Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Clinical Evaluation
2.2. Thrombospondin-1 Circulating Levels Measurement
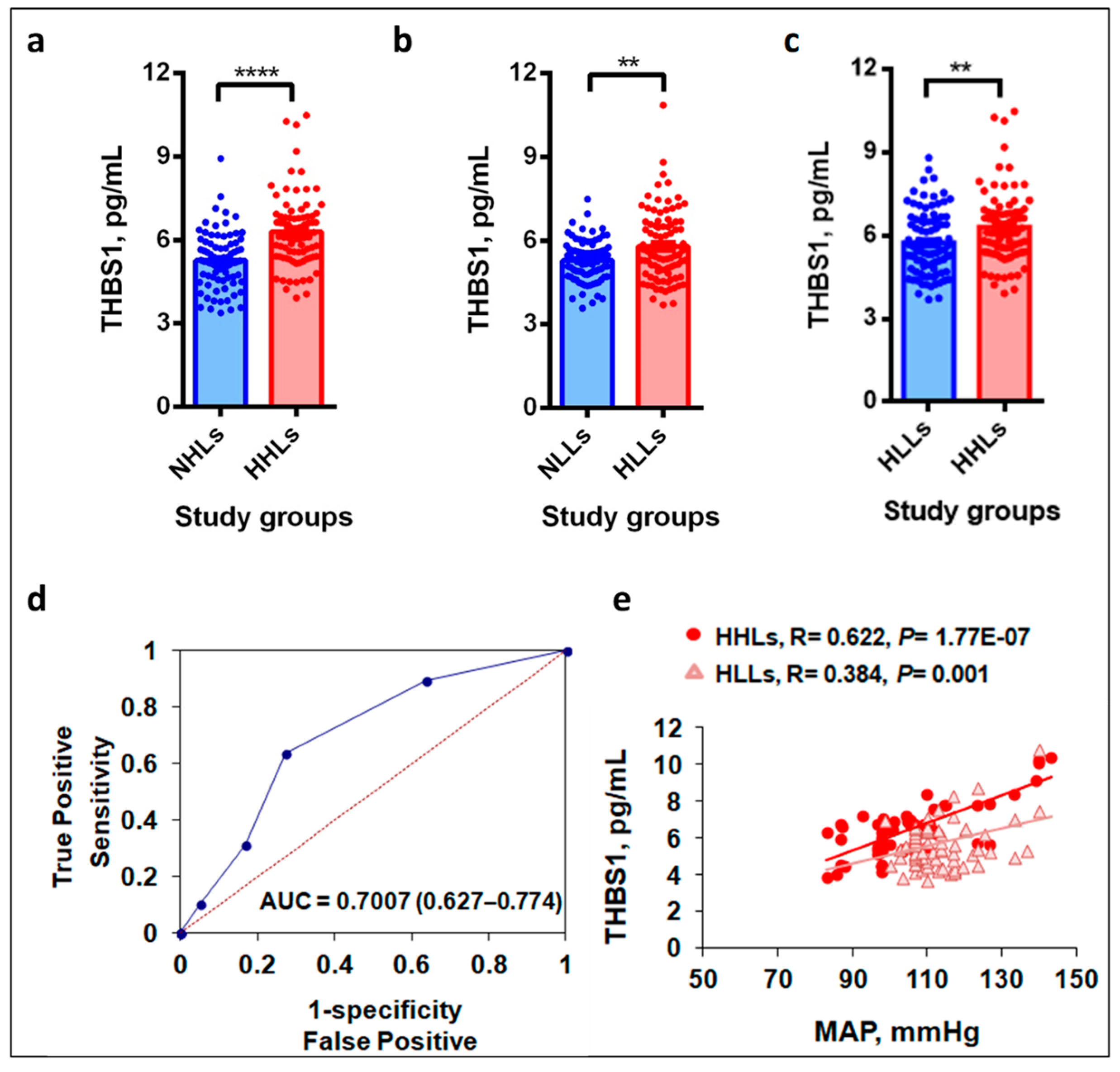
2.3. Receiver Operating Characteristic Curve
2.4. Correlation Analysis
2.5. Selection and Screening of the THBS1 and CD47 Gene Variants
2.5.1. SNP Genotyping in Highland and Sea-Level Populations
2.5.2. SNP–SNP Interactions and Allelic Influences of THBS1 and CD47 Gene Variants
2.6. Evaluation of THBS and CD Family Variants
2.6.1. Identifying Hypertension Associated Variants
2.6.2. Multi-Locus Interactions for Association Analysis
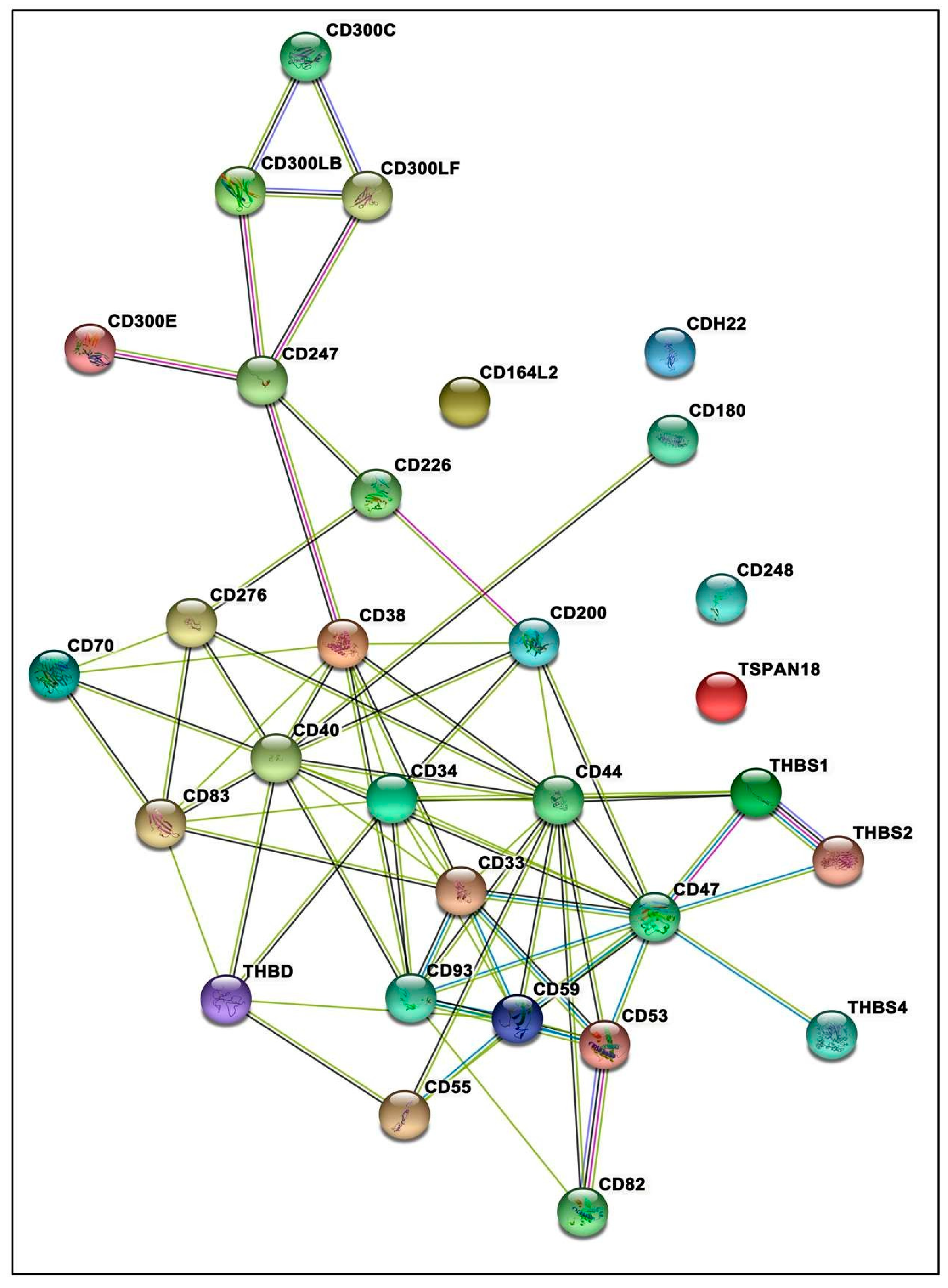
2.7. Protein–Protein Interactions Network
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Demographic and Clinical Characteristics
3.2. Plasma THBS1 Levels Upregulate in Hypertensive Study Groups
3.3. Hypobaric Hypoxic Environment Enhances THBS1 Levels
3.4. THBS1 Levels Correlate with MAP
3.5. Genotype Distributions of THBS1 rs2228263 and Its Receptor CD47 rs9879947
3.5.1. Effect of Risk Allele on the Circulating Levels
3.5.2. Genetic Interactions between the THBS1 and CD47 SNPs Revealed the Causal Role of Risk Alleles
3.6. THBS-CD Family Genes Variants Regulate Hypertension under Hypobaric Hypoxia
3.6.1. Variants of THBS-CD Family Genes Associate with Hypertension at HA
3.6.2. Within Gene and Gene–Gene Interactions Define Etiology of Hypertension
3.6.3. Haplotypes Associate with Hypertension
3.7. Protein–Protein Interactions Inclined toward Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan-Rowe, L.; Nikitorowicz, J.; Shiwen, X.; Leask, A.; Tsui, J.; Abraham, D.; Stratton, R. Thrombospondin 1 in hypoxia-conditioned media blocks the growth of human microvascular endothelial cells and is increased in systemic sclerosis tissues. Fibrogenes. Tissue Repair 2011, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.C.; Lawler, J. The thrombospondins. Int. J. Biochem. Cell Biol. 2004, 36, 961–968. [Google Scholar] [CrossRef]
- Rogers, N.M.; Sharifi-Sanjani, M.; Csányi, G.; Pagano, P.J.; Isenberg, J.S. Thrombospondin-1 and CD47 Regulation of Cardiac, Pulmonary and Vascular Responses in Health and Disease. Matrix Biol. 2014, 37, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.S.; Gutierrez, J. Thrombospondin 1 in Metabolic Diseases. Front. Endocrinol. 2021, 12, 638536. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, A.; Schneider, C.; Martiny, L.; Dedieu, S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front. Pharmacol. 