Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 1
Abstract
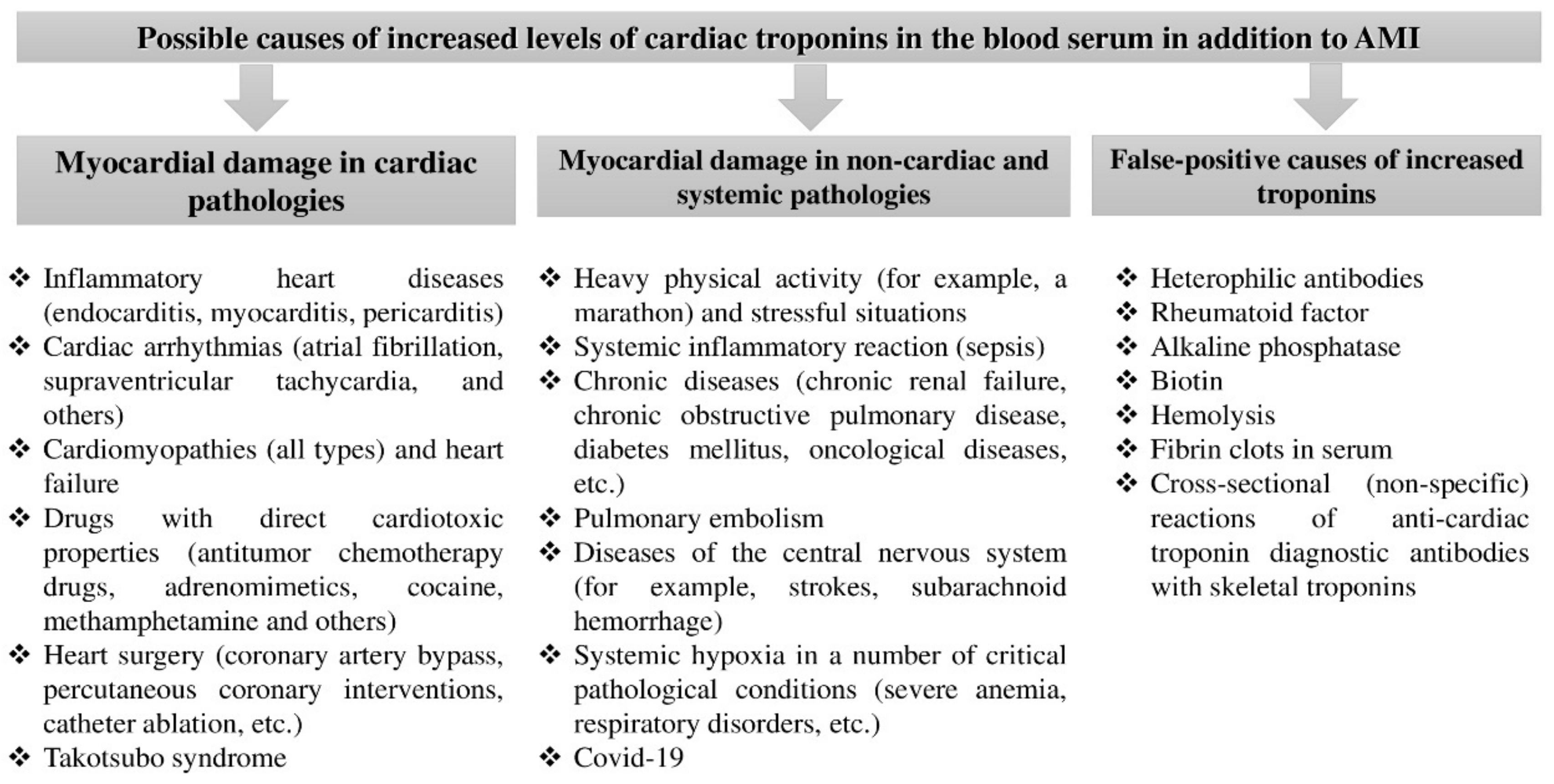
1. Background
1.1. Classification of Causes Accompanied by an Elevation in the Concentration of Cardiac Troponin Isoforms in the Absence of Myocardial Infarction
1.2. Causes and Mechanisms of Elevated Cardiac Troponins during Physical Activity
1.3. Causes and Mechanisms of Elevated Cardiac Troponins in Inflammatory Heart Diseases (Endo-, Peri-, and Myo-Carditis)
1.4. Diagnostic Valuae and Mechanisms of Elevation in Cardiac Troponins in PE
1.5. Causes and Mechanisms of Elevated Cardiac Troponins in Chronic Kidney Disease
1.6. Diagnostic Value and Mechanisms of Elevated Cardiac Troponins in Sepsis
2. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Biomarkers of acute myocardial infarction: Diagnostic and prognostic value. Part 1. J. Clin. Pract. 2020, 11, 75–84. (In Russian) [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Biomarkers of acute myocardial infarction: Diagnostic and prognostic value. Part 2. J. Clin. Pract. 2020, 11, 70–82. (In Russian) [Google Scholar] [CrossRef]
- Chaulin, A. Cardiac troponins: Contemporary biological data and new methods of determination. Vasc. Health Risk Manag. 2021, 17, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Karslyan, L.S.; Bazyuk, E.V.; Nurbaltaeva, D.A.; Duplyakov, D.V. Clinical and diagnostic value of cardiac markers in human biological fluids. Kardiologiia 2019, 59, 66–75. (In Russian) [Google Scholar] [CrossRef]
- Kanderian, A.S.; Francis, G.S. Cardiac troponins and chronic kidney disease. Kidney Int. 2006, 69, 1112–1114. [Google Scholar] [CrossRef]
- Nørlund, H.; Bovin, A. False positive troponin I due to heterophile antibodies. Ugeskr Laeger 2017, 179, V05170412. (In Danish) [Google Scholar]
- Li, S.F.; Zapata, J.; Tillem, E. The prevalence of false-positive cardiac troponin I in ED patients with rhabdomyolysis. Am. J. Emerg. Med. 2005, 23, 860–863. [Google Scholar] [CrossRef]
- Kaplan, A.; Orhan, N.; Ilhan’, E. False positive troponin levels due to heterophil antibodies in a pregnant woman. Turk. J. Emerg. Med. 2016, 15, 47–50. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Arrhythmogenic effects of doxorubicin. Complex Issues Cardiovasc. Dis. 2020, 9, 69–80. (In Russian) [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. MicroRNAs in atrial fibrillation: Pathophysiological aspects and potential biomarkers. Int. J. Biomed. 2020, 10, 198–205. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Svechkov, N.A.; Volkova, S.L.; Grigoreva, Y.V. Diagnostic value of cardiac troponins in elderly patients without myocardial infarction. Mod. Probl. Sci. Educ. 2020, 6, 12–24. (In Russian) [Google Scholar] [CrossRef]
- Jarolim, P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin. Chem. Lab. Med. 2015, 53, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A. Clinical and diagnostic value of highly sensitive cardiac troponins in arterial hypertension. Vasc. Health Risk Manag. 2021, 17, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Abashina, O.E.; Duplyakov, D.V. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russ. Open Med. J. 2020, 9, e0305. [Google Scholar] [CrossRef]
