Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradients: A Case Study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Sampling Area
2.3. Study Design
2.4. Statistical Analysis
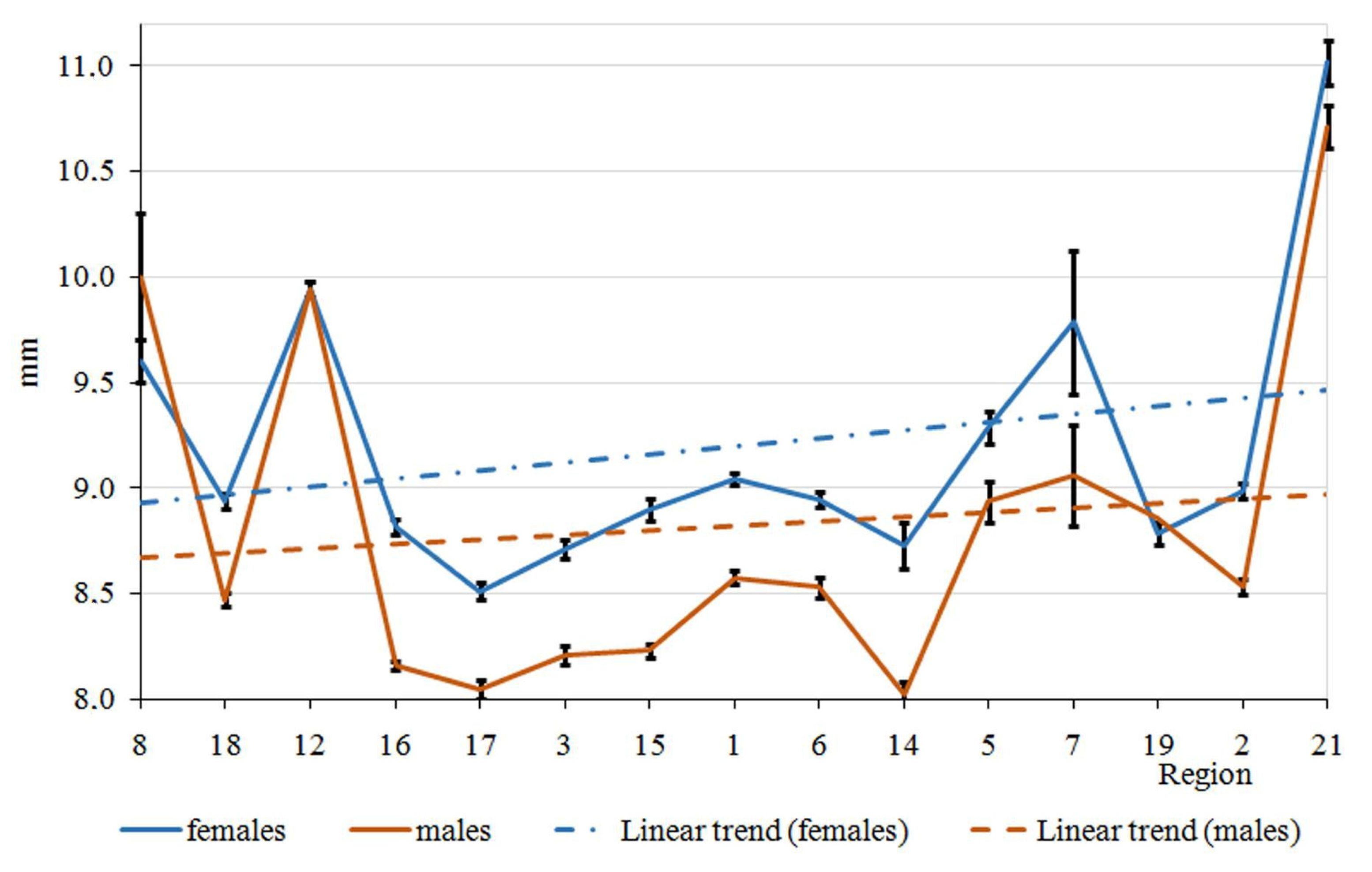
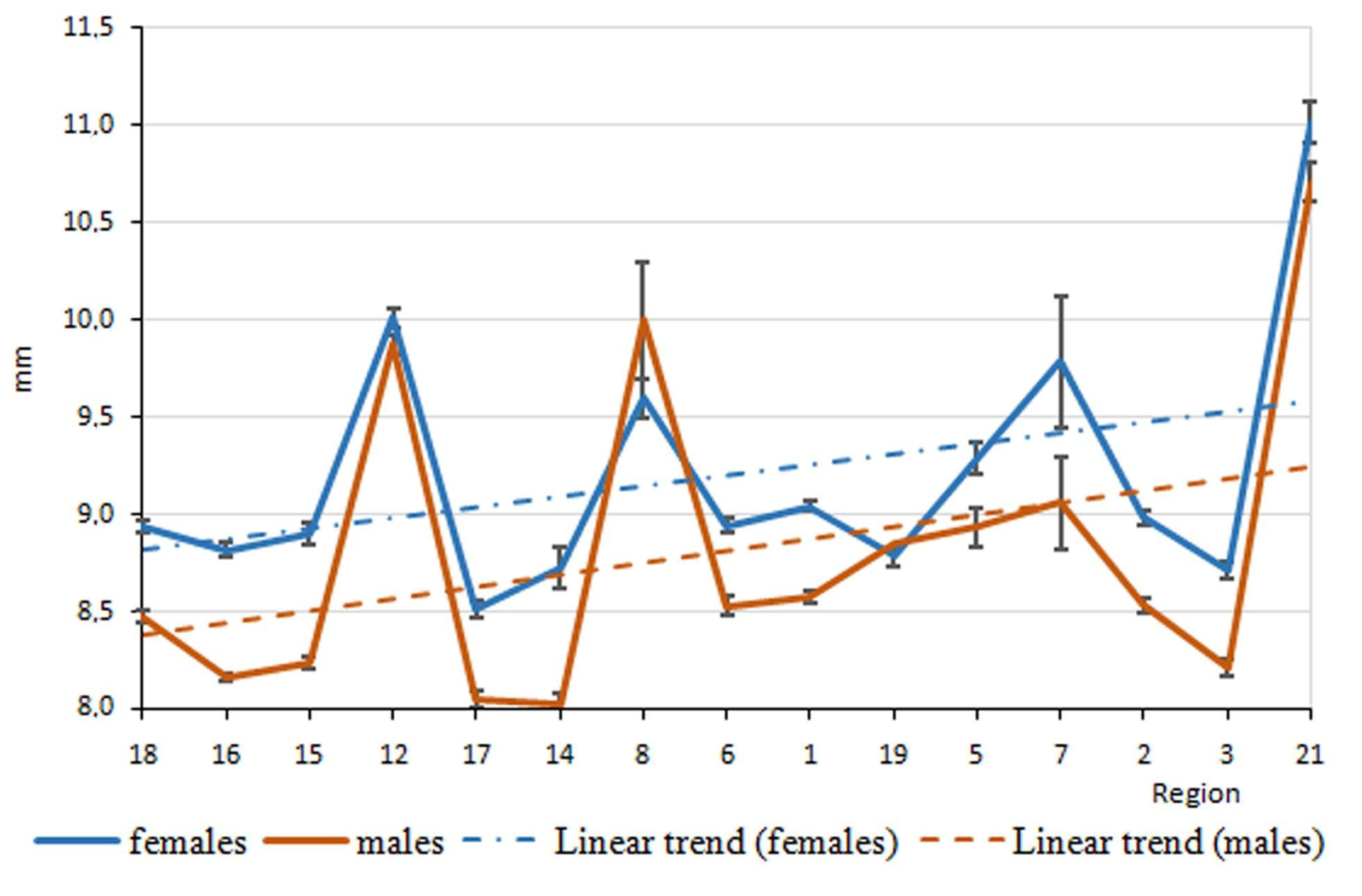
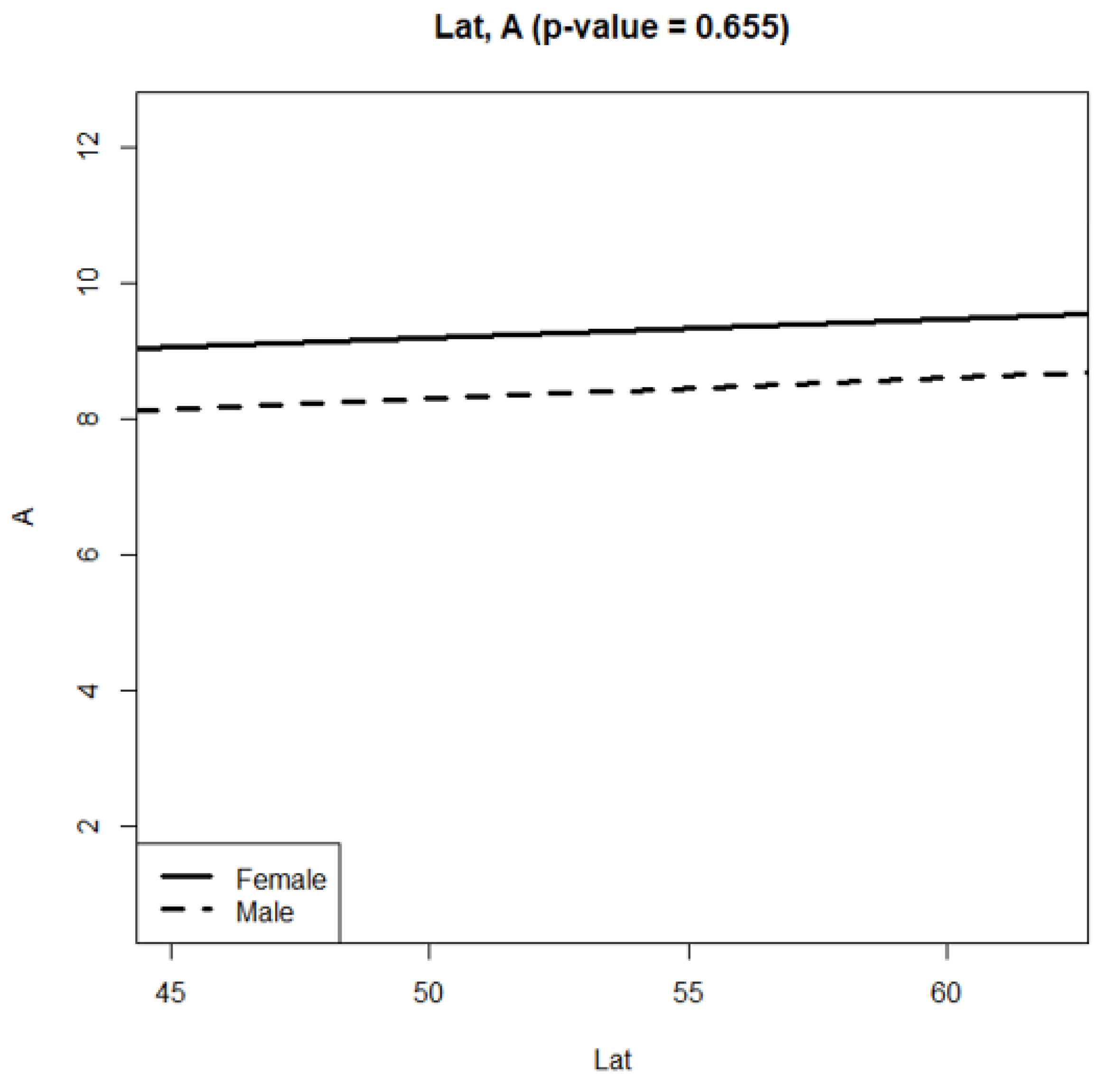
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, J.N. Relentless Evolution; The University of Chicago Press: Chicago, IL, USA, 2013; 499p. [Google Scholar]
- Chown, S.L.; Addo-Bediako, A.; Gaston, K.J. Physiological Variation in Insects: Large-Scale Patterns and Their Implications. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 587–602. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body Size Variation in Insects: A Macroecological Perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J. Temperature, Growth Rate, and Body Size in Ectotherms: Fitting Pieces of a Life-History Puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Høye, T.; Hammel, J. Climate Change and Altitudinal Variation in Sexual Size Dimorphism of Arctic Wolf Spiders. Clim. Res. 2010, 41, 259–265. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial Insects along Elevation Gradients: Species and Community Responses to Altitude. Biol. Rev. 2005, 80, 489. [Google Scholar] [CrossRef]
- Bowden, J.J.; Høye, T.T.; Buddle, C.M. Fecundity and Sexual Size Dimorphism of Wolf Spiders (Araneae: Lycosidae) along an Elevational Gradient in the Arctic. Polar Biol. 2013, 36, 831–836. [Google Scholar] [CrossRef][Green Version]
- Penell, A.; Raub, F.; Höfer, H. Estimating Biomass from Body Size of European Spiders Based on Regression Models. J. Arachnol. 2018, 46, 413. [Google Scholar] [CrossRef]
- Smith, C.C.; Fretwell, S.D. The Optimal Balance between Size and Number of Offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Fox, C.W.; Czesak, M.E. Evolutionary Ecology of Progeny Size in Arthropods. Annu. Rev. Entomol. 2000, 45, 341–369. [Google Scholar] [CrossRef]
