Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Design to Estimate Trophic Preferences
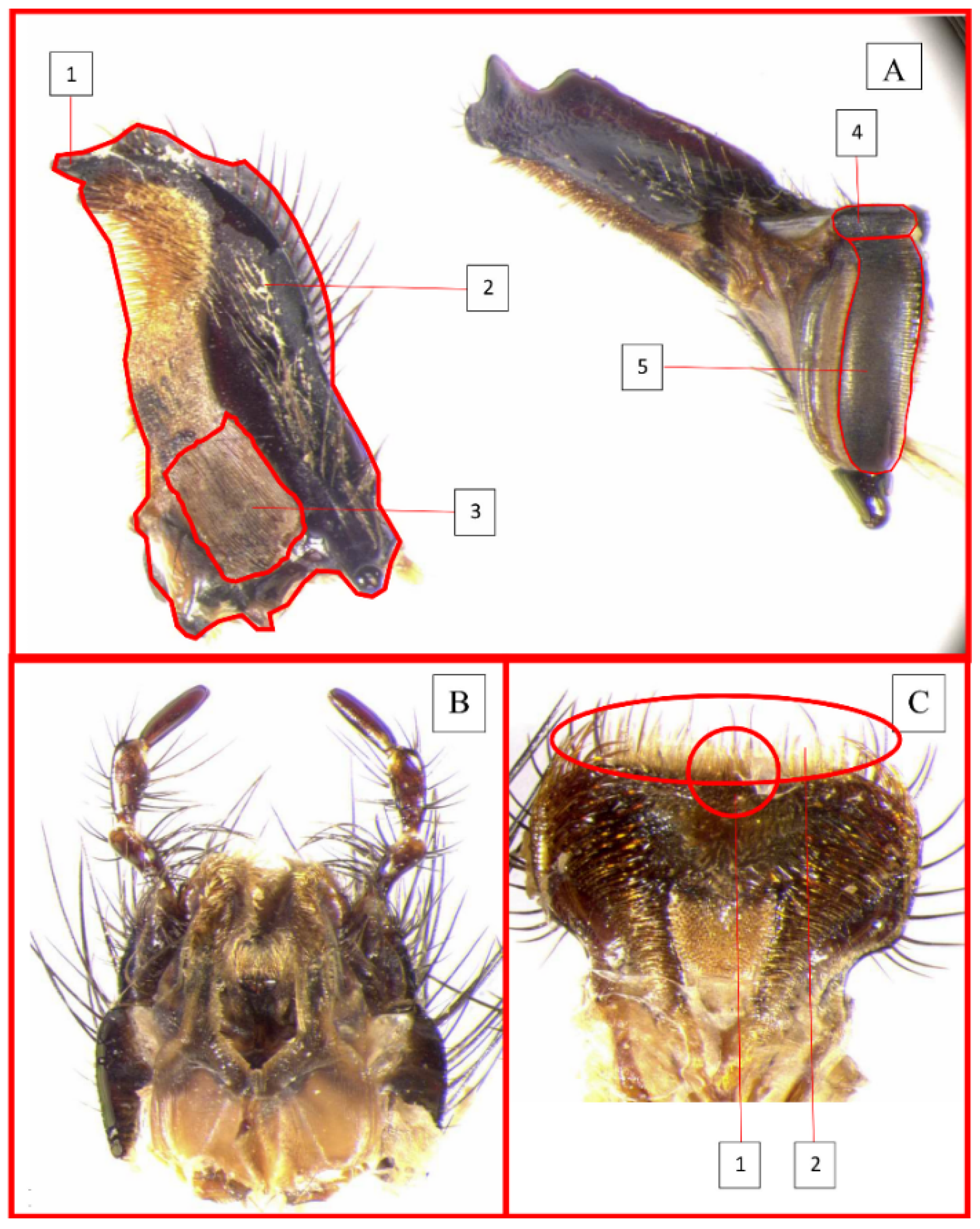
2.2. Functional Traits
2.3. Data Analysis
2.3.1. Trophic Preferences
2.3.2. Functional Diversity
3. Results
3.1. Trophic Preferences
3.2. Functional Diversity
4. Discussion
4.1. Trophic Preferences
4.2. Mouthparts
4.3. Body Size and Nesting Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roskov, Y.; Ower, G.; Orrell, T.; Nicolson, D.; Bailly, N.; Kirk, P.M.; Bourgoin, T.; DeWalt, R.E.; Decock, W.; van Nieukerken, E.; et al. P. Scarabs: World Scarabaeidae Database (version Jan 2019). In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Species Naturalis: Leiden, The Netherlands, 2019; ISSN 2405-884X; Available online: www.catalogueoflife.org/annual-checklist/2019 (accessed on 2 January 2020).