- Body, R.; Carlton, E. Understanding cardiac troponin part 1: Avoiding troponinitis. Emerg. Med. J. 2018, 35, 120–125. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Karslyan, L.S.; Nurbaltaeva, D.A.; Grigoriyeva, E.V.; Duplyakov, D.V. Cardial troponins metabolism under normal and pathological conditions. Sib. Med. Rev. 2019, 6, 5–14. [Google Scholar] [CrossRef]
- Anand, A.; Shah, A.S.V.; Beshiri, A.; Jaffe, A.S.; Mills, N.L. Global adoption of high-sensitivity cardiac Troponins and the universal definition of myocardial infarction. Clin. Chem. 2019, 65, 484–489. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Douglas, P.S.; O’Toole, M.; Rimm, E.; Ginsburg, G.S. Cardiac troponin T and I, echocardiographic wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am. J. Cardiol. 1999, 83, 1085–1089. [Google Scholar] [CrossRef]
- Mingels, A.; Jacobs, L.; Michielsen, E.; Swaanenburg, J.; Wodzig, W.; Van Dieijen-Visser, M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin. Chem. 2009, 55, 101–108. [Google Scholar] [CrossRef]
- Scherr, J.; Braun, S.; Schuster, T.; Hartmann, C.; Moehlenkamp, S.; Wolfarth, B.; Pressler, A.; Halle, M. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med. Sci. Sport. Exerc. 2011, 43, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; Leckie, T.; Watkins, E.; Fitzpatrick, D.; Galloway, R.; Grimaldi, R.; Baker, P. Post marathon cardiac troponin T is associated with relative exercise intensity. J. Sci. Med. Sport 2018, 21, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, A.I.; Hamer, M.; Gaze, D.; Collinson, P.; Steptoe, A. The association between cortisol response to mental stress and high sensitivity cardiac troponin T plasma concentration in healthy adults. J. Am. Coll. Cardiol. 2013, 62, 1694–1701. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. On the potential effect of circadian rhythms of cardiac troponins on the diagnosis of acute myocardial infarction. Signa Vitae 2021, 17, 79–84. [Google Scholar] [CrossRef]
- Chaulin, A.; Karslyan, L.S.; Grigorieva, E.V.; Nurbaltaeva, D.A.; Duplyakov, D.V. Metabolism of cardiac troponins (literature review). Complex Issues Cardiovasc. Dis. 2019, 8, 103–115. (In Russian) [Google Scholar] [CrossRef]
- O’Hanlon, R.; Wilson, M.; Wage, R.; Smith, G.; Alpendurada, F.D.; Wong, J.; Dahl, A.; Oxborough, D.; Godfrey, R.; Sharma, S.; et al. Troponin release following endurance exercise: Is inflammation the cause? A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2010, 12, 38. [Google Scholar] [CrossRef]
- Paana, T.; Jaakkola, S.; Bamberg, K.; Saraste, A.; Tuunainen, E.; Wittfooth, S.; Kallio, P.; Heinonen, O.J.; Knuuti, J.; Pettersson, K.; et al. Cardiac troponin elevations in marathon runners. Role of coronary atherosclerosis and skeletal muscle injury. The MaraCat Study. Int. J. Cardiol. 2019, 295, 25–28. [Google Scholar] [CrossRef]
