Gut Hormones as Potential Therapeutic Targets or Biomarkers of Response in Depression: The Case of Motilin
Abstract
1. Introduction
2. Materials and Methods
3. Results
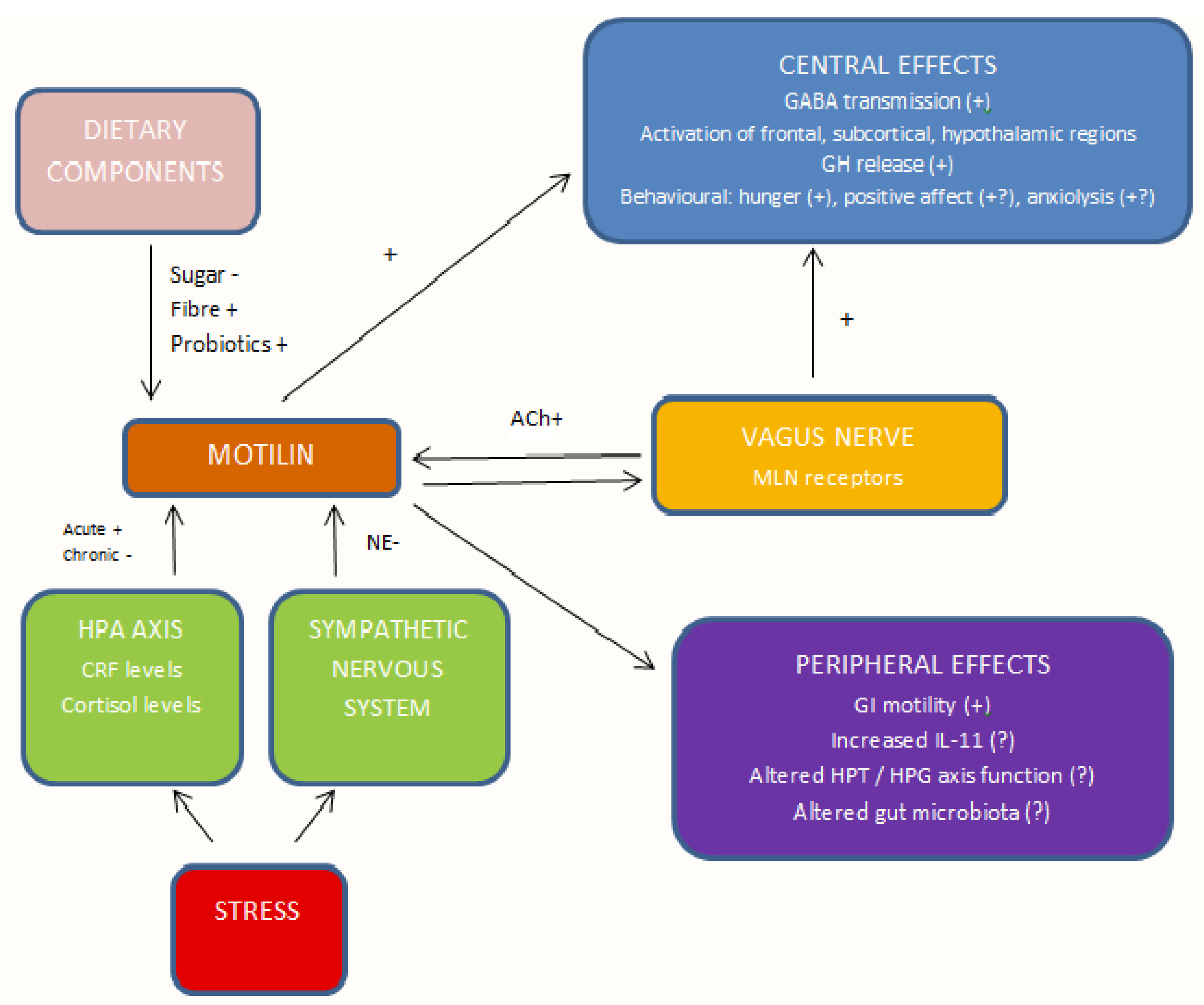
3.1. Conceptual Analysis of the Links between Motilin and Depression
3.1.1. Gastrointestinal Motility
3.1.2. Neuroendocrine Axis Functioning
3.1.3. Stress and Stress Responses
3.1.4. Monoamine Transmitters
3.1.5. Other Neurotransmitters
3.1.6. Immune and Inflammatory Pathways
3.1.7. Neurotrophic Factors
3.1.8. Diet
3.1.9. Antidepressants
3.1.10. Summary
3.2. Correlations between MLN rs2281820 Allele Frequencies and the Prevalence of Depression across Countries
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatry Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Guo, X.; McCutcheon, R.A.; Pillinger, T.; Mizuno, Y.; Natesan, S.; Brown, K.; Howes, O. The magnitude and heterogeneity of antidepressant response in depression: A meta-analysis of over 45,000 patients. J. Affect. Disord. 2020, 276, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Jain, S.B. Clinical implications of the STAR*D trial. Handb. Exp. Pharmacol. 2019, 250, 51–99. [Google Scholar]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Krause, M.; Gustmiedl, K.; Bighelli, I.; Schneider-Thoma, J.; Chaimani, A.; Leucht, S. Efficacy and tolerability of pharmacological and non-pharmacological interventions in older patients with major depressive disorder: A systematic review, pairwise and network meta-analysis. Eur. Neuropsychopharmacol. 2019, 29, 1003–1022. [Google Scholar] [CrossRef]
- Santoft, F.; Axelsson, E.; Ost, L.-G.; Hedman-Lagerlof, M.; Fust, J.; Hedman-Lagerlof, E. Cognitive behaviour therapy for depression in primary care: Systematic review and meta-analysis. Psychol. Med. 2019, 49, 1266–1274. [Google Scholar] [CrossRef]
- Patel, V.; Araya, R.; Chatterjee, S.; Chisholm, D.; Cohen, A.; De Silva, M.; Hosman, C.; McGuire, H.; Rojas, G.; van Ommeren, M. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet 2007, 370, 991–1005. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Taylor, D.; Sparshatt, A.; Varma, S.; Olofinjana, O. Antidepressant efficacy of agomelatine: Meta-analysis of published and unpublished studies. BMJ 2014, 348, g1888. