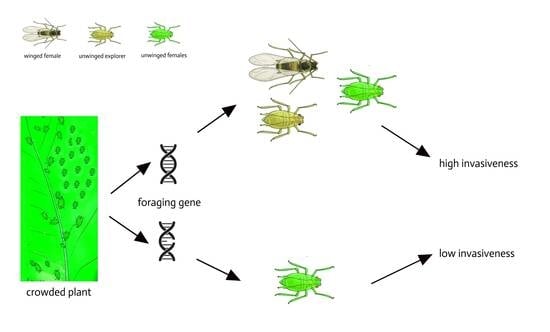
The foraging Gene Is Involved in the Presence of Wings and Explorative Behaviours in Parthenogenetic Females of the Aphid Myzus persicae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Rearing
2.2. RNA Extraction and foraging Gene Amplification
2.3. qPCR Experiments and Statistical Analysis
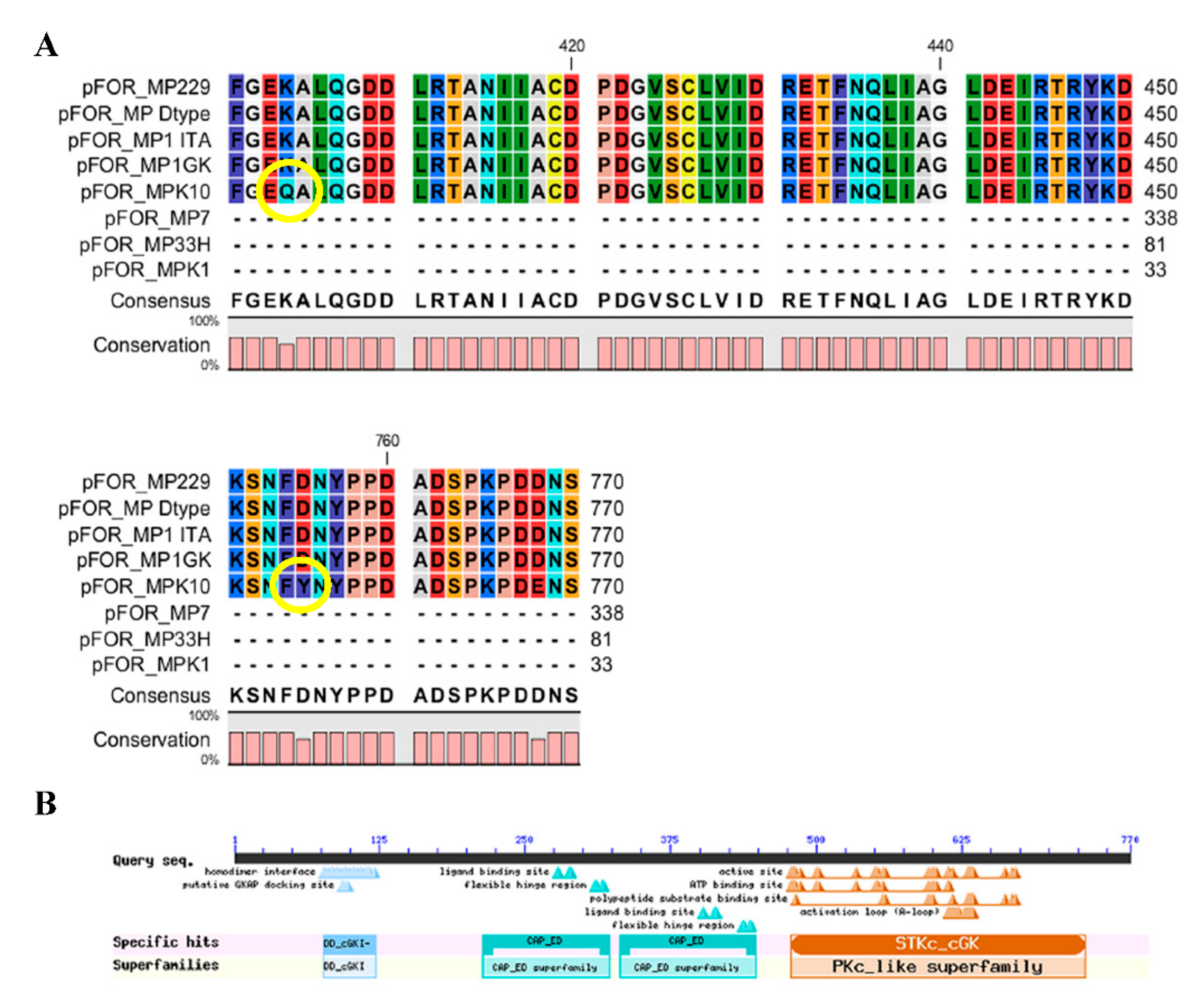
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osborne, K.; Robichon, A.; Burgess, E.; Butland, S.; Shaw, R.A.; Coulthard, A.; Pereira, H.S.; Greenspan, R.J.; Sokolowski, M.B. Natural behavior polymorphism due to a cGMP-dependant protein kinase of Drosophila. Science 1997, 277, 834–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shahar, Y.; Robichon, A.; Sokolowski, M.B.; Robinson, G.E. Influence of gene action across different time scales on behaviour. Science 2002, 296, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hammock, E.A. Gene regulation as a modulator of social preference in voles. Adv. Genet. 2007, 59, 107–127. [Google Scholar]
- Mansourian, S.; Fandino, R.A.; Riabinina, O. Progress in the use of genetic methods to study insect behavior outside Drosophila. Curr. Opin. Insect Sci. 2019, 36, 45–56. [Google Scholar] [CrossRef]
- Lucas, C.; Ben-Shahar, Y. The foraging gene as a modulator of division of labour in social insects. J. Neurogenet. 2021, 35, 168–178. [Google Scholar] [CrossRef]
- Lucas, C.; Kornfein, R.; Chakaborty-Chatterjee, M.; Schonfeld, J.; Geva, N.; Sokolowski, M.B.; Avali, A. The locus foraging gene. Arch. Insect Biochem. Physiol. 2011, 74, 249–251. [Google Scholar] [CrossRef]
