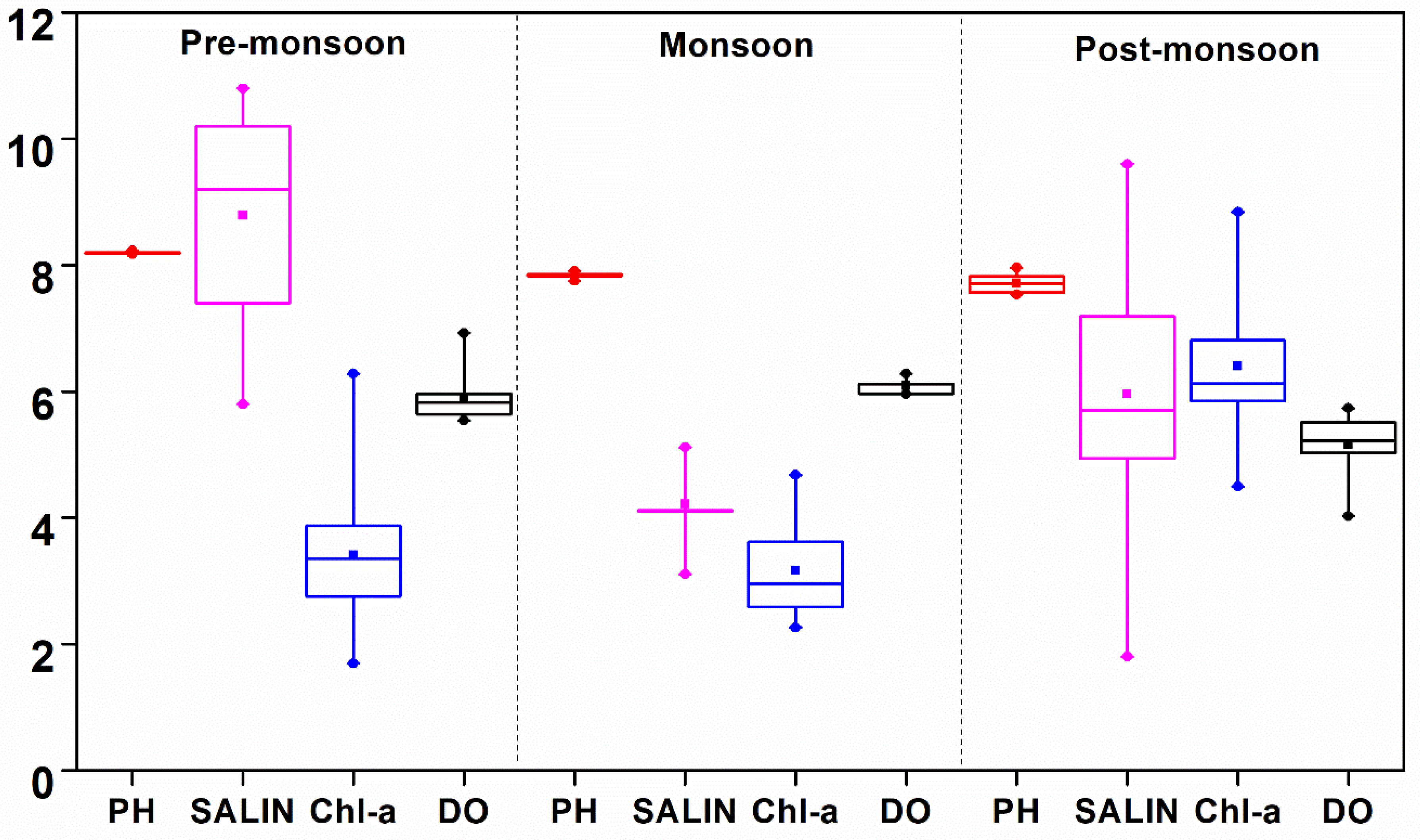
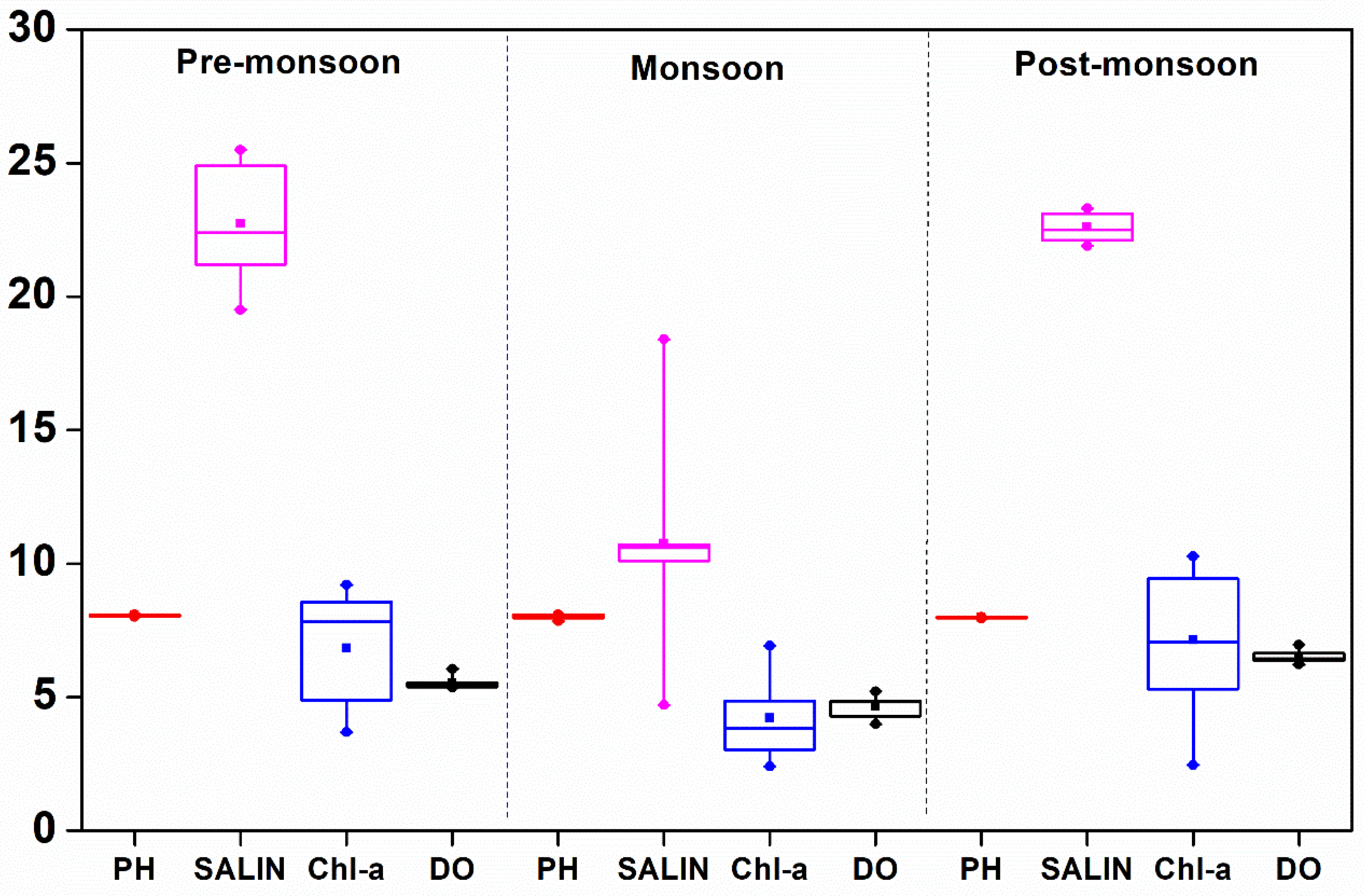
| pH | Mean | 8.197 ± 0.015 8.180–8.230 | 7.847 ± 0.048 7.750–7.911 | 7.716 ± 0.153 7.541–7.962 | 8.059 ± 0.038 8.011–8.101 | 8.02 ± 0.088 7.840–8.101 | 7.974 ± 0.021 7.940–8.001 |
| Range | |||||||
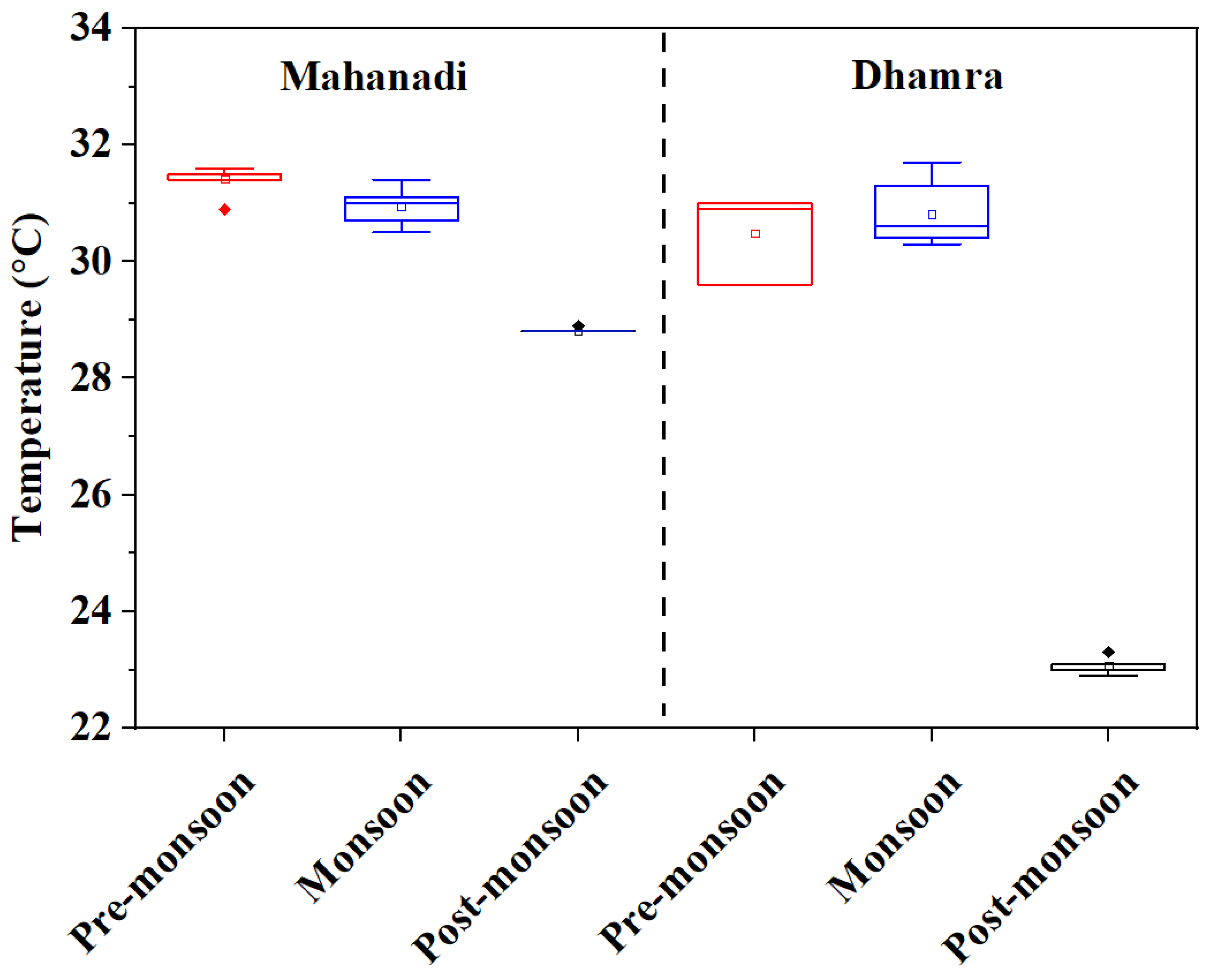
| Water temperature (°C) | Mean | 31.4 ± 0.21 30.9–31.6 | 30.9 ± 0.32 30.5–31.4 | 28.8 ± 0.01 28.8–28.9 | 30.5 ± 0.71 29.6–31.0 | 30.8 ± 0.62 30.3–31.7 | 23.1 ± 0.13 22.9–23.3 |
| Range | |||||||
| Salinity (psu) | Mean | 8.8 ± 1.8 5.8–10.8 | 4.2 ± 0.6 3.1–5.1 | 6 ± 2.3 1.8–9.6 | 22.7 ± 2.1 19.5–25.5 | 10.8 ± 3.5 4.7–18.4 | 22.6 ± 0.5 21.9–23.3 |
| Range | |||||||
| D.O. (mg L−1) | Mean | 5.9 ± 0.4 5.5–6.9 | 6.1 ± 0.1 6.0–6.3 | 5.2 ± 0.5 4.0–5.7 | 5.5 ± 0.2 5.3–6.1 | 4.6 ± 0.4 4.0–5.2 | 6.5 ± 0.2 6.2–7.0 |
| Range | |||||||
| Nitrite (µmol L−1) | Mean | 1.562 ± 0.661 0.52–2.678 | 0.262 ± 0.035 0.209–0.313 | 0.100 ± 0.038 0.048–0.152 | 0.737 ± 0.572 0.052–1.650 | 0.309 ± 0.088 0.174–0.483 | 0.247 ± 0.25 BDL–0.774 |
| Range | |||||||
| Nitrate (µmol L−1) | Mean | 7.839 ± 4.771 2.639–15.038 | 14.731 ± 0.752 13.542–15.653 | 4.259 ± 2.579 1.977–10.728 | 9.028 ± 1.387 7.358–10.915 | 8.114 ± 1.81 6.454–12.202 | 16.391 ± 1.621 14.235–19.134 |
| Range | |||||||
| Ammonia (µmol L−1) | Mean | 0.055 ± 0.108 BDL–0.338 | 1.217 ± 0.36 0.832–1.756 | 1.351 ± 1.658 BDL–5.273 | 7.976 ± 11.246 BDL–34.315 | 1.127 ± 0.973 0.211–3.464 | 5.369 ± 6.809 0.658–20.516 |
| Range | |||||||