- Gaston, K.J.; Jackson, S.F.; Cantú-Salazar, L.; Cruz-Piñón, G. The Ecological Performance of Protected Areas. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 93–113. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R.B. Size, Temperature, and Fitness: Three Rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Koivula, M. Useful Model Organisms, Indicators, or Both? Ground Beetles (Coleoptera, Carabidae) Reflecting Environmental Conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef]
- Meiri, S.; Yom-Tov, Y.; Geffen, E. What Determines Conformity to Bergmann’s Rule? Glob. Ecol. Biogeogr. 2007, 16, 788–794. [Google Scholar] [CrossRef]
- Shelomi, M. Where Are We Now? Bergmann’s Rule SensuLato in Insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.; Saveliev, A. Intra-Specific Body Size Variation of Ground Beetles (Coleoptera: Carabidae) in Latitudinal Gradient. Period. Biol. 2016, 118, 273–280. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.; Saveliev, A. Impact of Environmental Factors on the Body Shape Variation and Sexual Shape Dimorphism in Carabus granulatus L. (Coleoptera: Carabidae). Zool. Syst. 2017, 42, 71–89. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1994; Volume 25, pp. 1–58. [Google Scholar] [CrossRef]
- Badejo, O.; Skaldina, O.; Sorvari, J. Spatial and Temporal Variation in Thermal Melanism in the Aposematic Common Wasp (Vespula vulgaris) in Northern Europe. Ann. Zool. Fenn. 2018, 55, 67–78. [Google Scholar] [CrossRef]
- Polidori, C.; Gutiérrez-Cánovas, C.; Sánchez, E.; Tormos, J.; Castro, L.; Sánchez-Fernández, D. Climate Change-driven Body Size Shrinking in a Social Wasp. Ecol. Entomol. 2020, 45, 130–141. [Google Scholar] [CrossRef]
- Dayan, T.; Simberloff, D. Size Patterns among Competitors: Ecological Character Displacement and Character Release in Mammals, with Special Reference to Island Populations. Mammal Rev. 1998, 28, 99–124. [Google Scholar] [CrossRef]
- Watt, C.; Mitchell, S.; Salewski, V. Bergmann’s Rule; a Concept Cluster? Oikos 2010, 119, 89–100. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher Predation Risk for Insect Prey at Low Latitudes and Elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef]