- Fitzpatrick, M.J.; Sokolowski, M.B. In search of food: Exploring the evolutionary link between cGMP-dependent protein kinase (PKG) and behaviour. Integr. Comp. Biol. 2004, 44, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fussnecker, B.; Grozinger, C. Dissecting the role of Kr-h1 brain gene expression in foraging behaviour in honeybees (Apis mellifera). Insect Mol. Biol. 2008, 17, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Garabagi, F.; Wade French, B.; Schaafsma, A.W.; Pauls, K.P. Increased expression of a cGMP-dependent protein kinase in rotation-adapted western corn rootworm (Diabrotica virgifera virgifera L.). Insect Biochem. Mol. Biol. 2008, 38, 697–704. [Google Scholar] [CrossRef]
- Tobback, J.; Mommaerts, V.; Vandersmissen, H.P.; Smagghe, G.; Huybrechts, R. Age- and task-dependent foraging gene expression in the bumblebee Bombus terrestris. Arch. Insect Biochem. Physiol. 2011, 76, 30–42. [Google Scholar] [CrossRef]
- Sokolowski, M.B. Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behav. Genet. 1980, 10, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shahar, Y. The foraging gene, behavioural plasticity and honeybee division of labor. J. Comp. Physiol. A 2005, 191, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Tarès, S.; Arthaud, L.; Amichot, M.; Robichon, A. Environment exploration and colonization behaviour of the pea aphid associated with the expression of the foraging gene. PLoS ONE 2013, 8, e65104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia, D.H.; Silva, A.X.; Ballesteros, G.I.; Figueroa, C.C.; Niemeyer, H.M.; Ramírez, C.C. Differences in learning and memory of host plant features between specialist and generalist phytophagous insects. Anim. Behav. 2015, 106, 1–10. [Google Scholar] [CrossRef]