- Ahrens, D.; Schwarzer, J.; Vogler, A.P. The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc. R. Soc. B 2014, 281, 20141470. [Google Scholar] [CrossRef]
- Gunter, N.L.; Weir, T.A.; Slipinksi, A.; Bocak, L.; Cameron, S.L. If dung beetles (Scarabaeidae: Scarabaeinae) arose in association with dinosaurs, did they also suffer a mass co-extinction at the K-Pg boundary? PLoS ONE 2016, 11, e0153570. [Google Scholar] [CrossRef]
- Schweiger, A.H.; Svenning, J.-C. Down-sizing of dung beetle assemblages over the last 53000 years is consistent with a dominant effect of megafauna losses. Oikos 2018, 127, 1243–1250. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Gómez-Cifuentes, A.; Vaz-de-Mello, F.Z.; Zurita, G.A. Influence of land use on the trophic niche overlap of dung beetles in the semideciduous Atlantic forest of Argentina. Insect Conserv. Divers. 2018, 11, 554–564. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Velazco, S.J.E.; Zurita, G.A. Dung beetle trophic ecology: Are we misunderstanding resources attraction? Ecol. Entomol. 2020, 46, 552–561. [Google Scholar] [CrossRef]
- Halffter, G.; Matthews, E.G. The natural history of dung beetles of the Subfamily Scarabaeinae. In Folia Entomológica Mexicana; Carretera, Mexico, D.F., Ed.; 1966; 12–14; p. 312. [Google Scholar]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; p. XII + 481. [Google Scholar]
- Jones, A.G.; Forgie, S.A.; Scott, D.J.; Beggs, J.R. Generalist dung attraction response in a New Zealand dung beetle that evolved with an absence of mammalian herbivores. Ecol. Entomol. 2012, 37, 124–133. [Google Scholar] [CrossRef]
- Frank, K.; Krell, F.T.; Slade, E.M.; Raine, E.H.; Chiew, L.Y.; Schmitt, T.; Vairappan, C.S.; Walter, P.; Blüthgen, N. Global dung webs: High trophic generalism of dung beetles along the latitudinal diversity gradient. Ecol. Lett. 2018, 21, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Lumaret, J.P.; Iborra, O. Separation of trophic niches by dung beetles (Coleoptera, Scarabaeoidea) in overlapping habitats. Pedobiologia 1996, 40, 392–404. [Google Scholar]
- Galante, E.; Cartagena, M.C. Comparison of Mediterranean dung beetles (Coleoptera: Scarabaeoidea) in cattle and rabbit dung. Environ. Entomol. 1999, 28, 420–424. [Google Scholar] [CrossRef]
- Larsen, T.H.; Lopera, A.; Forsyth, A. Extreme trophic and habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 2006, 60, 315–324. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Pittino, R.; Luiselli, L. Exploring co-extinction correlates: The effects of habitat, biogeography and anthropogenic factors on ground squirrels-dung beetles associations. Biodivers. Conserv. 2011, 20, 3059–3076. [Google Scholar] [CrossRef]
- Sánchez-Huerta, J.L.; Tonelli, M.; Zunino, M.; Halffter, G. Redescription of Onthophagus halffteri Zunino (Coleoptera: Scarabaeidae: Scarabaeinae) with ecological and distributional notes. Coleopt. Bull. 2015, 69, 225–230. [Google Scholar] [CrossRef]
- Martín-Piera, F.; Lobo, J.M. A comparative discussion of trophic preferences in dung beetle communities. Misc. Zool. 1996, 19, 13–31. [Google Scholar]
- Finn, J.A.; Giller, P.S. Experimental investigations of colonisation by north temperate dung beetles of different types of domestic herbivore dung. Appl. Soil Ecol. 2002, 20, 1–13. [Google Scholar] [CrossRef]
- Dormont, L.; Epinat, G.; Lumaret, J.P. Trophic preferences mediated by olfactory cues in dung beetles colonizing cattle and horse dung. Environ. Entomol. 2004, 33, 370–377. [Google Scholar] [CrossRef]
- Errouissi, F.; Haloti, S.; Jay-Robert, P.; Janati-Idrissi, A.; Lumaret, J.P. Effects of the attractiveness for dung beetles of dung pat origin and size along a climatic gradient. Environ. Entomol. 2004, 33, 45–53. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Ieradi, M. Use of habitat resources by scarab dung beetles in an African savanna. Environ. Entomol. 2010, 39, 1756–1764. [Google Scholar] [CrossRef]