- Berrigan, D.; Charnov, E.L. Reaction Norms for Age and Size at Maturity in Response to Temperature: A Puzzle for Life Historians. Oikos 1994, 70, 474. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Harshman, L.G. Desiccation and Starvation Resistance in Drosophila: Patterns of Variation at the Species, Population and Intrapopulation Levels. Heredity 1999, 83, 637–643. [Google Scholar] [CrossRef]
- Cushman, J.H.; Lawton, J.H.; Manly, B.F.J. Latitudinal Patterns in European Ant Assemblages: Variation in Species Richness and Body Size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Demont, M. Bergmann and Converse Bergmann Latitudinal Clines in Arthropods: Two Ends of a Continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Dunham, A.E. The Temperature-Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Stillwell, R.C.; Young, K.A.; Fox, C.W.; Ashton, K.G. When RenschMeets Bergmann: Does Sexual Size Dimorphism Change Systematically with Latitude? Evolution 2006, 60, 2004–2011. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.A.; Saveliev, A.A.; Ukhova, N.L.; Vorobyova, I.G.; Solodovnikov, I.A.; Anciferov, A.L.; Gordienko, T.A.; Shagidullin, R.R.; Vavilov, D.N. Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradient (The Case Study in Pterostichus oblongpunctatus Fabricius, 1787). GSC Biol. Pharm. Sci. 2020, 13, 149–161. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.; Ananina, T.; Avtaeva, T.; Saveliev, A. Sexual Size Dimorphism in Ground Beetles and its Variation in Altitude Gradient. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2021; Volume 191. [Google Scholar] [CrossRef]
- Bousquet, Y. Tribe Pterostichini. In Catalogue of Palearctic Coleoptera. Archostemata—Myxophaga—Adephaga; Löbl, J., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2003; Volume 1, pp. 462–521. [Google Scholar]
- Niemelä, J.; Spence, J.R. Community Impacts of an Exotic Carabid: Pterostichus melanarius in Western Canadian Forests. In Carabid Beetles; Desender, K., Dufrene, M., Loreau, M., Luff, M.L., Maelfait, J.P., Eds.; Springer Nature: Basingstoke, UK, 1994; pp. 331–335. [Google Scholar]
- Niemela, J.; Spence, J.R.; Carcamo, H. Establishment and Interactions of Carabid Populations: An Experiment with Native and Introduced Species. Ecography 1997, 20, 643–652. [Google Scholar] [CrossRef]