- Rivi, M.; Monti, V.; Mazzoni, E.; Cassanelli, S.; Panini, M.; Bizzaro, D.; Mandrioli, M.; Manicardi, G.C. Karyotype variations in Italian populations of the peach-potato aphid Myzus persicae (Hemiptera: Aphididae). Bull. Entomol. Res. 2012, 102, 663–671. [Google Scholar] [CrossRef]
- Kati, A.N.; Mandrioli, M.; Skouras, P.J.; Malloch, G.L.; Voudouris, C.C.; Venturelli, M.; Manicardi, G.C.; Tsitsipis, J.A.; Fenton, B.; Margaritopoulos, J.T. Recent changes in the distribution of carboxylesterase genes and associated chromosomal rearrangements in Greek populations of the tobacco aphid Myzus persicae nicotianae. Biol. J. Linn. Soc. 2014, 113, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Leung, H.T.; Pak, W.L.; Sokolowski, M.B.; Robinson, G.E. cGMP- dependent changes in phototaxis: A possible role for the foraging gene in honeybee division of labor. J. Exp. Biol. 2003, 206, 2507–2515. [Google Scholar] [CrossRef] [Green Version]
- Heylen, K.; Gobin, B.; Billen, J.; Hu, T.T.; Arckens, L. Amfor expression in the honeybee brain: A trigger mechanism for nurse-forager transition. J. Insect Physiol. 2008, 54, 1400–1403. [Google Scholar] [CrossRef]
- Ingram, K.K.; Oefner, P.; Gordon, D.M. Task-specific expression of the foraging gene in harvester ants. Mol. Ecol. 2005, 14, 813–818. [Google Scholar] [CrossRef]