- Marshall, L.; Lee, K.K.; Stewart, S.D.; Wild, A.; Fujisawa, T.; Ferry, A.V.; Stables, C.L.; Lithgow, H.; Chapman, A.; Anand, A.; et al. Effect of exercise intensity and duration on cardiac troponin release. Circulation 2020, 141, 83–85. [Google Scholar] [CrossRef]
- Bjørkavoll-Bergseth, M.; Kleiven, Ø.; Auestad, B.; Eftestøl, T.; Oskal, K.; Nygård, M.; Skadberg, Ø.; Aakre, K.M.; Melberg, T.; Gjesdal, K.; et al. Duration of elevated heart rate is an important predictor of exercise-induced troponin elevation. J. Am. Heart Assoc. 2020, 9, e014408. [Google Scholar] [CrossRef] [PubMed]
- Kleiven, Ø.; Omland, T.; Skadberg, Ø.; Melberg, T.H.; Bjørkavoll-Bergseth, M.; Auestad, B.; Bergseth, R.; Greve, O.J.; Aakre, K.M.; Ørn, S. Occult obstructive coronary artery disease is associated with prolonged cardiac troponin elevation following strenuous exercise. Eur. J. Prev. Cardiol. 2020, 27, 1212–1221. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Banfi, G.; Plebani, M. Cardiac troponins and physical exercise. It’s time to make a point. Biochem. Med. 2011, 21, 55–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hickman, P.E.; Potter, J.M.; Aroney, C.; Koerbin, G.; Southcott, E.; Wu, A.H.; Roberts, M. Cardiac troponin may be released by ischemia alone, without necrosis. Clin. Chim. Acta 2010, 411, 318–323. [Google Scholar] [CrossRef]
- Schwartz, P.; Piper, H.M.; Spahr, R.; Spieckermann, P.G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am. J. Pathol. 1984, 115, 349–361. [Google Scholar]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.-U.; Zamorano, J.L. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Sicari, R.; Cortigiani, L. The clinical use of stress echocardiography in ischemic heart disease. Cardiovasc. Ultrasound 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Samaha, E.; Brown, J.; Brown, F.; Martinez, S.C.; Scott, M.; Jaffe, A.S.; Davila-Roman, V.G.; Nagele, P. High-sensitive cardiac troponin T increases after stress echocardiography. Clin. Biochem. 2019, 63, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bunin, V.A.; Kozlov, K.L.; Linkova, N.S.; Paltseva, E.M. An increase in troponin-I concentration in the saliva of patients with ischemic heart disease correlates with the stage of disease development. Kompleksnye Problemy Serdecno-Sosudistyh Zabolevanij 2017, 6 (Suppl. S4), 13–14. (In Russian) [Google Scholar]
- Chaulin, A.M.; Duplyakova, P.D.; Bikbaeva, G.R.; Tukhbatova, A.A.; Grigorieva, E.V.; Duplyakov, D.V. Concentration of high-sensitivity cardiac troponin I in the oral fluid in patients with acute myocardial infarction: A pilot study. Russ. J. Cardiol. 2020, 25, 3814. [Google Scholar] [CrossRef]
- Manjunath, L.; Yeluru, A.; Rodriguez, F. 27-year-old man with a positive troponin: A case report. Cardiol. Ther. 2018, 7, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lauer, B.; Niederau, C.; Kühl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.-E.; Schultheiss, H.-P. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef]