- Noriega, J.A. Dung beetles (Coleoptera: Scarabaeinae) attracted to Lagothrix lagotricha (Humboldt) and Alouatta seniculus (Linnaeus) (Primates: Atelidae) dung in a Colombian Amazon forest. Psyche 2012, 2012, 437589. [Google Scholar] [CrossRef]
- Puker, A.; Correa, C.M.A.; Korasaki, V.; Ferreira, K.R.; Oliveira, N.G. Dung beetles (Coleoptera: Scarabaeidae) attracted to dung of the largest herbivorous rodent on earth: A comparison with human feces. Environ. Entomol. 2013, 42, 1218–1225. [Google Scholar] [CrossRef][Green Version]
- Bogoni, J.A.; Hernández, M.I.M. Attractiveness of native mammal’s feces of different trophic guilds to dung beetles (Coleoptera: Scarabaeinae). J. Insect Sci. 2014, 14, 299. [Google Scholar] [CrossRef]
- Frank, K.; Brückner, A.; Hilpert, A.; Heethoff, M.; Blüthgen, N. Nutrient quality of vertebrate dung as a diet for dung beetles. Sci. Rep. 2017, 7, 12141. [Google Scholar] [CrossRef]
- Barbero, E.; Palestrini, C.; Rolando, A. Dung beetle conservation: Effects of habitat and resource selection (Coleoptera: Scarabaeoidea). J. Insect Conserv. 1999, 3, 75–84. [Google Scholar] [CrossRef]
- Hanski, I. Nutritional ecology of dung- and carrion-feeding insects. In Nutritional Ecology of Insects, Mites, and Spiders; Slansky, F., Rodriguez, J.G., Eds.; John Wiley & Sons: New York, NY, USA, 1987; pp. 837–884. [Google Scholar]
- Holter, P. Herbivore dung as food for dung beetles: Elementary coprology for entomologists. Ecol. Entomol. 2016, 41, 367–377. [Google Scholar] [CrossRef]
- Chame, M. Terrestrial mammal feces: A morphometric summary and description. Mem. Inst. Oswaldo Cruz 2003, 98 (Suppl. I), 71–94. [Google Scholar] [CrossRef]
- Dormont, L.; Jay-Robert, P.; Bessière, J.M.; Rapior, S.; Lumaret, J.P. Innate olfactory preferences in dung beetles. J. Exp. Biol. 2010, 213, 3177–3186. [Google Scholar] [CrossRef] [PubMed]
- Stavert, J.R.; Gaskett, A.C.; Scott, D.J.; Beggs, J.R. Dung beetles in an avian-dominated island ecosystem: Feeding and trophic ecology. Oecologia 2014, 176, 259–271. [Google Scholar] [CrossRef]
- Frank, K.; Brückner, A.; Blüthgen, N.; Schmitt, T. In search of cues: Dung beetle attraction and the significance of volatile composition of dung. Chemoecology 2018, 28, 145–152. [Google Scholar] [CrossRef]
- Inouchi, J.; Shibuya, T.; Hatanaka, T. Food odor responses of single antennal olfactory cells in the Japanese dung beetle, Geotrupes auratus (Coleoptera: Geotrupidae). Appl. Entomol. Zool. 1988, 23, 167–174. [Google Scholar] [CrossRef]
- Wurmitzer, C.; Blüthgen, N.; Krel, F.T.; Maldonado, B.; Ocampo, F.; Müller, J.K.; Schmitt, T. Attraction of dung beetles to herbivore dung synthetic compounds in a comparative field study. Chemoecology 2017, 27, 75–84. [Google Scholar] [CrossRef]
- Hunt, J.; Simmons, L.W. Optimal maternal investment in the dung beetle Onthophagus taurus? Behav. Ecol. Sociobiol. 2004, 55, 302–312. [Google Scholar] [CrossRef]
- Arellano, L.; Castillo-Guevara, C.; Huerta, C.; Germán-García, A.; Lara, C. Effect of using different types of animal dung for feeding and nesting by the dung beetle Onthophagus lecontei (Coleoptera: Scarabaeinae). Can. J. Zool. 2015, 93, 337–343. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Kadiri, N.; Bertrand, M. Changes in resources: Consequences for the dynamics of dung beetle communities. J. Appl. Ecol. 1992, 29, 349–356. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Piattella, E. Changes in food resources and conservation of scarab beetles: From sheep to dog dung in a green urban area of Rome (Coleoptera, Scarabaeoidea). Biol. Conserv. 2005, 123, 547–556. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Effects of the progressive abandonment of grazing on dung beetle biodiversity: Body size matters. Biodivers. Conserv. 2018, 27, 189–204. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Grazing abandonment and dung beetle assemblage composition: Reproductive behaviour has something to say. Ecol. Indic. 2019, 96, 361–367. [Google Scholar] [CrossRef]