- Ingram, K.K.; Kleeman, L.; Peteru, S. Differential regulation of the foraging gene associated with task behaviours in harvester ants. BMC Ecol. 2011, 11, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.; Sokolowski, M.B. Molecular basis for changes in behavioural state in ant social behaviours. Proc. Natl. Acad. Sci. USA 2009, 106, 6351–6356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anreiter, I.; Sokolowski, M.B. The foraging gene and its behavioural effects: Pleiotropy and plasticity. Annu. Rev. Genet. 2019, 53, 373–392. [Google Scholar] [CrossRef] [PubMed]
- L’Etoile, N.D.; Coburn, C.N.; Eastham, J.; Kistler, A.; Gallegos, G. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron 2002, 36, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Hatano, E.; Kunert, G.; Weisser, W.W. Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS ONE 2010, 5, e11188. [Google Scholar] [CrossRef]
- Nardelli, A.; Peona, V.; Toschi, A.; Mandrioli, M.; Manicardi, G. Afit: A bioinformatic tool for measuring aphid fitness and invasiveness. Bull. Entomol. Res. 2017, 107, 458–465. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. The molecular basis of an insect host shift. Proc. Nat. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, M.; Salvatore, D.; Ferrari, A.; Patelli, N.; Manicardi, G.C. Comparative analysis of intra- and inter-specific genomic variability in the peach potato aphid, Myzus persicae. Insects 2019, 10, e368. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, M.; Manicardi, G.C. Evolutionary insights into the aphid genome: Aphid genomics between quality problems and intriguing perspectives. Int. Rev. Cell Mol. Biol. 2020, 354, 215–230. [Google Scholar]