- Soongswang, J.; Durongpisitkul, K.; Ratanarapee, S.; Leowattana, W.; Nana, A.; Laohaprasitiporn, D.; Akaniroj, S.; Limpimwong, N.; Kangkagate, C. Cardiac troponin T: A marker in the diagnosis of clinically suspected acute myocarditis and chronic dilated cardiomyopathy in children. Pediatr. Cardiol. 2002, 23, 531–535. [Google Scholar] [CrossRef]
- Soongswang, J.; Durongpisitkul, K.; Nana, A.; Laohaprasitiporn, D.; Kangkagate, C.; Punlee, C.; Limpimwong, N. Cardiac troponin T: A marker in the diagnosis of acute myocarditis in children. Pediatr. Cardiol. 2005, 26, 45–49. [Google Scholar] [CrossRef]
- Yozgat, C.Y.; Yesilbas, O.; Uzuner, S.; Saritas, B.; Ergor, S.N.; Temur, H.O.; Yozgat, Y. Recurrent elevation of troponin levels in acute myocarditis: Is it a sign of ventricular tachycardia? Indian J. Pediatr. 2020, 87, 1076–1077. [Google Scholar] [CrossRef]
- Putschoegl, A.; Auerbach, S. Diagnosis, evaluation, and treatment of myocarditis in children. Pediatr. Clin. N. Am. 2020, 67, 855–874. [Google Scholar] [CrossRef]
- Ukena, C.; Kindermann, M.; Mahfoud, F.; Geisel, J.; Lepper, P.M.; Kandolf, R.; Böhm, M.; Kindermann, I. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin. Res. Cardiol. 2014, 103, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Hsiao, H.-J.; Hsia, S.-H.; Lin, J.-J.; Hwang, M.-S.; Chung, H.-T.; Chen, C.-L.; Huang, Y.-C.; Tsai, M.-H. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS ONE 2019, 14, e0214087. [Google Scholar] [CrossRef]
- Dancea, A.B. Myocarditis in infants and children: A review for the paediatrician. Paediatr. Child Health 2001, 6, 543–545. [Google Scholar] [CrossRef]
- Abrar, S.; Ansari, M.J.; Mittal, M.; Kushwaha, K. Predictors of mortality in paediatric myocarditis. J. Clin. Diagn. Res. 2016, 10, SC12–SC16. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Shimada, T.; Hattori, H.; Shimada, M.; Mason, J.W. Autoantibodies against cardiac troponin I in patients presenting with myocarditis. CVD Prev. Control 2011, 6, 41–46. [Google Scholar] [CrossRef]
- Watkin, R.W.; Lang, S.; Smith, J.M.; Elliott, T.S.; Littler, W.A. Role of troponin I in active infective Endocarditis. Am. J. Cardiol. 2004, 94, 1198–1199. [Google Scholar] [CrossRef]
- Tsenovoy, P.; Aronow, W.S.; Joseph, J.; Kopacz, M.S. Patients with infective endocarditis and increased cardiac troponin I levels have a higher incidence of in-hospital mortality and valve replacement than those with normal cardiac troponin I levels. Cardiology 2009, 112, 202–204. [Google Scholar] [CrossRef]
- Purcell, J.B.; Patel, M.; Khera, A.; de Lemos, J.A.; Forbess, L.W.; Baker, S.; Cabell, C.H.; Peterson, G.E. Relation of troponin elevation to outcome in patients with infective endocarditis. Am. J. Cardiol. 2006, 101, 1479–1481. [Google Scholar] [CrossRef]
