Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of Waste Materials
2.2. Enzymatic Hydrolysis
2.3. Glucose Quantification
2.4. Growth Curves with Media Containing Glucose Juice
2.5. Limonene Production and Isolation
2.6. Product Quantification with Gas-Chromatography (GC)
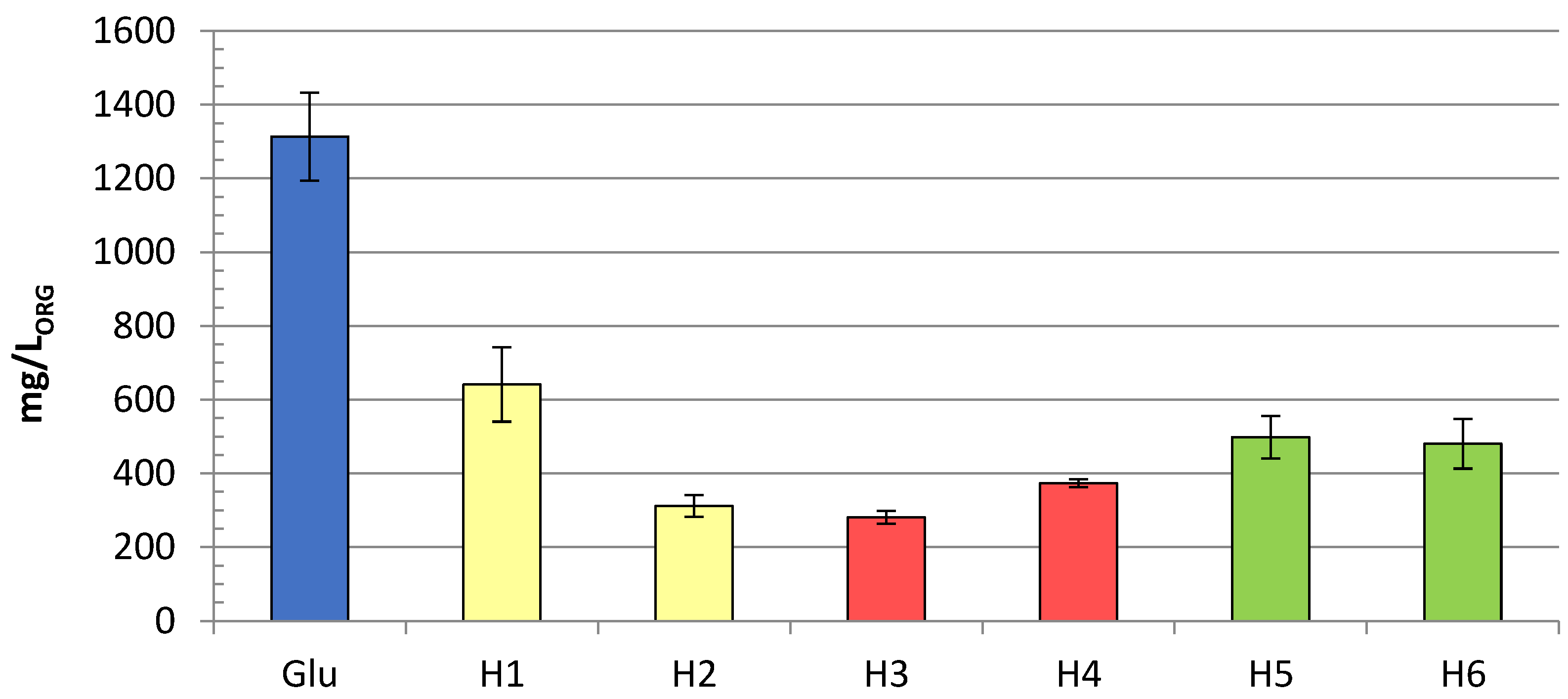
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bovea, M.D.; Ibanez-Fores, V.; Gallardo, A.; Colomer-Mendoza, F.J. Environmental assessment of alternative municipal solid waste management strategies. A spanish case study. Waste Manag. 2010, 30, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Mahvi, A.H.; Norouzian Baghani, A.; Sorooshian, A.; Delikhoon, M.; Sheikhi, R.; Ashournejad, Q. Municipal solid waste recycling: Impacts on energy savings and air pollution. J. Air Waste Manag. Assoc. 2021, 71, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.M.; Colvero, D.A.; Gomes, A.P.; Wenzel, H.; Schalch, V.; Cimpan, C. Environmental assessment of existing and alternative options for management of municipal solid waste in brazil. Waste Manag. 2018, 78, 857–870. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2020, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Tanguay-Rioux, F.; Heroux, M.; Legros, R. Physical properties of recyclable materials and implications for resource recovery. Waste Manag. 2021, 136, 195–203. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. Int. Solid Wastes Public Clean. Assoc. ISWA 2019, 37, 112–119. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, C. Chapter five—A “circular” world: Reconciling profitability with sustainability. In The Circular Economy; Sillanpää, M., Ncibi, C., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 207–279. [Google Scholar]
- Wojnowska-Baryla, I.; Bernat, K.; Zaborowska, M. Strategies of recovery and organic recycling used in textile waste management. Int. J. Environ. Res. Public Health 2022, 19, 5859. [Google Scholar] [CrossRef]
- Korolkow, J. Bedarf und Wiederverwendung von Bekleidung und Textilien in Deutschland; Bundesverband Sekundärrohstoffe Und Entsorgung: Bonn, Germany, 2015. [Google Scholar]