- Shelby, E.A.; Moss, J.B.; Andreason, S.A.; Simmons, A.M.; Moore, A.J.; Moore, P.J. Debugging: Strategies and considerations for efficient RNAi-mediated control of the whitefly Bemisia tabaci. Insects 2020, 11, e723. [Google Scholar] [CrossRef]
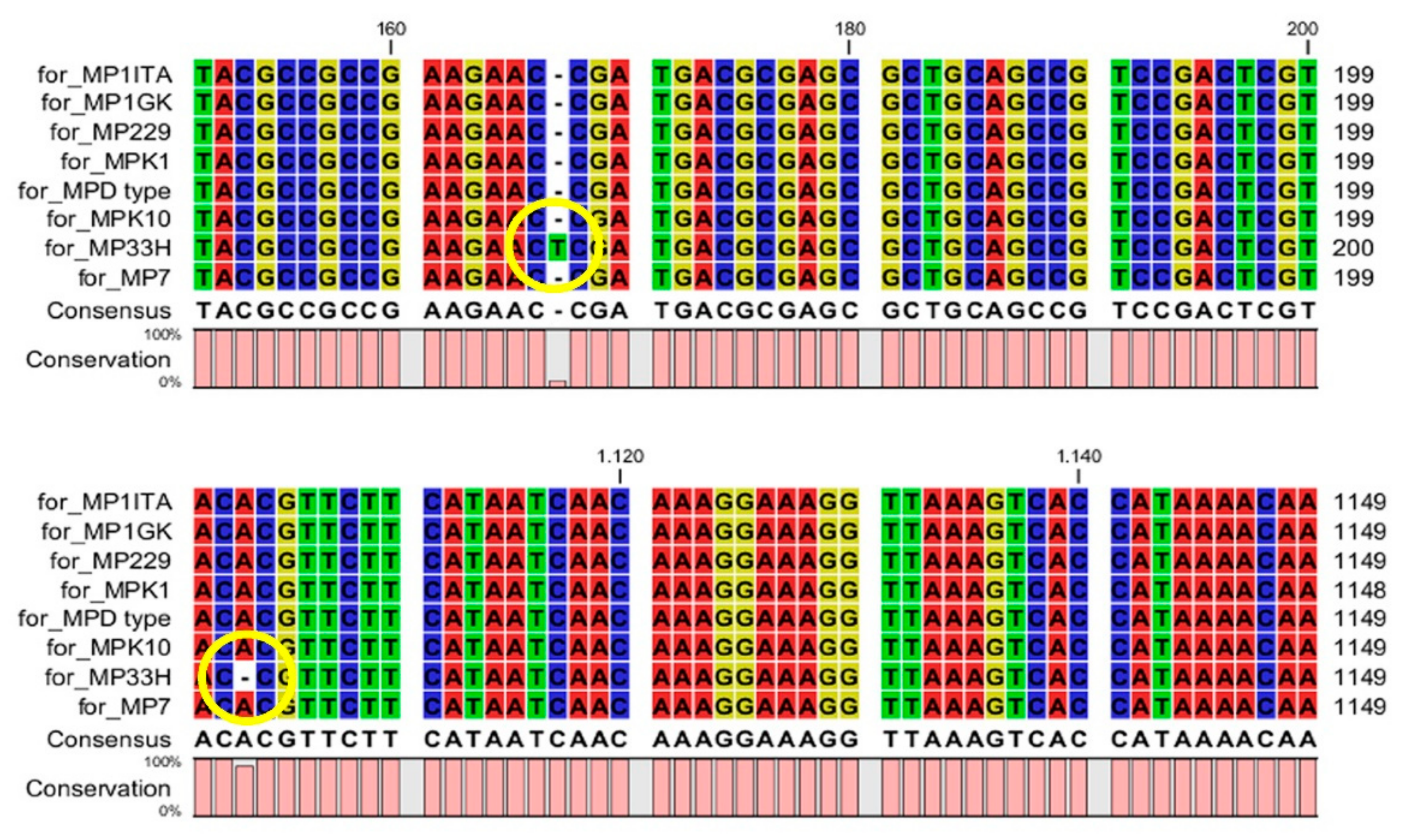
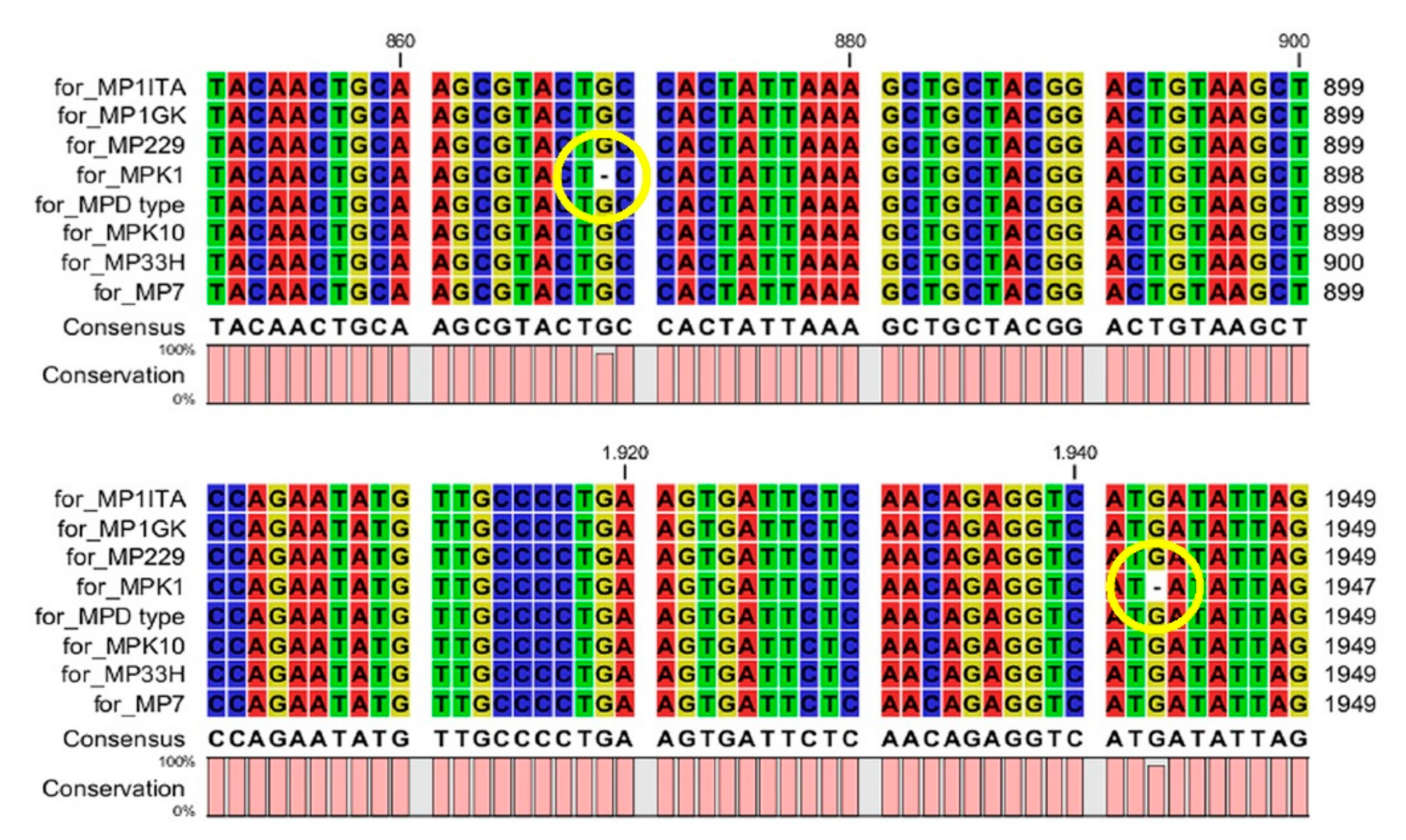
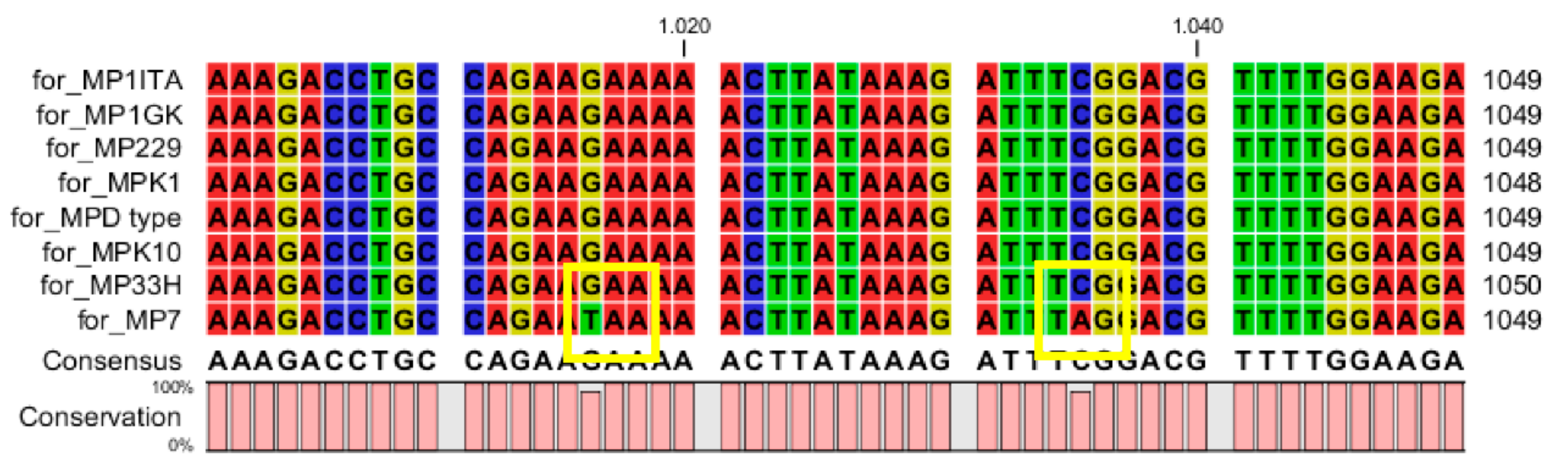


| Clone | Year first Identification | Host | Description | Insecticide Resistance | Reproductive Mode |
|---|---|---|---|---|---|
| 1ITA | 2003 | peach | susceptible to insecticides, generalist, common | S | holocyclic |
| K1 | 2004 | potato | long-term resistant, identified as M. p. nicotianae, common | R3 resistance due to esterase gene duplication, kdr mutation | holocyclic |
| K10 | 2004 | potato | long-term resistant, generalist, common | R3 resistance due to esterase gene duplication, kdr mutation | obligate parthenogenetic |
| 1GK | 2002 | shepherd’s purse | susceptible, identified as M. p. nicotianae, common | S | holocyclic |
| 229 | 2004 | potato | long-term resistant, generalist, common | R3 resistance due to esterase gene duplication | holocyclic |
| 33H | 2012 | potato | susceptible to insecticides, generalist, common | S | obligate parthenogenetic |
| 7 | 2008 | peach | susceptible to insecticides, generalist, common | S | obligate parthenogenetic |
| D type | 2001 | potato | long-term resistant, generalist, common | R3 resistance due to esterase gene duplication, kdr mutation, skdr mutation | obligate parthenogenetic |
| 1ITA | 100% | |||||||
| 1GK | 99.91% | 100% | ||||||
| 229 | 100% | 99.91% | 100% | |||||
| K1 | 99.82% | 99.74% | 99.82% | 100% | ||||
| D type | 100% | 99.91% | 100% | 99.82% | 100% | |||