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Lopes, M.; Fregni, F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 2008, 11, 1169–1180. [Google Scholar] [CrossRef]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatry Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Chavez-Castillo, M.; Nunez, V.; Nava, M.; Ortega, A.; Rojas, M.; Bermudez, V.; Rojas-Quintero, J. Depression as a neuroendocrine disorder: Emerging neuropsychopharmacological approaches beyond monoamines. Adv. Pharmacol. Sci. 2019, 2019, 7943481. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.E.M.; Gardner, A.C.; Kwon, S.; Chea, W.; Muthukumaraswamy, S.D. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatry Res. 2018, 105, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Hiroaki-Sato, V.A. A brief history of antidepressant drug development: From tricyclics to beyond ketamine. Acta Neuropsychiatr. 2018, 30, 307–322. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Wei, C.; Wang, J.; Wu, A. Intranasal ketamine for depression in adults: A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials. Front. Psychol. 2021, 12, 648691. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Aamir, A.; Diwan, M.N.; Awan, H.A.; Ullah, I.; Irfan, M.; De Berardis, D. Treating postpartum depression: What do we know about brexanolone? Diseases 2021, 9, 52. [Google Scholar] [CrossRef]
- Sylvia, K.E.; Demas, G.E. A gut reaction: Microbiome-brain-immune interactions modulate social and affective behaviors. Horm. Behav. 2018, 99, 41–49. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2021, 43, 293–305. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Huang, J.; Cai, Y.; Su, Y.; Zhang, M.; Shi, Y.; Zhu, N.; Jin, F.; Peng, D.; Fang, Y. Gastrointestinal symptoms during depressive episodes in 3256 patients with major depressive disorders: Findings from the NSSD. J. Affect. Disord. 2021, 286, 27–32. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitryptiline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Ciocan, D.; Cassard, A.-M.; Becquemont, L.; Verstuyft, C.; Voican, C.S.; El Asmar, K.; Colle, R.; David, D.; Trabado, S.; Feve, B.; et al. Blood microbiota and metabolomic signature of major depression before and after antidepressant treatment: A prospective case-control study. J. Psychiatry Neurosci. 2021, 46, E358–E368. [Google Scholar] [CrossRef] [PubMed]
- Tetel, M.J.; de Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018, 30, e12548. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.A.; Harmatz, E.S.; Goosens, K.A. Ghrelin as a stress hormone: Implications for psychiatric illness. Biol. Psychiatry 2020, 88, 531–540. [Google Scholar] [CrossRef]