- Thomas, C.F.G.; Parkinson, L.; Marshall, E.J.P. Isolating the Components of Activity-Density for the Carabid Beetle Pterostichus melanarius in Farmland. Oecologia 1998, 116, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sukhodolskyay, R.A. Population Characteristic of Ground Beetles Dwelling the Cottages. In Animals and Plants Synantropization; Pleshanov, A.S., Ed.; Institute of Geography, Siberian Branch of the Russian Academy of Science: Irkutsk, Russia, 2007; pp. 99–102. [Google Scholar]
- Fournier, E.; Loreau, M. Activity and Satiation State in Pterostichus melanarius: An Experiment in Different Agricultural Habitats: P. melanarius Foraging Activity in Agricultural Habitats. Ecol. Entomol. 2001, 26, 235–244. [Google Scholar] [CrossRef]
- Kryzhanovskij, O.L.; Belousov, I.A.; Kabak, I.I.; Kataev, B.M.; Makarov, K.V.; Shilenkov, V.G. A Checklist of the Ground-Beetles of Russia and Adjacent Lands (Insecta, Coleoptera, Carabidae); Pensoft-series Faunistica; Pensoft: Sofia, Bulgaria, 1995; 275p. [Google Scholar]
- Freude, H.; Harde, K.W.; Lohse, G.A. Die Käfer Mitteleuropas. Band 2. Adephaga. 1. Carabidae (Laufkäfer); Elsevier: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Putchkov, A. Ground Beetles of the Ukraine (Coleoptera, Carabidae). ZooKeys 2011, 100, 503–515. [Google Scholar] [CrossRef]
- Puchkov, A.V. Ground-Beetles (Coleoptera, Carabidae) of Transformed Cenoses of Ukraine; I.I. Schmalhausen Institute of Zoology of National Academy of Sciences of Ukraine: Kiev, Ukraine, 2018; 448p, (In Ukrainian). [Google Scholar] [CrossRef]
- Sharova, I.C.; Denisova, M.I. Sezonnaya dinamika lesnykh populyatsiy zhuzhelits roda Pterostichus (Coleoptera, Carabidae) [Seasonal dynamics of forest populations of ground beetles of the genus Pterostichus (Coleoptera, Carabidae)]. Zool. Zhurnal 1997, 76, 418–427. [Google Scholar]
- Matalin, A.V. Geographic Variability of the Life Cycle in Pterostichus melanarius (Coleoptera, Carabidae). Entomol. Rev. 2006, 86, 409–422. [Google Scholar] [CrossRef]
- Kielty, J.P.; Allen-Williams, L.J.; Underwood, N. Prey Preferences of Six Species of Carabidae (Coleoptera) and One Lycosidae (Araneae) Commonly Found in UK Arable Crop Fields. J. Appl. Entomol. 1999, 123, 193–200. [Google Scholar] [CrossRef]