2015, 6, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buda, V.; Andor, M.; Cristescu, C.; Tomescu, M.C.; Muntean, D.M.; Bâibâță, D.E.; Bordejevic, D.A.; Danciu, C.; Dalleur, O.; Coricovac, D.; et al. Thrombospondin-1 Serum Levels in Hypertensive Patients with Endothelial Dysfunction after One Year of Treatment with Perindopril. Drug. Des. Devel. Ther. 2019, 13, 3515–3526. [Google Scholar] [CrossRef] [Green Version]
- Kuebler, W.M. What mediates the effects of thrombospondin-1 in pulmonary hypertension? New evidence for a dual-pronged role of CD47. Cardiovasc. Res. 2017, 113, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Kaur, S.; Isenberg, J.S. Regulation of cellular redox signaling by matricellular proteins in vascular biology, immunology, and cancer. Antioxid. Redox Signal. 2017, 27, 874–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Mickael, C.; Kassa, B.; Gebreab, L.; Robinson, J.C.; Koyanagi, D.E.; Sanders, L.; Barthel, L.; Meadows, C.; Fox, D.; et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun. 2017, 8, 15494. [Google Scholar] [CrossRef]
- Kumar, R.; Kohli, S.; Ali, Z.; Duhan, K.; Ram, R.; Gupta, M.; Tyagi, S.; Mohammad, G.; Pasha, M.A.Q. CYBA (p22phox) variants associate with blood pressure and oxidative stress markers in hypertension: A replication study in populations of diverse altitudes. Hypertens. Res. 2015, 38, 498–506. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-Induced Pulmonary Vascular Remodeling: Cellular and Molecular Mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.J.; Iwuchukwu, C.; Maier, K.G.; Gahtan, V. Thrombospondin-1-induced vascular smooth muscle cell migration and proliferation are functionally dependent on microRNA-21. Surgery 2014, 155, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Quertermous, T.; Boothroyd, D.B.; Priest, J.R.; Glassford, A.J.; Myers, R.M.; Fortmann, S.P.; Iribarren, C.; Tabor, H.K.; Assimes, T.L.; et al. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am. Heart J. 2007, 154, 1035–1042. [Google Scholar] [CrossRef]
- Tan, A.C.; Fan, J.; Karikari, C.; Bibikova, M.; Garcia, E.W.; Zhou, L.; Barker, D.; Serre, D.; Feldmann, G.; Hruban, R.H.; et al. Allele-specific expression in the germline of patients with familial pancreatic cancer: An unbiased approach to cancer gene discovery. Cancer Biol. Ther. 2008, 7, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.A.; Novelli, E.M.; Isenberg, J.S.; Garrett, M.E.; Chu, Y.; Soldano, K.; Ataga, K.I.; Telen, M.J.; Ashley-Koch, A.; Gladwin, M.T.; et al. Thrombospondin-1 gene polymorphism is associated with estimated pulmonary artery pressure in patients with sickle cell anemia. Clin. Trial Am. J. Hematol. 2017, 92, E31–E34. [Google Scholar] [CrossRef] [Green Version]