| K10 | 99.74% | 99.65% | 99.74% | 99.56% | 99.74% | 100% | ||
| 33H | 99.87% | 99.78% | 99.87% | 99.69% | 99.87% | 99.61% | 100% | |
| 7 | 99.87% | 99.78% | 99.87% | 99.69% | 99.87% | 99.61% | 99.74% | 100% |
| 1ITA | 1GK | 229 | K1 | D type | K10 | 33H | 7 |
| Clone | Presence (+) or Absence (−) of Severe Mutations | Abundance (+) or Absence (−) of Alate Females | Presence (+) or Absence (−) of Explorative Behavior |
|---|---|---|---|
| 1ITA | − | + | − |
| 1GK | − | ++ | + |
| 229 | − | ++ | + |
| 33H | + | − | − |
| K10 | − | ++ | + |
| K1 | + | − | − |
| 7 | + | − | − |
| D type | − | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrioli, M.; Manicardi, G.C. The foraging Gene Is Involved in the Presence of Wings and Explorative Behaviours in Parthenogenetic Females of the Aphid Myzus persicae. Life 2022, 12, 369. https://doi.org/10.3390/life12030369
Mandrioli M, Manicardi GC. The foraging Gene Is Involved in the Presence of Wings and Explorative Behaviours in Parthenogenetic Females of the Aphid Myzus persicae. Life. 2022; 12(3):369. https://doi.org/10.3390/life12030369
Chicago/Turabian StyleMandrioli, Mauro, and Gian Carlo Manicardi. 2022. "The foraging Gene Is Involved in the Presence of Wings and Explorative Behaviours in Parthenogenetic Females of the Aphid Myzus persicae" Life 12, no. 3: 369. https://doi.org/10.3390/life12030369
APA StyleMandrioli, M., & Manicardi, G. C. (2022). The foraging Gene Is Involved in the Presence of Wings and Explorative Behaviours in Parthenogenetic Females of the Aphid Myzus persicae. Life, 12(3), 369. https://doi.org/10.3390/life12030369