- Korolev, O.V.; Brygadyrenko, V.V. Comparative Analysis of Pterostichus melanarius (Coleoptera, Carabidae) Trophic Preferences in Different Condition of Laboratory Keeping. Optim. Prot. Ecosyst. 2012, 6, 178–190. [Google Scholar]
- Korolev, O.V.; Brygadyrenko, V.V. Trophic Relations of Pterostichus melanarius (Coleoptera, Carabidae) with Dominant Species of Invertebrates in Forest Ecosystems of Steppe Dnieper Region. Visnyk Dnipropetr. Univ. Biol. Ecol. 2012, 20, 48–54. [Google Scholar]
- Brygadyrenko, V.V.; Korolev, O.V. Peculiarities of Trophic Spectrum of Pterostichus melanarius (Coleoptera: Carabidae) in Laboratory Conditions. Visnyk Bilocerk. Derzhavnogo Agrar. Univ. 2006, 43, 67–71. [Google Scholar]
- Brygadyrenko, V.V.; Korolev, O.V. Morphological Polymorphism in an Urban Population of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae). GRAELLSIA 2015, 71, e025. [Google Scholar] [CrossRef][Green Version]
- Sukhodolskaya, R.A. Intraspecific Body Size Variation in Ground Beetles (Coleoptera, Carabidae) in Urban-Suburban-Rural-Natural Gradient. Acta Biol. Univ. Daugavp. 2013, 13, 121–128. [Google Scholar]
- Sukhodolskaya, R. Variation in Body Size and Body Shape in Ground Beetle Pterostichus melanarius Ill. (Coleoptera, Carabidae). J. Agri-Food Appl. Sci. 2014, 2, 196–205. [Google Scholar]
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Brygadyrenko, V.V. Modeling the Bioclimatic Range of Pterostichus melanarius (Coleoptera, Carabidae) in Conditions of Global Climate Change. Biosys. Divers. 2021, 29, 140–150. [Google Scholar] [CrossRef]
- Cooper, M. Latitudinal and Longitudinal Gradients in Old World Forest Millipedes; LAP LAMBERT Academic Publishing: Beau Bassin, Mauritius, 2021; 77p. [Google Scholar]
- Vandewoestijne, S.; Van Dyck, H. Flight Morphology along a Latitudinal Gradient in a Butterfly: Do Geographic Clines Differ between Agricultural and Woodland Landscapes? Ecography 2011, 34, 876–886. [Google Scholar] [CrossRef]
- Śniegula, S.; Gołąb, M.J. Test for Latitudinal Variation of Life History, Behavior and Mortality in the Strictly Univoltine Damselfly Sympecma fusca (Zygoptera: Lestidae): Latitudinal Variation of Insect Traits. Entomol. Sci. 2015, 18, 479–488. [Google Scholar] [CrossRef]