| Phosphate (µmol L−1) | Mean | 2.554 ± 1.198 1.348–5.037 | 5.819 ± 1.423 4.250–8.392 | 0.967 ± 0.504 0.324–1.758 | 0.467 ± 0.124 0.284–0.659 | 1.485 ± 0.194 1.289–1.889 | 0.363 ± 0.203 0.137–0.811 |
| Range | |||||||
| FCO2 (Flux Density) | PH | Water Temperature | Salinity | Chl a | F0 | Fm | Yield | PBS | PS II | PBS/PSII | DO | NO2− | NO3− | NH3 | PO43− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCO2 (Flux density) | 1 | |||||||||||||||
| PH | −0.597 ** | 1 | ||||||||||||||
| Water temperature | 0.152 | 0.186 | 1 | |||||||||||||
| salinity | −0.504 ** | 0.313 * | −0.535 ** | 1 | ||||||||||||
| Chl a | −0.327* | −0.016 | −0.448 ** | 0.438 ** | 1 | |||||||||||
| F0 | −0.359 ** | −0.017 | −0.459 ** | 0.441 ** | 0.990 ** | 1 | ||||||||||
| Fm | −0.351 ** | −0.002 | −0.468 ** | 0.454 ** | 0.994 ** | 0.996 ** | 1 | |||||||||
| Yield | −0.331 * | −0.024 | −0.401 ** | 0.432 ** | 0.962 ** | 0.9 57** | 0.965 ** | 1 | ||||||||
| PBS | −0.222 | −0.114 | −0.456 ** | 0.289 * | 0.902 ** | 0.896 ** | 0.906 ** | 0.890 ** | 1 | |||||||
| PS II | −0.262 | −0.076 | −0.457 ** | 0.381 ** | 0.994 ** | 0.984 ** | 0.985 ** | 0.949 ** | 0.892 ** | 1 | ||||||
| PBS/PSII | 0.133 | −0.160 | 0.077 | −0.228 | −0.206 | −0.216 | −0.198 | −0.159 | 0.196 | −0.238 | 1 | |||||
| DO | 0.265 | 0.056 | −0.452 ** | 0.271 * | 0.043 | 0.029 | 0.049 | 0.013 | −0.077 | 0.072 | −0.394 ** | 1 | ||||
| NO2− | −0.136 | 0.217 | 0.114 | 0.207 | −0.087 | −0.109 | −0.096 | 0.008 | −0.196 | −0.105 | −0.229 | −0.017 | 1 | |||