- Imazio, M.; Demichelis, B.; Cecchi, E.; Belli, R.; Ghisio, A.; Bobbio, M.; Trinchero, R. Cardiac troponin I in acute pericarditis. J. Am. Coll. Cardiol. 2003, 42, 2144–2148. [Google Scholar] [CrossRef] [PubMed]
- Gamaza-Chulián, S.; León-Jiménez, J.; Recuerda-Núñez, M.; Camacho-Freire, S.; Gutiérrez-Barrios, A.; Vargas-Machuca, J.C. Cardiac troponin-T in acute pericarditis. J. Cardiovasc. Med. 2014, 15, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galie, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J.; Kavsak, P. Measurement of high-sensitivity cardiac troponin in pulmonary embolism: Useful test or a clinical distraction. Semin. Thromb. Hemost. 2019, 45, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Svechkov, N.A.; Grigoreva, Y.U.V. Laboratory biomarkers of right ventricular dysfunction. Mod. Probl. Sci. Educ. 2021, 1, 24–38. (In Russian) [Google Scholar] [CrossRef]
- Giannitsis, E.; Müller-Bardorff, M.; Kurowski, V.; Weidtmann, B.; Wiegand, U.; Kampmann, M.; Katus, H.A. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000, 102, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, G.; Dogan, O.T.; Berk, S.; Epozturk, K.; Ozsahin, S.L.; Akkurt, I. Significance of serum cardiac troponin I levels in pulmonary embolism. J. Thorac. Dis. 2012, 4, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism. A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Müller-Bardorff, M.; Weidtmann, B.; Giannitsis, E.; Kurowski, V.; A Katus, H. Release kinetics of cardiac troponin T in survivors of confirmed severe pulmonary embolism. Clin. Chem. 2002, 48, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Friesen, D.; Aschoff, J.; Dellas, C.; Hasenfuss, G.; Katus, H.; Konstantinides, S.; Giannitsis, E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur. Heart J. 2010, 31, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Binder, L.; Hruska, N.; Luthe, H.; Buchwald, A.B. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J. Am. Coll. Cardiol. 2000, 36, 1632–1636. [Google Scholar] [CrossRef]
- Cotugno, M.; Orgaz-Molina, J.; Rosa-Salazar, V.; Guirado-Torrecillas, L.; García-Pérez, B. Right ventricular dysfunction in acute pulmonary embolism: NT-proBNP vs. troponin T. Med. Clin. 2017, 148, 339–344. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Cho, J.Y.; Sim, D.S.; Yoon, H.J.; Yoon, N.S.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.; et al. D-dimer/troponin ratio in the differential diagnosis of acute pulmonary embolism from non-ST elevation myocardial infarction. Korean J. Intern. Med. 2019, 34, 1263. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Abashina, O.E.; Duplyakov, D.V. High-sensitivity cardiac troponins: Detection and central analytical characteristics. Cardiovasc. Ther. Prev. 2021, 20, 2590. (In Russian) [Google Scholar] [CrossRef]
- Chaulin, A.M.; Grigorieva, Y.V.; Pavlova, T.V.; Duplyakov, D.V. Diagnostic significance of complete blood count in cardiovascular patients; Samara State Medical University. Russ. J. Cardiol. 2020, 25, 3923. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Comorbidity in chronic obstructive pulmonary disease and cardiovascular disease. Cardiovasc. Ther. Prev. 2021, 20, 2539. (In Russian) [Google Scholar] [CrossRef]