- Gittings, T.; Giller, P.S. Resource quality and the colonization and succession of coprophagous dung beetles. Ecography 1998, 21, 581–592. [Google Scholar] [CrossRef]
- Bürgis, H. Gourmets unter den Käfern: Die kotfresser (Coprophaga). I. Hartkotfresser vom Geotrupes-Typ. A. Lebensweise und mundwerkzeuge des Stierkäfers. Mikrokosmos 1982, 71, 298–303. [Google Scholar]
- Bürgis, H. Gourmets unter den Käfern: Die kotfresser (Coprophaga). I. Hartkotfresser vom Geotrupes-Typ. B. Nahrungsaufnahme der Hartkotfresser. Mikrokosmos 1982, 71, 341–344. [Google Scholar]
- Bürgis, H. Gourmets unter den Käfern: Die kotfresser (Coprophaga). II. Weichkotfresser vom Aphodius-Typ. A. Lebensweise und mundwerkzeuge des Mondhornkäfers. Mikrokosmos 1984, 73, 45–50. [Google Scholar]
- Bürgis, H. Gourmets unter den Käfern: Die kotfresser (Coprophaga). II. Weichkotfresser vom Aphodius-Typ. B. Die nahrungsaufnahme der adulten Weichkotfresser. Mikrokosmos 1984, 73, 368–374. [Google Scholar]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. In ELS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional. Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Fountain-Jones, N.; Baker, S.C.; Jordan, G.J. Moving beyond the guild concept: Developing a practical functional trait framework for terrestrial beetles. Ecol. Entomol. 2015, 40, 1–13. [Google Scholar] [CrossRef]
- Milotić, T.; Baltzinger, C.; Eichberg, C.; Eycott, A.E.; Heurich, M.; Müller, J.; Noriega, J.A.; Menendez, R.; Stadler, J.; Ádám, R.; et al. Functionally richer communities improve ecosystem functioning: Dung removal and secondary seed dispersal by dung beetles in the Western Palearctic. J. Biogeogr. 2019, 46, 70–82. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Effects of grazing intensity and the use of veterinary medical products on dung beetle biodiversity in the sub-mountainous landscape of Central Italy. PeerJ 2017, 5, e2780. [Google Scholar] [CrossRef]
- Larsen, T.H.; Forsyth, A. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 2005, 37, 322–325. [Google Scholar] [CrossRef]
- Silva, P.G.D.; Hernández, M.I.M. Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS ONE 2015, 10, e0126112. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.M.; Martín-Piera, F.; Veiga, C.M. Las trampas pitfall con cebo, sus posibilidades en el estudio de las comunidades coprófagas de Scarabaeoidea (Col.): I. Características determinantes de su capacidad de captura. Rev. D’écologie Biol. Sol 1988, 25, 77–100. [Google Scholar]
- Errouissi, F.; Lumaret, J.P. Field effects of faecal residues from ivermectin slow-release boluses on the attractiveness of cattle dung to dung beetles. Med Vet. Entomol. 2010, 24, 433–440. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Morelli, F.; Zunino, M. Dung beetles: Functional identity, not functional diversity, accounts for ecological process disruption caused by the use of veterinary medical products. J. Insect Conserv. 2020, 24, 643–654. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD Multivariate Analysis of Ecological Data, Version 4.0; MjM Software: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Mason, N.W.H.; MacGillivray, K.; Steel, J.B.; Wilson, J.B. An index of functional diversity. J. Veg. Sci. 2003, 14, 571–578. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouillot, D.; Mason, N.W.H.; Dumay, O.; Wilson, J.B. Functional regularity: A neglected aspect of functional diversity. Oecologia 2005, 142, 353–359. [Google Scholar] [CrossRef]
- Casanoves, F.; Pla, L.; Di Rienzo, J.A.; Díaz, S. FDiversity: A software package for the integrated analysis of functional diversity. Methods Ecol. Evol. 2011, 2, 233–237. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Macchiavelli, R.E.; Casanoves, F. Modelos Lineales Mixtos: Aplicaciones en InfoStat, 1st ed.; Grupo InfoStat: Córdoba, Argentina, 2011; p. 193. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. 2020. Available online: http://www.infostat.com.ar (accessed on 2 January 2020).