| NO3− | 0.339 * | 0.008 | −0.443 ** | 0.292 * | −0.016 | −0.057 | −0.022 | −0.037 | 0.074 | −0.011 | 0.156 | 0.628 ** | −0.100 | 1 | ||
| NH3 | −0.123 | 0.086 | −0.109 | 0.395 ** | 0.360 ** | 0.361 ** | 0.365 ** | 0.334 * | 0.244 | 0.328 * | −0.122 | 0.070 | −0.102 | 0.036 | 1 | |
| PO43− | 0.718 ** | −0.104 | 0.432 ** | −0.612 ** | −0.571 ** | −0.587 ** | −0.570 ** | −0.566 ** | −0.428 ** | −0.534 ** | 0.219 | 0.157 | −0.096 | 0.257 | −0.218 | 1 |
| FCO2 (Flux Density) | PH | Water Temperature | Salinity | Chl a | F0 | Fm | Yield | PBS | PS II | PBS/PSII | DO | NO2− | NO3− | NH3 | PO43− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCO2 (Flux density) | 1 | |||||||||||||||
| PH | −0.716 ** | 1 | ||||||||||||||
| Water temperature | −0.601 ** | 0.479 * | 1 | |||||||||||||
| salinity | 0.532 ** | −0.001 | −0.485 * | 1 | ||||||||||||
| Chl a | 0.105 | 0.283 | −0.288 | 0.435 * | 1 | |||||||||||
| F0 | 0.089 | 0.286 | −0.302 | 0.436 * | 0.993 ** | 1 | ||||||||||
| Fm | 0.098 | 0.273 | −0.309 | 0.427 * | 0.996 ** | 0.997 ** | 1 | |||||||||
| Yield | 0.070 | 0.297 | −0.223 | 0.407 * | 0.954 ** | 0.951 ** | 0.960 ** | 1 | ||||||||
| PBS | −0.149 | 0.223 | −0.283 | 0.063 | 0.867 ** | 0.871 ** | 0.881 ** | 0.855 ** | 1 | |||||||
| PS II | 0.145 | 0.247 | −0.334 | 0.473 * | 0.997 ** | 0.991 ** | 0.994 ** | 0.945 ** | 0.853 ** | 1 | ||||||
| PBS/PSII | −0.544 ** | −0.099 | 0.288 | −0.918 ** | −0.462 * | −0.457 * | −0.442 * | −0.411 * | 0.000 | −0.492 ** | 1 | |||||
| DO | 0.608 ** | −0.250 | −0.829 ** | 0.781 ** | 0.460* | 0.464 * | 0.464 * | 0.378 | 0.240 | 0.509 ** | −0.644 ** | 1 | ||||