- Mukhametnabiev, T. Manual Carabid Morphometric Measurement for Method by Sukhodolskaya. Available online: https://github.com/CRTmatrix/-Manual-Carabid-morphometric-measurement-for-method-by-Sukhodolskaya- (accessed on 1 December 2021).
- Outomuro, D.; Golab, M.J.; Johansson, F.; Sniegula, S. Body and Wing Size, but Not Wing Shape, Vary along a Large-Scale Latitudinal Gradient in a Damselfly. Sci. Rep. 2021, 11, 18642. [Google Scholar] [CrossRef]
- Miller, S.E.; Sheehan, M.J. Ecogeographical Patterns of Body Size Differ among North American Paper Wasp Species. Insect. Soc. 2021, 68, 109–122. [Google Scholar] [CrossRef]
- Hein, N.; Pétillon, J.; Pape, R.; Feilhauer, H.; Vanselow, K.A.; Löffler, J. Broad-Scale Rather than Fine-Scale Environmental Variation Drives Body Size in a Wandering Predator (Araneae, Lycosidae). Arct. Antarct. Alp. Res. 2019, 51, 315–326. [Google Scholar] [CrossRef]
- Günter, F.; Beaulieu, M.; Freiberg, K.F.; Welzel, I.; Toshkova, N.; Žagar, A.; Simčič, T.; Fischer1, K. Genotype-environment Interactions Rule the Response of a Widespread Butterfly to Temperature Variation. J. Evol. Biol. 2020, 33, 920–929. [Google Scholar] [CrossRef]
- Ellers, J.; Boggs, C.L. The Evolution of Wing Color in ColiasButterflies: Heritability, Sex Linkage, and Population Divergence. Evolution 2002, 56, 836. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual Issues in Local Adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Craig Stillwell, R.; Fox, C.W. Geographic Variation in Body Size, Sexual Size Dimorphism and Fitness Components of a Seed Beetle: Local Adaptation versus Phenotypic Plasticity. Oikos 2009, 118, 703–712. [Google Scholar] [CrossRef]
- Marangoni, F.; Tejedo, M.; Cogălniceanu, D. Can Age and Growth Patterns Explain the Geographical Variation in the Body Size of Two Toad Species? An. Acad. Bras. Ciênc. 2021, 93, e20190470. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J.; Preziosi, R.F. Sexual Selection and the Evolution of Allometry for Sexual Size Dimorphism in the Water Strider, Aquarius Remigis. Am. Nat. 1994, 144, 101–118. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. The Evolution of Body Size: What Keeps Organisms Small? Q. Rev. Biol. 2000, 75, 385–407. [Google Scholar] [CrossRef]