- Dormont, L.; Rapior, S.; McKey, D.B.; Lumaret, J.P. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 2007, 17, 23–30. [Google Scholar] [CrossRef]
- Dellacasa, G.; Dellacasa, M. Coleoptera Aphodiidae, Aphodiinae; Fauna d’Italia, Coleoptera Aphodiidae Aphodiinae; Calderini de Il Sole 24 Ore.: Milano, Italia, 2006; Volume XLI, p. 484. [Google Scholar]
- Lumaret, J.P. Atlas Des Coléoptères Scarabéides Laparosticti de France; Muséum National d’Histoire Naturelle; Secrétariat de la Faune et la Flore: Paris, France, 1990; p. 419. [Google Scholar]
- Verdú, J.R.; Galante, E. Behavioural and morphological adaptations for a low-quality resource in semi-arid environments: Dung beetles (Coleoptera, Scarabaeoidea) associated with the European rabbit (Oryctolagus cuniculus L.). J. Nat. Hist. 2004, 38, 705–715. [Google Scholar] [CrossRef]
- Dellacasa, G.; Dellacasa, M.; Mann, D.J. The Morphology of the Labrum (Epipharynx, Ikrioma and Aboral Surface) of Adult Aphodiini (Coleoptera: Scarabaeidae: Aphodiinae), and Its Implications for Systematics. Insecta Mundi 2010, 132, 1–21. [Google Scholar]
- Holter, P.; Scholtz, C.H. Re-establishment of biting mouthparts in desert-living dung beetles (Scarabaeidae: Scarabaeinae) feeding on plant litter-old structures reacquired or new ones evolved? J. Morphol. 2011, 272, 1007–1016. [Google Scholar] [CrossRef]
- Hata, K.; Edmonds, W.D. Structure and function of the mandibles of adult dung beetles (Coleoptera: Scarabaeidae). Int. J. Insect Morphol. Embryol. 1983, 12, 1–12. [Google Scholar] [CrossRef]
- Holter, P. Particle feeding in Aphodius dung beetles (Scarabaeidae): Old hypotheses and new experimental evidence. Funct. Ecol. 2000, 14, 631–637. [Google Scholar] [CrossRef]
- Scholtz, C.H.; Davis, A.L.V.; Kryger, U. Evolutionary Biology and Conservation of Dung Beetles; Pensoft Publishers: Sofia, Bulgaria, 2009; p. 567. [Google Scholar]
- Moczek, A.P. Horn polyphenism in the beetle Onthophagus taurus: Larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behav. Ecol. 1998, 9, 636–641. [Google Scholar] [CrossRef]
- Kishi, S.; Nishida, T. Adjustment of parental investment in the dung beetle Onthophagus atripennis (Col. Scarabaeidae). Ethology 2006, 112, 1239–1245. [Google Scholar] [CrossRef]
- Edwards, P.B. Seasonal variation in the dung of African grazing mammals, and its consequences for coprophagous insects. Funct. Ecol. 1991, 5, 617–628. [Google Scholar] [CrossRef]
- Tonelli, M. Some considerations on the terminology applied to dung beetle functional groups. Ecol. Entomol. 2021, 46, 772–776. [Google Scholar] [CrossRef]
- Milotić, T.; Quidé, S.; Van Loo, T.; Hoffmann, M. Linking functional group richness and ecosystem functions of dung beetles: An experimental quantification. Oecologia 2017, 183, 177–190. [Google Scholar] [CrossRef]
- Tocco, C.; Balmer, J.P.; Villet, M.H. Trophic preference of southern African dung beetles (Scarabaeoidea: Scarabaeinae and Aphodiinae) and its influence on bioindicator surveys. Afr. J. Ecol. 2018, 56, 938–948. [Google Scholar] [CrossRef]
- Kadlec, J.; Mikatova, S.; Maslo, P.; Sipkova, H.; Sipek, P.; Sladecek, F.X.J. Delaying insect access alters community composition on small carrion: A quantitative approach. Entomol. Exp. Appl. 2019, 167, 729–740. [Google Scholar] [CrossRef]
| Trait | Trait Type | Data Type | Functional Link |