| NO2− | 0.117 | 0.027 | 0.116 | 0.221 | −0.091 | −0.121 | −0.106 | 0.036 | −0.210 | −0.096 | −0.226 | −0.056 | 1 | |||
| NO3− | 0.625 ** | −0.541 ** | −0.903 ** | 0.502 ** | 0.299 | 0.319 | 0.315 | 0.213 | 0.251 | 0.351 | −0.343 | 0.841 ** | −0.193 | 1 | ||
| NH3 | 0.077 | 0.245 | 0.032 | 0.298 | 0.324 | 0.331 | 0.320 | 0.284 | 0.127 | 0.308 | −0.329 | 0.148 | −0.121 | −0.073 | 1 | |
| PO43− | −0.589 ** | −0.005 | 0.580 ** | −0.886 ** | −0.505 ** | −0.499 ** | −0.498 ** | −0.450 * | −0.156 | −0.536 ** | 0.884 ** | −0.796 ** | −0.198 | −0.512 ** | −0.251 | 1 |
| FCO2 (Flux Density) | PH | Water Temperature | Salinity | Chl a | F0 | Fm | Yield | PBS | PS II | PBS/PSII | DO | NO2− | NO3− | NH3 | PO43− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCO2 (Flux density) | 1 | |||||||||||||||
| PH | −0.543 ** | 1 | ||||||||||||||
| Water temperature | 0.066 | 0.734 ** | 1 | |||||||||||||
| salinity | −0.577 ** | 0.390* | 0.197 | 1 | ||||||||||||
| Chl a | −0.294 | −0.353 | −0.740 ** | −0.318 | 1 | |||||||||||
| F0 | −0.354 | −0.338 | −0.760 ** | −0.232 | 0.983 ** | 1 | ||||||||||
| Fm | −0.343 | −0.340 | −0.762 ** | −0.263 | 0.987 ** | 0.998 ** | 1 | |||||||||
| Yield | −0.285 | −0.373 | −0.702 ** | −0.300 | 0.957 ** | 0.950 ** | 0.958 ** | 1 | ||||||||
| PBS | 0.053 | −0.545 ** | −0.733 ** | −0.587 ** | 0.919 ** | 0.891 ** | 0.903 ** | 0.898 ** | 1 | |||||||
| PS II | −0.241 | −0.384* | −0.742 ** | −0.366 | 0.998 ** | 0.976 ** | 0.981 ** | 0.952 ** | 0.940 ** | 1 | ||||||