- Rahimtoola, A.; Bergin, J.D. Acute pulmonary embolism: An update on diagnosis and management. Curr. Probl. Cardiol. 2005, 30, 61–114. [Google Scholar] [CrossRef]
- Punukollu, G.; Khan, I.A.; Gowda, R.M.; Lakhanpal, G.; Vasavada, B.C.; Sacchi, T.J. Cardiac troponin I release in acute pulmonary embolism in relation to the duration of symptoms. Int. J. Cardiol. 2005, 99, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Duplyakov, D.V. High-sensitivity cardiac troponins: Circadian rhythms. Cardiovasc. Ther. Prev. 2021, 20, 2639. (In Russian) [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakova, P.D.; Duplyakov, D.V. Circadian rhythms of cardiac troponins: Mechanisms and clinical significance. Russ. J. Cardiol. 2020, 25, 4061. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Cardiac troponins: Analytical characteristics and diagnostic capabilities of modern (high-sensitive) determination methods. J. Clin. Diagn. Res. 2021, 15, BE01–BE06. [Google Scholar] [CrossRef]
- Katrukha, I.A.; Kogan, A.E.; Vylegzhanina, A.V.; Serebryakova, M.; Koshkina, E.V.; Bereznikova, A.V.; Katrukha, A.G. Thrombin-mediated degradation of human cardiac troponin T. Clin. Chem. 2017, 63, 1094–1100. [Google Scholar] [CrossRef]
- Streng, A.S.; de Boer, D.; van Doorn, W.P.; Kocken, J.M.; Bekers, O.; Wodzig, W.K. Cardiac troponin T degradation in serum is catalysed by human thrombin. Biochem. Biophys. Res. Commun. 2016, 481, 165–168. [Google Scholar] [CrossRef]
- Pervan, P.; Svaguša, T.; Prkačin, I.; Savuk, A.; Bakos, M.; Perkov, S. Urine high-sensitive troponin I measuring in patients with hypertension. Signa Vitae 2017, 13, 62–64. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lee, S.Y.; Li, Y.H.; Lin, C.Y.; Shieh, M.D.; Ciou, D.S. Urine high-sensitivity troponin I predict incident cardiovascular events in patients with diabetes mellitus. J. Clin. Med. 2020, 9, 3917. [Google Scholar] [CrossRef]
- Ziebig, R.; Lun, A.; Hocher, B.; Priem, F.; Altermann, C.; Asmus, G.; Kern, H.; Krause, R.; Lorenz, B.; Möbes, R.; et al. Renal elimination of troponin T and troponin I. Clin. Chem. 2003, 49, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. Diagnostic value of highly sensitive cardiac troponins and mechanisms of their increase in serum and urine in arterial hypertension. Riv. Ital. Med. Lab. 2021, 17, 99–107. [Google Scholar] [CrossRef]
- Dubin, R.F.; The CRIC Study Investigators; Li, Y.; He, J.; Jaar, B.G.; Kallem, R.; Lash, J.P.; Makos, G.; Rosas, S.E.; Soliman, E.Z.; et al. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: A cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013, 14, 229. [Google Scholar] [CrossRef]