|---|---|---|---|
| Fresh body mass | BM | Quan | Resource use |
| Metabolic rate | |||
| Thermoregulatory pattern | |||
| Competition | |||
| Sphericity | BM | Quan | Resource use |
| Head area/Total area Ratio | BM | Quan | Resource use |
| Hind tibiae length | BM | Quan | Resource use |
| Metamesosternal area | BM | Quan | Dispersal capability |
| Abdomen length | BM | Quan | Resource use |
| Wing load | BM | Quan | Dispersal capability |
| Foraging strategy | |||
| Habitat use | |||
| Thermoregulatory pattern | |||
| Number of teeth in the mandibles profile | MM | Quan | Resource use |
| Conjunctive/total mandible area ratio | MM | Quan | Resource use |
| Percentage of filtering/masticator area of mandibular molars | MM | Quan | Resource use |
| Zygum | MM | Qual | Resource use |
| Trophic diversity | E | Quan | Resource use |
| Nest type | E | Qual | Resource use |
| Competition | |||
| Habitat use | |||
| Nest depth | E | Qual | Resource use |
| Competition | |||
| Habitat use | |||
| Horizontal nest distance | E | Qual | Resource use |
| Competition | |||
| Habitat use | |||
| Nesting patterns | E | Qual | Resource use |
| Competition | |||
| Habitat use | |||
| Daily activity | E | Qual | Resource use |
| Competition | |||
| Habitat use | |||
| Phenology | E | Qual | Resource use |
| Competition | |||
| Habitat use |
| Family | Subfamily | Tribe | Species | Horse | Cow | Total |
|---|---|---|---|---|---|---|
| Scarabaeidae | Aphodiinae | Aphodiini | Acanthobodilus immundus (Creutzer, 1799) | 6 | 17 | 23 |
| Scarabaeidae | Aphodiinae | Aphodiini | Acrossus luridus (Fabricius, 1775) | 66 | 163 | 229 |
| Scarabaeidae | Aphodiinae | Aphodiini | Acrossus rufipes (Linnaeus, 1758) | 0 | 4 | 4 |
| Scarabaeidae | Aphodiinae | Aphodiini | Agrilinus constans (Duftschmid, 1805) | 0 | 1 | 1 |
| Scarabaeidae | Aphodiinae | Aphodiini | Agrilinus convexus (Erichson, 1848) | 5 | 18 | 23 |
| Scarabaeidae | Aphodiinae | Aphodiini | Amidorus thermicola (Sturm, 1800) | 2 | 0 | 2 |
| Scarabaeidae | Aphodiinae | Aphodiini | Aphodius coniugatus (Panzer, 1795) | 1 | 10 | 11 |
| Scarabaeidae | Aphodiinae | Aphodiini | Aphodius fimetarius (Linnaeus, 1758) | 44 | 114 | 158 |
| Scarabaeidae | Aphodiinae | Aphodiini | Aphodius foetidus (Herbst, 1783) | 4 | 7 | 11 |
| Scarabaeidae | Aphodiinae | Aphodiini | Biralus mahunkaorum (Ádám, 1983) | 0 | 1 | 1 |
| Scarabaeidae | Aphodiinae | Aphodiini | Bodilopsis rufa (Moll, 1782) | 21 | 808 | 829 |
| Scarabaeidae | Aphodiinae | Aphodiini | Bodiloides ictericus (Laicharting, 1781) | 0 | 11 | 11 |
| Scarabaeidae | Aphodiinae | Aphodiini | Calamosternus granarius (Linnaeus, 1767) | 20 | 3 | 23 |
| Scarabaeidae | Aphodiinae | Aphodiini | Calamosternus mayeri (Pilleri, 1953) | 0 | 1 | 1 |
| Scarabaeidae | Aphodiinae | Aphodiini | Chilothorax conspurcatus (Linnaeus, 1758) | 778 | 3 | 781 |
| Scarabaeidae | Aphodiinae | Aphodiini | Chilothorax lineolatus (Illiger, 1803) | 2 | 1 | 3 |
| Scarabaeidae | Aphodiinae | Aphodiini | Chilothorax paykulli (Bedel, 1907) | 48 | 5 | 53 |
| Scarabaeidae | Aphodiinae | Aphodiini | Colobopterus erraticus (Linnaeus, 1758) | 401 | 2612 | 3013 |
| Scarabaeidae | Aphodiinae | Aphodiini | Coprimorphus scrutator (Herbst, 1789) | 45 | 134 | 179 |
| Scarabaeidae | Aphodiinae | Aphodiini | Esymus merdarius (Fabricius, 1775) | 57 | 50 | 107 |