| PBS/PSII | 0.907 ** | −0.506 ** | 0.005 | −0.721 ** | −0.183 | −0.235 | −0.211 | −0.140 | 0.208 | −0.128 | 1 | |||||
| DO | 0.290 | 0.280 | 0.683 ** | 0.021 | −0.650 ** | −0.669 ** | −0.666 ** | −0.569 ** | −0.563 ** | −0.644 ** | 0.250 | 1 | ||||
| NO2− | −0.526 ** | 0.801 ** | 0.624 ** | 0.498 ** | −0.344 | −0.349 | −0.351 | −0.321 | −0.582 ** | −0.378 | −0.611 ** | 0.255 | 1 | |||
| NO3− | 0.646 ** | 0.036 | 0.528 ** | −0.326 | −0.545 ** | −0.632 ** | −0.606 ** | −0.502 ** | −0.302 | −0.510 ** | 0.625 ** | 0.574 ** | −0.040 | 1 | ||
| NH3 | 0.328 | −0.523 ** | −0.375 | −0.319 | 0.219 | 0.241 | 0.250 | 0.248 | 0.361 | 0.244 | 0.366 | −0.135 | −0.470 * | 0.038 | 1 | |
| PO43− | 0.637 ** | 0.086 | 0.505 ** | −0.317 | −0.651 ** | −0.673 ** | −0.652 ** | −0.643 ** | −0.369 | −0.614 ** | 0.694 ** | 0.490 ** | −0.085 | 0.727 ** | 0.058 | 1 |
| Regression Equation | r2 (p ≤ 0.05) | |
|---|---|---|
| Mahanadi | ||
| Pre-Monsoon | FCO2 = −235.2 + 30.5 pH − 1.4 salinity − 0.12 Fm + 5.3NH4 | r2 = 0.94 |
| Monsoon | FCO2 = 4436.2 − 552.4 pH | r2 = 0.69 |
| Post-Monsoon | FCO2 = 9397.4 − 171.1 pH − 279.8 Water temperature +179.1 NO2 | r2 = 0.92 |
| Dhamra | ||
| Pre-Monsoon | FCO2 = 160.3 − 5.41 temperature | r2 = 0.95 |
| Monsoon | FCO2 = 867.7 − 108.7 pH − 0.92 salinity | r2 = 0.93 |
| Post-Monsoon | FCO2 = 363.7 − 58.6 pH + 5.4 Water temperature 7 − 0.91 salinity | r2 = 0.98 |
| Mahanadi Annual | FCO2 = 620.4 − 160.5 pH +24.9 temperature +17.5 Chl a − 836.8 yield + 29.3 PBS:PSII | r2 = 0.94 |