- Fairbairn, D.J. Allometry for Sexual Size Dimorphism: Testing Two Hypotheses for Rensch’s Rule in the Water Strider Aquarius remigis. Am. Nat. 2005, 166, S69–S84. [Google Scholar] [CrossRef]
- Kraushaar, U.; Blanckenhorn, W.U. Population Variation in Sexual Selection and its Effect on Size Allometry in Two Dung Fly Species with Contrasting Sexual Size Dimorphism. Evolution 2002, 56, 307–321. [Google Scholar] [CrossRef]
- Holtby, L.B.; Healey, M.C. Sex-Specific Life History Tactics and Risk-Taking in Coho Salmon. Ecology 1990, 71, 678–690. [Google Scholar] [CrossRef]
- Lande, R. SEXUAL Dimorphism, Sexual Selection, and Adaptation in Polygenic Characters. Evolution 1980, 34, 292–305. [Google Scholar] [CrossRef]
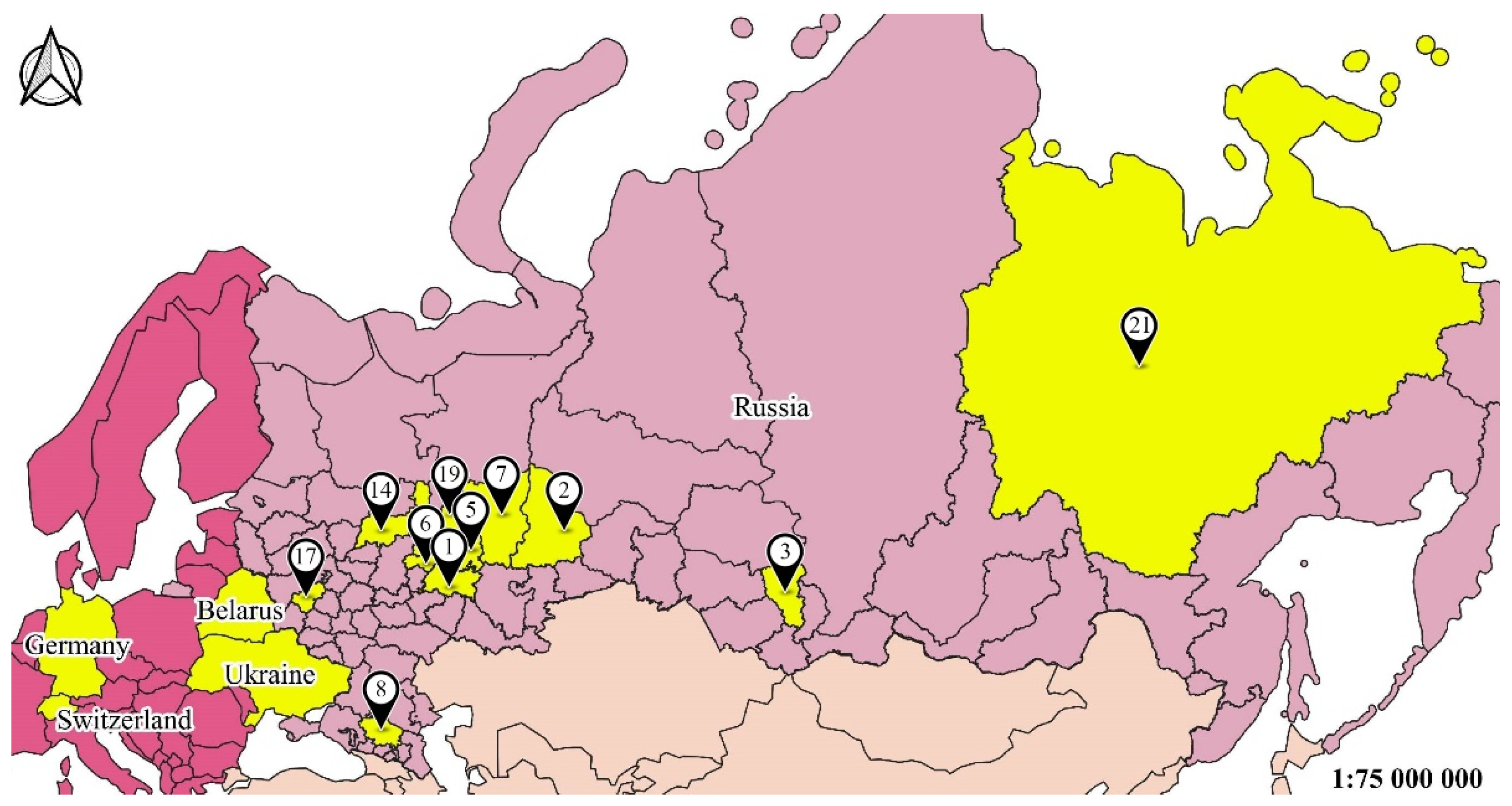


| N | Region | Latitude, °N | Longitude, °E | Number of Sites | Type of Habitats | Sample Size |
|---|---|---|---|---|---|---|
| 1 1 | Tatarstan Republic | 55°47′ | 49°06′ | 53 | Meadow, birch, oak, elm | 11,312 |
| 2 | Sverdlovsk region | 58°42′ | 61°20′ | 6 | Meadow, pine, birch | 458 |
| 3 | Kemerovo region | 54°56′ | 87°14′ | 20 | Meadows, birch, lawn | 1954 |
| 5 | Udmurtia Republic | 57°17′ | 52°45′ | 16 | Birch, oak, elm | 396 |
| 6 | Mariy El Republic | 56°42′ | 47°52′ | 14 | Meadow, birch, oak | 67 |
| 7 | Cis-Ural | 57°01′ | 57°9′ | 21 | Birch, oak, elm | 58 |
| 8 | Stavropol region | 45°02′ | 41°55′ | 6 | Meadow, birch | 76 |
| 12 | Ukraine | 48°27′ | 34°56′ | Artificial forest plantation | 225 | |
| 14 | Kostroma region | 57°48′ | 41°19′ | 3 | Fir dominated ecosystem recovery after felling | 60 |
| 15 | Belarus | 55°13′ | 30°18′ | 3 | Forest with pine, oak, alder, willow | 369 |
| 16 | Germany | 52°45′ | 9°23′ | 18 | Oilseed rape fields | 1339 |
| 17 | Kaluga region | 54°32′ | 36°16′ | 8 | Gardens, old-growth forest | 233 |
| 18 | Switzerland | 47°23′ | 8°05′ | 8 | Oilseed rape fields | 450 |
| 19 | Kirov region | 58°001′ | 48°027′ | 3 | Spruce, lime and oak forests | 57 |
| 21 | Yakutsk region | 62°01′ | 129°43′ | 2 | Meadows, lawn | 48 |