| Scarabaeidae | Aphodiinae | Aphodiini | Esymus pusillus (Herbst, 1789) | 6 | 31 | 37 |
| Scarabaeidae | Aphodiinae | Aphodiini | Euorodalus paracoenosus (Balthasar & Hrubant, 1960) | 2 | 0 | 2 |
| Scarabaeidae | Aphodiinae | Aphodiini | Labarrus lividus (Olivier, 1789) | 663 | 8 | 671 |
| Scarabaeidae | Aphodiinae | Aphodiini | Limarus zenkeri (Germar, 1813) | 2 | 0 | 2 |
| Scarabaeidae | Aphodiinae | Aphodiini | Loraphodius suarius (Faldermann, 1836) | 33 | 19 | 52 |
| Scarabaeidae | Aphodiinae | Aphodiini | Melinopterus consputus (Creutzer, 1799) | 61,128 | 40,406 | 101,534 |
| Scarabaeidae | Aphodiinae | Aphodiini | Melinopterus prodromus (Brahm, 1790) | 6859 | 531 | 7390 |
| Scarabaeidae | Aphodiinae | Aphodiini | Melinopterus reyi (Reitter, 1892) | 12 | 4 | 16 |
| Scarabaeidae | Aphodiinae | Aphodiini | Melinopterus stolzi (Reitter, 1906) | 0 | 2 | 2 |
| Scarabaeidae | Aphodiinae | Aphodiini | Nialus varians (Duftschmid, 1805) | 0 | 9 | 9 |
| Scarabaeidae | Aphodiinae | Aphodiini | Nimbus contaminatus (Herbst, 1783) | 435 | 371 | 806 |
| Scarabaeidae | Aphodiinae | Aphodiini | Nimbus johnsoni (Baraud, 1976) | 12 | 9 | 21 |
| Scarabaeidae | Aphodiinae | Aphodiini | Nimbus obliteratus (Panzer, 1823) | 2175 | 829 | 3004 |
| Scarabaeidae | Aphodiinae | Aphodiini | Otophorus haemorroidalis (Linnaeus, 1758) | 9 | 63 | 72 |
| Scarabaeidae | Aphodiinae | Aphodiini | Phalacronothus biguttatus (Germar, 1824) | 2 | 4 | 6 |
| Scarabaeidae | Aphodiinae | Aphodiini | Planolinus fasciatus (Olivier, 1789) | 0 | 2 | 2 |
| Scarabaeidae | Aphodiinae | Aphodiini | Sigorus porcus (Fabricius, 1792) | 216 | 179 | 395 |
| Scarabaeidae | Aphodiinae | Aphodiini | Teuchestes fossor (Linnaeus, 1758) | 1 | 11 | 12 |
| Scarabaeidae | Aphodiinae | Aphodiini | Trichonotulus scrofa (Fabricius, 1787) | 45 | 202 | 247 |
| Scarabaeidae | Scarabaeinae | Onitini | Bubas bison (Linnaeus, 1767) | 30 | 76 | 106 |
| Scarabaeidae | Scarabaeinae | Coprini | Copris lunaris (Linnaeus, 1758) | 11 | 12 | 23 |
| Scarabaeidae | Scarabaeinae | Oniticellini | Euoniticellus fulvus (Goeze, 1777) | 6129 | 4130 | 10,259 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Caccobius schreberi (Linnaeus, 1767) | 14 | 16 | 30 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus coenobita (Herbst, 1783) | 80 | 126 | 206 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus fracticornis (Preyssler, 1790) | 3935 | 6124 | 10,059 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus grossepunctatus Reitter, 1905 | 46 | 108 | 154 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus illyricus (Scopoli, 1763) | 1 | 1 | 2 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus joannae Goljan, 1953 | 297 | 560 | 857 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus lemur (Fabricius, 1781) | 194 | 493 | 687 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus medius (Kugelann, 1792) | 5273 | 5601 | 10,874 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus opacicollis Reitter, 1892 | 11 | 27 | 38 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus ruficapillus Brullé, 1832 | 41 | 166 | 207 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus taurus (Schreber, 1759) | 365 | 506 | 871 |
| Scarabaeidae | Scarabaeinae | Onthophagini | Onthophagus verticicornis (Laicharting, 1781) | 564 | 761 | 1325 |