| Dhamra Annual | FCO2 = 965.9 − 116.1 pH − 0.39 salinity − 4.6 PBS:PSII | r2 = 0.90 |
| Mahanadi and Dhamra Annual | FCO2 = 1079.8 − 162.6 pH + 4.0 temperature + 13.8 DO +1.9 nitrate +10.1 phosphate | r2 = 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanaik, S.; Mohapatra, P.K.; Mohapatra, D.; Swain, S.; Panda, C.R.; Dash, P.K. The Interaction of Seasons and Biogeochemical Properties of Water Regulate the Air–Water CO2 Exchanges in Two Major Tropical Estuaries, Bay of Bengal (India). Life 2022, 12, 1536. https://doi.org/10.3390/life12101536
Pattanaik S, Mohapatra PK, Mohapatra D, Swain S, Panda CR, Dash PK. The Interaction of Seasons and Biogeochemical Properties of Water Regulate the Air–Water CO2 Exchanges in Two Major Tropical Estuaries, Bay of Bengal (India). Life. 2022; 12(10):1536. https://doi.org/10.3390/life12101536
Chicago/Turabian StylePattanaik, Suchismita, Pradipta Kumar Mohapatra, Debasish Mohapatra, Sanhita Swain, Chitta Ranjan Panda, and Pradeep Kumar Dash. 2022. "The Interaction of Seasons and Biogeochemical Properties of Water Regulate the Air–Water CO2 Exchanges in Two Major Tropical Estuaries, Bay of Bengal (India)" Life 12, no. 10: 1536. https://doi.org/10.3390/life12101536
APA StylePattanaik, S., Mohapatra, P. K., Mohapatra, D., Swain, S., Panda, C. R., & Dash, P. K. (2022). The Interaction of Seasons and Biogeochemical Properties of Water Regulate the Air–Water CO2 Exchanges in Two Major Tropical Estuaries, Bay of Bengal (India). Life, 12(10), 1536. https://doi.org/10.3390/life12101536