- Wilhelm, J.; Hettwer, S.; Schuermann, M.; Bagger, S.; Gerhardt, F.; Mundt, S.; Muschik, S.; Zimmermann, J.; Amoury, M.; Ebelt, H.; et al. Elevated troponin in septic patients in the emergency department: Frequency, causes, and prognostic implications. Clin. Res. Cardiol. 2014, 103, 561–567. [Google Scholar] [CrossRef]
- Røsjø, H.; The FINNSEPSIS Study Group; Varpula, M.; Hagve, T.-A.; Karlsson, S.; Ruokonen, E.; Pettilä, V.; Omland, T. Circulating high-sensitive troponin T in severe sepsis and septic shock: Distribution, associated factors, and relation to outcome. Int. Care Med. 2011, 37, 77–85. [Google Scholar] [CrossRef]
- de Filippi, C.R.; Herzog, C.A. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin. Chem. 2016, 63, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, D.; Quiroga, B.; Panizo, N.; Rodríguez-Ferrero, M.; Macías, N.; Reque, J.; Anaya, F.; Luño, J. High-sensitivity troponin T levels in kidney transplant recipients. Transplant. Proc. 2012, 44, 2545–2547. [Google Scholar] [CrossRef] [PubMed]
- Ricchiutti, V.; Apple, F.S. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin. Chem. 1999, 45, 2129–2135. [Google Scholar] [CrossRef]
- Haller, C.; Zehelein, J.; Remppis, A.; Müller-Bardorff, M.; Katus, H.A. Cardiac troponin T in patients with end stage renal disease: Absence of expression in truncal skeletal muscle. Clin. Chem. 1998, 44, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ooi, D.S.; Isolato, P.A.; Veinot, J.P. Correlation of antemortem serum creatine kinase, creatinekinase-MB, troponin I, and troponin T with cardiac pathology. Clin. Chem. 2000, 46, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Duplyakov, D.V. Cardiac troponins: Current data on the diagnostic value and analytical characteristics of new determination methods. Cor Vasa 2021, 63, 486–493. [Google Scholar] [CrossRef]
- Ali, S.A.; Kazmi, S.; Jalal-Ud-Din, M.; Qasim, M.I.; Jadoon, Z.G. Frequency of elevated troponin T in patients of hronic renal failure without clinically suspected acute myocardial infarction. J. Ayub Med. Coll. Abbottabad JAMC 2019, 31, 364–367. [Google Scholar]
- Chaulin, A.M.; Duplyakov, D.V. Increased natriuretic peptides not associated with heart failure. Russ. J. Cardiol. 2020, 25, 4140. [Google Scholar] [CrossRef]
- Guest, T.M.; Ramanathan, A.V.; Tuteur, P.G.; Schechtman, K.B.; Ladenson, J.H.; Jaffe, A.S. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA 1995, 273, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Persichini, R.; Monnet, X.; Teboul, J.-L. Management of myocardial dysfunction in severe sepsis. Semin. Respir. Crit. Care Med. 2011, 32, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. Phosphorylation and fragmentation of the cardiac troponin T: Mechanisms, role in pathophysiology and laboratory diagnosis. Int. J. Biomed. 2021, 11. In Press. [Google Scholar]
- Kumar, A.; Kumar, A.; Paladugu, B.; Mensing, J.; Parrillo, J.E. Transforming growth factor-beta 1 blocks in vitro cardiac myocyte depression induced by tumour necrosis factor-alpha, interleukin-1 beta, and human septic shock serum. Crit. Care Med. 2007, 35, 358–364. [Google Scholar] [CrossRef]