- Lascorz, J.; Bevier, M.; Schönfels, W.V.; Kalthoff, H.; Aselmann, H.; Beckmann, J.; Egberts, J.; Buch, S.; Becker, T.; Schreiber, S.; et al. Association study identifying polymorphisms in CD47 and other extracellular matrix pathway genes as putative prognostic markers for colorectal cancer. Int. J. Colorectal Dis. 2013, 28, 173–181. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Richalet, J.P.; Gratadour, P.; Robach, P.; Pham, I.; Déchaux, M.; Joncquiert-Latarjet, A.; Mollard, P.; Brugniaux, J.; Cornolo, J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005, 171, 275–281. [Google Scholar] [CrossRef]
- Narvaez-Guerra, O.; Herrera-Enriquez, K.; Medina-Lezama, J.; Chirinos, J.A. Systemic Hypertension at High Altitude. Hypertension 2018, 72, 567–578. [Google Scholar] [CrossRef]
- Mishra, A.; Mohammad, G.; Norboo, T.; Newman, J.H.; Pasha, M.A.Q. Lungs at high-altitude: Genomic insights into hypoxic responses. J. Appl. Physiol. 2015, 119, 1–15. [Google Scholar] [CrossRef]
- Petousi, N.; Robbins, P.A. Human adaptation to the hypoxia of high altitude: The Tibetan paradigm from the pregenomic to the postgenomic era. J. Appl. Physiol. 2014, 116, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Mohammad, G.; Thinlas, T.; Pasha, M.A.Q. EGLN1 variants influence expression and SaO2 levels to associate with high-altitude pulmonary oedema and adaptation. Clin. Sci. 2013, 124, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M.; Cavalleri, G.L.; Deng, L.; Elston, R.C.; Gao, Y.; Knight, J.; Li, C.; Li, J.C.; Liang, Y.; McCormack, M.; et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. USA 2010, 107, 11459–11464. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Kohli, S.; Dua, S.; Thinlas, T.; Mohammad, G.; Pasha, M.A.Q. Genetic differences and aberrant methylation in the apelin system predict the risk of high-altitude pulmonary edema. Proc. Natl. Acad. Sci. USA 2015, 112, 6134–6139. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.; Frantz, C.; Bals, R.; Wilkens, H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir. Res. 2016, 17, 96. [Google Scholar] [CrossRef] [Green Version]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signaling by thrombospondin-1: Implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Lawler, P.R.; Lawler, J. Molecular Basis for the Regulation of Angiogenesis by Thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Ferrer, E.; Peinado, V.I.; Castañeda, J.; Prieto-Lloret, J.; Olea, E.; González-Martín, M.C.; Vega-Agapito, M.V.; Díez, M.; Domínguez-Fandos, D.; Obeso, A.; et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur. Respir. J. 2011, 38, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Daubon, T.; Léon, C.; Clarke, K.; Andrique, L.; Salabert, L.; Darbo, E.; Pineau, R.; Guérit, S.; Maitre, M.; Dedieu, S.; et al. Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nat. Commun. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Mickael, C.; Kassa, B.; Sanders, L.; Hernandez-Saavedra, D.; Koyanagi, D.E.; Kumar, S.; Pugliese, S.C.; Thomas, S.; McClendon, J.; et al. Interstitial macrophage-derived thrombospondin-1 contributes to hypoxia-induced pulmonary hypertension. Cardiovasc. Res. 2020, 116, 2021–2030. [Google Scholar] [CrossRef]
- Labrousse-Arias, D.; Castillo-González, R.; Rogers, N.M.; Torres-Capelli, M.; Barreira, B.; Aragonés, J.; Cogolludo, A.; Isenberg, J.S.; Calzada, M.J. HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc. Res. 2016, 109, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antezana, A.M.; Antezana, G.; Aparicio, O.; Noriega, I.; Velarde, F.L.; Richalet, J.P. Pulmonary hypertension in high-altitude chronic hypoxia: Response to nifedipine. Eur. Respir. J. 1998, 12, 1181–1185. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.C.; Blazy, I.; Déchaux, M.; Rabier, D.; Mason, N.P.; Richalet, J.P. Response of nitric oxide pathway to L-arginine infusion at the altitude of 4350 m. Eur. Respir. J. 2001, 18, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hemann, C.; Hille, R.; Stuehr, D.J.; et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593. [Google Scholar] [CrossRef] [Green Version]
- Leung, L.L. Role of thrombospondin in platelet aggregation. J. Clin. Investig. 