- Woolridge, A.C.; Ward, G.D.; Phillips, P.S.; Collins, M.; Gandy, S. Life cycle assessment for reuse/recycling of donated waste textiles compared to use of virgin material: An uk energy saving perspective. Resour. Conserv. Recycl. 2006, 46, 94–103. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. Environmental impacts of the textile industry and its assessment through life cycle assessment. In Roadmap to Sustainable Textiles and Clothing: Environmental and Social Aspects of Textiles and Clothing Supply Chain; Muthu, S.S., Ed.; Springer: Singapore, 2014; pp. 1–39. [Google Scholar]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Agency, E.E. European Environment Agency. 2020. Available online: https://www.Eea.Europa.Eu/media/infographics/textile-waste/view (accessed on 4 August 2021).
- Wang, Z.; Sundara Sekar, B.; Li, Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals. Bioresour. Technol. 2021, 323, 124551. [Google Scholar] [CrossRef]
- Degli-Innocenti, F. Is composting of packaging real recycling? Waste Manag. 2021, 130, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Bernet, N.; Steyer, J.P.; Delgenes, J.P.; Escudie, R. Cardboard proportions and total solids contents as driving factors in dry co-fermentation of food waste. Bioresour. Technol. 2018, 248, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Piribauer, B.; Bartl, A.; Ipsmiller, W. Enzymatic textile recycling—Best practices and outlook. Waste Manag. Res. Int. Solid Wastes Public Clean. Assoc. ISWA 2021, 39, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Haven, M.O.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and cellic(r) ctec2: Saccharification/fermentation of wheat straw, solid-liquid partition and potential of enzyme recycling by alkaline washing. Enzym. Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef]
- Quartinello, F.; Vecchiato, S.; Weinberger, S.; Kremenser, K.; Skopek, L.; Pellis, A.; Guebitz, G.M. Highly selective enzymatic recovery of building blocks from wool-cotton-polyester textile waste blends. Polymers 2018, 10, 1107. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid drugs, biofuels, and chemicals—Artemisinin, farnesene, and beyond. Adv. Biochem. Eng. Biotechnol. 2015, 148, 355–389. [Google Scholar]
- Vasic, K.; Knez, Z.; Leitgeb, M. Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Zebec, Z.; Wilkes, J.; Jervis, A.J.; Scrutton, N.S.; Takano, E.; Breitling, R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 2016, 34, 37–43. [Google Scholar] [CrossRef]
- Vickers, C.E.; Sabri, S. Isoprene. In Biotechnology of Isoprenoids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 289–317. [Google Scholar]