- Aberegg, S.K.; Kaufman, D.A. Troponin in sepsis. Ann. Am. Thorac. Soc. 2019, 16, 1335–1336. [Google Scholar] [CrossRef]
- Sheyin, O.; Davies, O.; Duan, W.; Perez, X. The prognostic significance of troponin elevation in patients with sepsis: A meta-analysis. Heart Lung 2015, 44, 75–81. [Google Scholar] [CrossRef]
- Sharma, A.C. Sepsis-induced myocardial dysfunction. Shock 2007, 28, 265–269. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Environmental factors and cardiovascular diseases. Hyg. Sanit. 2021, 100, 223–228. [Google Scholar] [CrossRef]
- Bessière, F.; Khenifer, S.; Dubourg, J.; Durieu, I.; Lega, J.-C. Prognostic value of troponins in sepsis: A meta-analysis. Intensive Care Med. 2013, 39, 1181–1189. [Google Scholar] [CrossRef]
| Diagnostic Algorithm 0 → 1 h | |||||
| Immunoassay, manufacturer | High-sensitivity troponin level, indicative of very low probability of NSTEMI, ng/L | High-sensitivity troponin level, indicative of low probability of NSTEMI, ng/L | Changes in high-sensitivity troponin levels in 1 h in order to exclude NSTEMI, ng/L | High-sensitivity troponin level, indicative of high probability of NSTEMI, ng/L | Changes in high-sensitivity troponin levels in 1 h in order to confirm NSTEMI, ng/L |
| hs-cTnT (Elecsys; Roche) | <5 | <12 | <3 | ≥52 | ≥5 |
| hs-cTnI (Architect; Abbott) | <4 | <5 | <2 | ≥64 | ≥6 |
| hs-cTnI (Centaur; Siemens) | <3 | <6 | <3 | ≥120 | ≥12 |
| hs-cTnI (Access; Beckman Coulter) | <4 | <5 | <4 | ≥50 | ≥15 |
| hs-cTnI (Clarity; Singulex) | <1 | <2 | <1 | ≥30 | ≥6 |
| Diagnostic Algorithm 0 → 2 h | |||||
| Immunoassay, manufacturer | High-sensitivity troponin level, indicative of very low probability of NSTEMI, ng/L | High-sensitivity troponin level, indicative of low probability of NSTEMI, ng/L | Changes in high-sensitivity troponin levels in 2 h in order to exclude NSTEMI, ng/L | High-sensitivity troponin level, indicative of high probability of NSTEMI, ng/L | Changes in high-sensitivity troponin levels in 2 h in order to confirm NSTEMI, ng/L |
| hs-cTnT (Elecsys; Roche) | <5 | <14 | <4 | ≥52 | ≥10 |
| hs-cTnI (Architect; Abbott) | <4 | <6 | <2 | ≥64 | ≥15 |
| hs-cTnI (Centaur; Siemens) | <3 | <8 | <7 | ≥120 | ≥20 |
| hs-cTnI (Access; Beckman Coulter) | <4 | <5 | <5 | ≥50 | ≥20 |
| hs-cTnI (Clarity; Singulex) | <1 | TBD | TBD | ≥30 | TBD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaulin, A.M. Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 1. Life 2021, 11, 914. https://doi.org/10.3390/life11090914
Chaulin AM. Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 1. Life. 2021; 11(9):914. https://doi.org/10.3390/life11090914
Chicago/Turabian StyleChaulin, Aleksey M. 2021. "Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 1" Life 11, no. 9: 914. https://doi.org/10.3390/life11090914
APA StyleChaulin, A. M. (2021). Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 1. Life, 11(9), 914. https://doi.org/10.3390/life11090914