1984, 74, 1764–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanamandra, U.; Boddu, R.; Pramanik, S.; Mishra, K.; Kapoor, R.; Ahuja, A.; Chatterjee, T.; Das, S. Prevalence and Clinical Characteristics of Post-Thrombotic Syndrome in High-Altitude-Induced Deep Vein Thrombosis: Experience of a Single Tertiary Care Center from Real-World Settings. High. Alt. Med. Biol. 2020, 21, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Congote, L.F.; DiFalco, M.R.; Gibbs, B.F. Thrombospondin 1, produced by endothelial cells under the action of erythropoietin, stimulates thymidine incorporation into erythroid cells and counteracts the inhibitory action of insulin-like growth factor binding protein 3. Cytokine 2005, 30, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; McMurray, J.L.; Mohammed, F.; Bicknell, R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019, 286, 3299–3332. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Stecca, B. Aberrant Sialylation in Cancer: Biomarker and Potential Target for Therapeutic Intervention? Cancers 2021, 13, 201. [Google Scholar] [CrossRef]

| Parameters | NHLs (n = 183) | HHLs (n = 166) | p-Value | NLLs (n = 486) | HLLs (n = 509) | p-Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 48 (26%) | 55 (33%) | 363 (75%) | 326 (64%) | ||
| Female | 135 (74%) | 111 (67%) | 123 (25%) | 183 (36%) | ||
| Clinical Characteristics | ||||||
| Age, years | 39.8 ± 9.0 | 40.9 ± 9.1 | NS | 38.0 ± 9.7 | 43.1 ± 7.7 | <0.0001 |
| BMI, kg/m2 | 21.5 ± 2.8 | 21.7 ± 2.7 | NS | 23.1 ± 2.6 | 24.4 ± 2.4 | <0.0001 |
| SBP, mm Hg | 111.3 ± 8.33 | 141.6 ± 16.0 | <0.0001 | 115.7 ± 6.0 | 157.1 ± 16.2 | <0.0001 |
| DBP, mm Hg | 72.6 ± 7.5 | 88.9 ± 11.8 | <0.0001 | 76.2 ± 5.1 | 96.6 ± 8.5 | <0.0001 |
| MAP, mm Hg | 85.5 ± 6.9 | 106.5 ± 12.4 | <0.0001 | 89.4 ± 5.1 | 116.8 ± 10.1 | <0.0001 |
| High Altitude | |||||||
| Gene (Variant Type) | SNP | Genotype/Allele | NHLs (n = 183) | HHLs (n = 166) | Logistic Regression Analysis | ||
| n, (% Distribution) | χ2 | p-value | OR (95% CI) | ||||
| THBS1 | rs2228263 | TT | 164 (89.6%) | 153 (92.1%) | - | - | Reference |
| (Synonymous) | TC | 16 (8.7%) | 8 (4.8%) | 2.03 | 0.154 | 0.52 (0.21–1.27) | |
| Co-dominant | CC | 3 (1.6%) | 5 (3.0%) | 0.88 | 0.346 | 2.04 (0.46–9.06) | |
| TT + TC | 180 (98.4%) | 161 (97.0%) | - | - | Reference | ||
| Recessive | CC | 3 (1.6%) | 5 (3.0%) | 1.08 | 0.298 | 2.19 (0.49–9.67) | |
| T | 344 (94.0%) | 314 (94.6%) | - | - | Reference | ||
| Allelic | C | 22 (6.0%) | 18 (5.4%) | 0.04 | 0.834 | 0.93 (0.48–1.78) | |
| CD47 | rs9879947 | GG | 92 (50.2%) | 80 (48.2%) | - | - | Reference |
| (3′UTR) | GA | 79 (43.1%) | 61 (36.7%) | 0.3 | 0.58 | 0.88 (0.56–1.38) | |
| Co-dominant | AA | 12 (6.5%) | 25 (15.1%) | 5.81 | 0.016 | 2.56(1.19–5.51) | |
| GG + GA | 171 (93.4%) | 141 (84.9%) | - | - | Reference | ||