- Leferink, N.G.H.; Jervis, A.J.; Zebec, Z.; Toogood, H.S.; Hay, S.; Takano, E.; Scrutton, N.S. A ‘plug and play’ platform for the production of diverse monoterpene hydrocarbon scaffolds in Escherichia coli. ChemistrySelect 2016, 1, 1893–1896. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Kim, E.M.; Batth, T.S.; Cho, N.; Hu, Q.; Chan, L.J.; Petzold, C.J.; Hillson, N.J.; Adams, P.D.; Keasling, J.D.; et al. Principal component analysis of proteomics (pcap) as a tool to direct metabolic engineering. Metab. Eng. 2015, 28, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Gutierrez, J.; Koma, D.; Hu, Q.; Yang, Y.; Chan, L.J.G.; Petzold, C.J.; Adams, P.D.; Vickers, C.E.; Nielsen, L.K.; Keasling, J.D.; et al. Toward industrial production of isoprenoids in Escherichia coli: Lessons learned from crispr-cas9 based optimization of a chromosomally integrated mevalonate pathway. Biotechnol. Bioeng. 2018, 115, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lutz, S. A gram-scale limonene production process with engineered Escherichia coli. Molecules 2020, 25, 1881. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Gourlay, K.; Saddler, J.N. The enzymatic hydrolysis of pretreated pulp fibers predominantly involves “peeling/erosion” modes of action. Biotechnol. Biofuels 2014, 7, 87. [Google Scholar] [CrossRef]
- Sipos, B.; Dienes, D.; Schleicher, Á.; Perazzini, R.; Crestini, C.; Siika-aho, M.; Réczey, K. Hydrolysis efficiency and enzyme adsorption on steam-pretreated spruce in the presence of poly(ethylene glycol). Enzym. Microb. Technol. 2010, 47, 84–90. [Google Scholar] [CrossRef]
- Basu, S.; Malik, S.; Joshi, G.; Gupta, P.K.; Rana, V. Utilization of bio-polymeric additives for a sustainable production strategy in pulp and paper manufacturing: A comprehensive review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100050. [Google Scholar] [CrossRef]
- Kajiwara, K.; Uemura, T.; Kishikawa, H.; Nishida, K.; Hashiguchi, Y.; Uehara, M.; Sakakida, M.; Ichinose, K.; Shichiri, M. Noninvasive measurement of blood glucose concentrations by analysing fourier transform infra-red absorbance spectra through oral mucosa. Med. Biol. Eng. Comput. 1993, 31, S17–S22. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, J.; Park, S.Y.; Yu, J.H.; Kim, K.H. Cellulase recycling in high-solids enzymatic hydrolysis of pretreated empty fruit bunches. Biotechnol. Biofuels 2019, 12, 138. [Google Scholar] [CrossRef]
- Azam Ali, M.; Shavandi, A. 6—Medical textiles testing and quality assurance. In Performance Testing of Textiles; Wang, L., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 129–153. [Google Scholar]