| Estimate | Std. Error | t Value | Pr(>|t|) | ||
|---|---|---|---|---|---|
| (Intercept) | 7.8008 | 0.1933 | 40.350 | <2 × 10−16 | *** |
| fSexMale | −0.9916 | 0.2692 | −3.683 | 0.0002 | *** |
| Lat | 0.0276 | 0.0035 | 7.869 | 4.16 × 10−15 | *** |
| fSexMale:Lat | 0.0022 | 0.0049 | 0.447 | 0.6549 |
| Estimate | Std. Error | t value | Pr(>|t|) | ||
|---|---|---|---|---|---|
| (Intercept) | 9.1650 | 0.0301 | 303.632 | <2 × 10−16 | *** |
| fSexMale | −0.9406 | 0.0418 | −22.488 | <2 × 10−16 | *** |
| Lon | 0.0032 | 0.0005 | 5.561 | 2.8 × 10−8 | *** |
| fSexMale:Lon | 0.0012 | 0.0008 | 1.564 | 0.118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzyanin, S.; Saveliev, A.; Ukhova, N.; Vorobyova, I.; Solodovnikov, I.; Anciferov, A.; Shagidullin, R.; Teofilova, T.; Nogovitsyna, S.; Brygadyrenko, V.; et al. Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradients: A Case Study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae). Life 2022, 12, 112. https://doi.org/10.3390/life12010112
Luzyanin S, Saveliev A, Ukhova N, Vorobyova I, Solodovnikov I, Anciferov A, Shagidullin R, Teofilova T, Nogovitsyna S, Brygadyrenko V, et al. Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradients: A Case Study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae). Life. 2022; 12(1):112. https://doi.org/10.3390/life12010112
Chicago/Turabian StyleLuzyanin, Sergey, Anatoly Saveliev, Nadezhda Ukhova, Iraida Vorobyova, Igor Solodovnikov, Anatoliy Anciferov, Rifgat Shagidullin, Teodora Teofilova, Sargylana Nogovitsyna, Viktor Brygadyrenko, and et al. 2022. "Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradients: A Case Study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae)" Life 12, no. 1: 112. https://doi.org/10.3390/life12010112
APA StyleLuzyanin, S., Saveliev, A., Ukhova, N., Vorobyova, I., Solodovnikov, I., Anciferov, A., Shagidullin, R., Teofilova, T., Nogovitsyna, S., Brygadyrenko, V., Alexanov, V., & Sukhodolskaya, R. (2022). Modeling Sexual Differences of Body Size Variation in Ground Beetles in Geographical Gradients: A Case Study of Pterostichus melanarius (Illiger, 1798) (Coleoptera, Carabidae). Life, 12(1), 112. https://doi.org/10.3390/life12010112