| Recessive | AA | 12 (6.6%) | 25 (15.1%) | 6.96 | 0.008 | 2.68 (1.28–5.57) | |
| G | 263 (71.9%) | 221 (66.6%) | - | - | Reference | ||
| Allelic | A | 103 (28.1%) | 111 (33.4%) | 2.46 | 0.116 | 1.29 (0.93–1.79) | |
| Low Altitude | |||||||
| Gene (Variant Type) | SNP | Genotype/Allele | NLLs (n = 486) | HLLs (n = 509) | Logistic Regression Analysis | ||
| n, (% Distribution) | χ2 | p-value | OR (95% CI) | ||||
| THBS1 | rs2228263 | TT | 412 (84.7%) | 431 (84.7%) | - | - | Reference |
| (Synonymous) | TC | 53 (11%) | 69 (13.6%) | 1.22 | 0.268 | 1.23 (0.85–1.79) | |
| Co-dominant | CC | 21 (4.3%) | 9 (1.7%) | 5.06 | 0.024 | 0.40 (0.18–0.89) | |
| TT + TC | 465 (95.7%) | 500 (98.3%) | - | - | Reference | ||
| Recessive | CC | 21 (4.3%) | 9 (1.7%) | 5.38 | 0.02 | 0.39 (0.18–0.80) | |
| T | 877 (90.2%) | 931 (91.4%) | - | - | Reference | ||
| Allelic | C | 95 (9.8%) | 87 (8.6%) | 0.96 | 0.326 | 0.86 (0.63–1.16) | |
| CD47 | rs9879947 | GG | 167 (34.4%) | 187 (36.8%) | - | - | Reference |
| (3′UTR) | GA | 244 (50.1%) | 232 (45.6%) | 1.36 | 0.244 | 0.85 (0.65–1.11) | |
| Co-dominant | AA | 75 (15.5%) | 90 (17.6%) | 0.12 | 0.729 | 1.06 (0.74–1.53) | |
| GG + GA | 411 (84.5%) | 419 (82.3%) | - | - | Reference | ||
| Recessive | AA | 75 (15.5%) | 90 (17.7%) | 0.86 | 0.351 | 1.16 (0.84–1.62) | |
| G | 578 (59.5%) | 606 (59.5%) | - | - | Reference | ||
| Allelic | A | 394 (40.5%) | 412 (40.5%) | 0 | 0.963 | 0.99 (0.83–1.18) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.; Chanana, N.; Mohammad, G.; Thinlas, T.; Gupta, M.; Syed, M.A.; Das, R.S.; Pasha, Q.; Mishra, A. Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude. Life 2021, 11, 893. https://doi.org/10.3390/life11090893
Sharma K, Chanana N, Mohammad G, Thinlas T, Gupta M, Syed MA, Das RS, Pasha Q, Mishra A. Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude. Life. 2021; 11(9):893. https://doi.org/10.3390/life11090893
Chicago/Turabian StyleSharma, Kavita, Neha Chanana, Ghulam Mohammad, Tashi Thinlas, Mohit Gupta, Mansoor Ali Syed, Rajat Subhra Das, Qadar Pasha, and Aastha Mishra. 2021. "Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude" Life 11, no. 9: 893. https://doi.org/10.3390/life11090893
APA StyleSharma, K., Chanana, N., Mohammad, G., Thinlas, T., Gupta, M., Syed, M. A., Das, R. S., Pasha, Q., & Mishra, A. (2021). Hypertensive Patients Exhibit Enhanced Thrombospondin-1 Levels at High-Altitude. Life, 11(9), 893. https://doi.org/10.3390/life11090893