- Hyatt, D.C.; Youn, B.; Zhao, Y.; Santhamma, B.; Coates, R.M.; Croteau, R.B.; Kang, C. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl. Acad. Sci. USA 2007, 104, 5360–5365. [Google Scholar] [CrossRef]
- Chubukov, V.; Mingardon, F.; Schackwitz, W.; Baidoo, E.E.; Alonso-Gutierrez, J.; Hu, Q.; Lee, T.S.; Keasling, J.D.; Mukhopadhyay, A. Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase ahpc. Appl. Environ. Microbiol. 2015, 81, 4690–4696. [Google Scholar] [CrossRef]
- Chueca, B.; Pagan, R.; Garcia-Gonzalo, D. Differential mechanism of Escherichia coli inactivation by (+)-limonene as a function of cell physiological state and drug’s concentration. PLoS ONE 2014, 9, e94072. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Vega, P.; Xu, Y.; Chen, C.Y.; Irudayaraj, J. Exploring the anti-quorum sensing activity of a d-limonene nanoemulsion for Escherichia coli o157:H7. J. Biomed. Mater. Res. Part A 2018, 106, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ortega, A.; Vinaixa, M.; Zebec, Z.; Takano, E.; Scrutton, N.S. A toolbox for diverse oxyfunctionalisation of monoterpenes. Sci. Rep. 2018, 8, 14396. [Google Scholar] [CrossRef] [PubMed]
- Thuan, N.H.; Tatipamula, V.B.; Canh, N.X.; Van Giang, N. Recent advances in microbial co-culture for production of value-added compounds. 3 Biotech 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
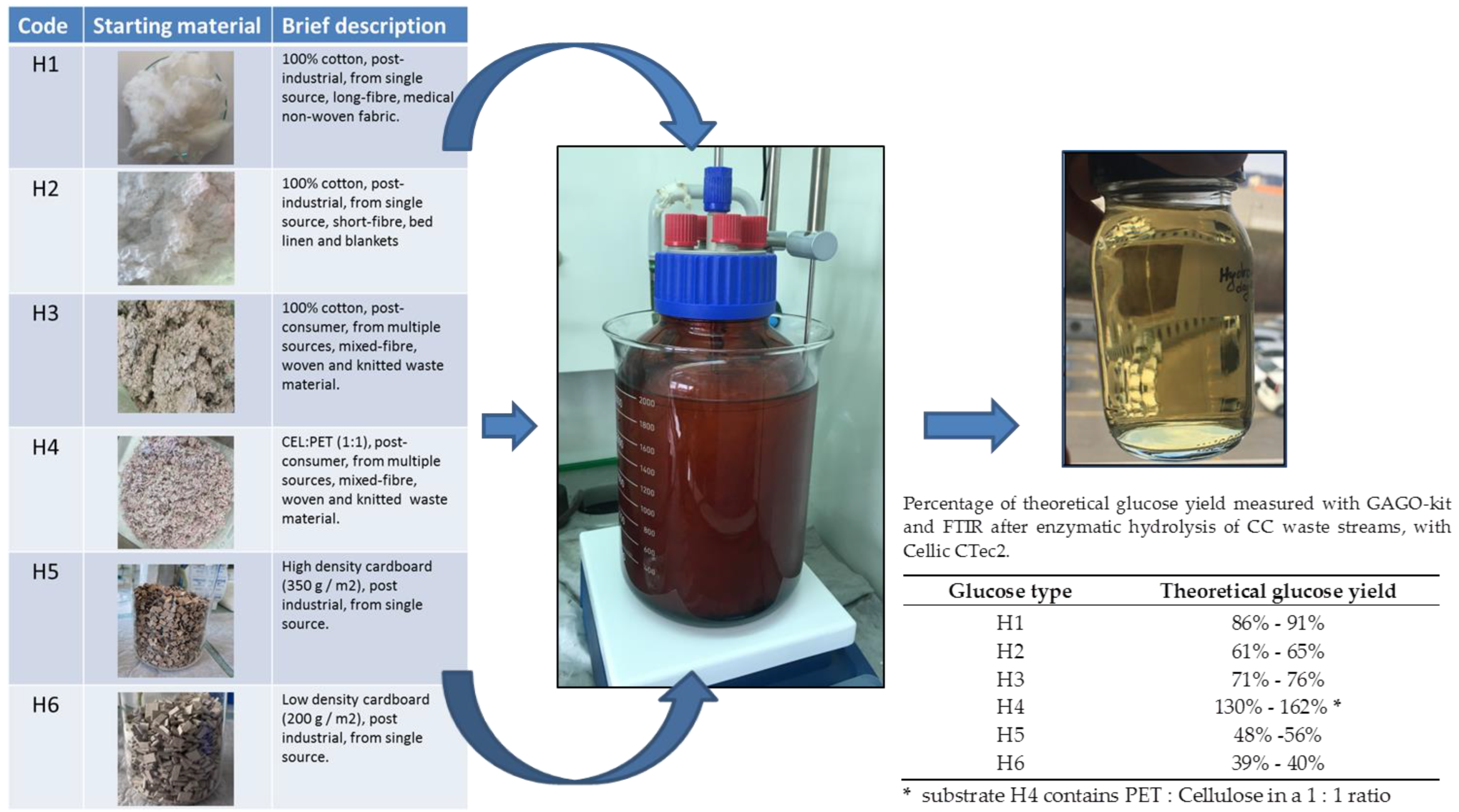
| Glucose Type | Glucose Conc. g/L GAGO 1 | Glucose Conc. g/L FTIR 2 |
|---|---|---|
| H1 | 43.23 ± 0.91 (86%) | 45.64 ± 1.18 (91%) |
| H2 | 30.91 ± 0.56 (61%) | 32.58 ± 0.93 (65%) |
| H3 | 38.33 ± 0.36 (76%) | 35.90 ± 0.89 (76%) |
| H4 | 40.65 ± 0.12 (162%) * | 32.52 ± 0.93 (130%) * |
| H5 | 24.14 ± 0.44 (48%) | 27.90 ± 0.78 (56%) |
| H6 | 21.04 ± 0.81 (42%) | 19.92 ± 0.83 (39%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebec, Ž.; Poberžnik, M.; Lobnik, A. Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli. Life 2022, 12, 1423. https://doi.org/10.3390/life12091423
Zebec Ž, Poberžnik M, Lobnik A. Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli. Life. 2022; 12(9):1423. https://doi.org/10.3390/life12091423
Chicago/Turabian StyleZebec, Žiga, Mojca Poberžnik, and Aleksandra Lobnik. 2022. "Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli" Life 12, no. 9: 1423. https://doi.org/10.3390/life12091423
APA StyleZebec, Ž., Poberžnik, M., & Lobnik, A. (2022). Enzymatic Hydrolysis of Textile and Cardboard Waste as a Glucose Source for the Production of Limonene in Escherichia coli. Life, 12(9), 1423. https://doi.org/10.3390/life12091423