| Scarabaeidae | Scarabaeinae | Sisyphini | Sisyphus schaefferi (Linnaeus, 1758) | 180 | 781 | 961 |
| Geotrupidae | Geotrupinae | Geotrupini | Geotrupes spiniger Marsham, 1802 | 91 | 174 | 265 |
| Geotrupidae | Geotrupinae | Geotrupini | Sericotrupes niger (Marsham, 1802) | 115 | 145 | 260 |
| Geotrupidae | Geotrupinae | Geotrupini | Trypocopris vernalis apenninicus (Mariani, 1958) | 3 | 6 | 9 |
| Total species (S) | 50 | 55 | 58 | |||
| Total individuals (N) | 90,480 | 66,456 | 156,936 |
| Indicator Species | Cow | Horse |
|---|---|---|
| Aphodius coniugatus (Panzer, 1795) | 42.4 | |
| Aphodius fimetarius (Linnaeus, 1758) | 67.3 | |
| Bodilopsis rufa (Moll, 1782) | 84.5 | |
| Colobopterus erraticus (Linnaeus, 1758) | 86.7 | |
| Esymus pusillus (Herbst, 1789) | 61.4 | |
| Geotrupes spiniger Marsham, 1802 | 65.7 | |
| Chilothorax conspurcatus (Linnaeus, 1758) | 59.8 | |
| Labarrus lividus (Olivier, 1789) | 92.2 |
| Trait Type | Trait Name | CWM | FDvar | FRO | |||
|---|---|---|---|---|---|---|---|
| Cow | Horse | Cow | Horse | Cow | Horse | ||
| Body Morphology | Fresh body mass | X | |||||
| Hind tibiae length | X | ||||||
| Abdomen length | X | ||||||
| Wing load | X | ||||||
| Mouthpart Morphology | Conjunctive/total mandible area ratio | X | |||||
| Percentage of filtering area of mandibular molars | X | ||||||
| Zygum developed | X | ||||||
| Zygum underdeveloped | X | ||||||
| Ethological | Nest type 0 (non-nester) | X | |||||
| Nest type 3 (Nest composed of a single brood mass located underground in a simple nest) | X | ||||||
| Nest type 7 (Nest composed of a single brood ball located underground in a simple nest) | X | ||||||
| Nest depth 0 (within excrement) | X | ||||||
| Horizontal nest distance 0 (within food source) | X | ||||||
| Horizontal nest distance 1 (starting within food source but with a horizontal extension) | X | ||||||
| Horizontal nest distance 3 (a great distance out from the food source) | X | ||||||
| Nesting patterns 2 (Telecoprid medium-little sized) | X | ||||||
| Nesting patterns 5 (Paracoprid with large body size) | X | ||||||
| Nesting patterns 8 (Paracoprid with small body size burying dung slowly and at shallow depth without well-developed brood mass) | X | ||||||
| Nesting patterns 11 (non-nester) | X | ||||||
| Phenology 1 (Autumn, winter and spring) | X | ||||||
| Phenology 8 (Summer and autumn) | X | ||||||
| Phenology 14 (All year) | X | X | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonelli, M.; Giménez Gómez, V.C.; Verdú, J.R.; Casanoves, F.; Zunino, M. Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process. Life 2021, 11, 873. https://doi.org/10.3390/life11090873
Tonelli M, Giménez Gómez VC, Verdú JR, Casanoves F, Zunino M. Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process. Life. 2021; 11(9):873. https://doi.org/10.3390/life11090873
Chicago/Turabian StyleTonelli, Mattia, Victoria C. Giménez Gómez, José R. Verdú, Fernando Casanoves, and Mario Zunino. 2021. "Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process" Life 11, no. 9: 873. https://doi.org/10.3390/life11090873
APA StyleTonelli, M., Giménez Gómez, V. C., Verdú, J. R., Casanoves, F., & Zunino, M. (2021). Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process. Life, 11(9), 873. https://doi.org/10.3390/life11090873