- Huang, H.-J.; Zhu, X.-C.; Han, Q.-Q.; Wang, Y.-L.; Yue, N.; Wang, J.; Yu, R.; Li, B.; Wu, G.-C.; Liu, Q.; et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 2017, 326, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, B.J.; Wang, X.F.; Zhong, L.L.; Cui, R.J. Ghrelin produces antidepressant-like effect in the estrogen deficient mice. Oncotarget 2017, 8, 58964–58973. [Google Scholar] [CrossRef][Green Version]
- Akter, S.; Pham, N.M.; Nanri, A.; Kurotani, K.; Kuwahara, K.; Jacka, F.N.; Yasuda, K.; Sato, M.; Mizoue, T. Association of serum leptin and ghrelin with depressive symptoms in a Japanese working population: A cross-sectional study. BMC Psychiatry 2014, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Algul, S.; Ozcelik, O. Evaluating the levels of nesfatin-1 and ghrelin hormones in patients with moderate and severe major depressive disorders. Psychiatry Investig. 2018, 15, 214–218. [Google Scholar] [CrossRef]
- Mani, B.K.; Zigman, J.M. Ghrelin as a survival hormone. Trends Endocrinol. Metab. 2017, 28, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.K.; Lumpkin, M.D.; Nilaver, G.; McCann, S.M. Motilin: A novel growth hormone releasing agent. Brain Res. Bull. 1984, 12, 57–62. [Google Scholar] [CrossRef]
- Peeters, T.L. Ghrelin and the gut. Endocr. Dev. 2013, 25, 41–48. [Google Scholar] [PubMed]
- Wierup, N.; Bjorkqvist, M.; Westrom, B.; Pierzynowski, S.; Sundler, F.; Sjolund, K. Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J. Clin. Endocrinol. Metab. 2007, 92, 3573–3581. [Google Scholar] [CrossRef]
- Depoortere, I. Motilin and motilin receptors: Characterization and functional significance. Verh. K Acad. Geneeskd. Belg. 2001, 63, 511–529. [Google Scholar]
- Arneth, B.M. Gut-brain axis biochemical signaling from the gastrointestinal tract to the central nervous system: Gut dysbiosis and altered brain function. Postgrad. Med. J. 2018, 94, 446–452. [Google Scholar] [CrossRef]
- Klimova, B.; Novotny, M.; Valis, M. The impact of nutrition and intestinal microbiome on elderly depression—A systematic review. Nutrients 2020, 12, 710. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwatz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Lim, L.L.; Yee, A.; Loh, H.S. Association between subclinical hypothyroidism and depression: An updated systematic review and meta-analysis. BMC Psychiatry 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Peet, M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: An ecological analysis. Br. J. Psychiatry 2004, 184, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Way, B.M.; Lieberman, M.D. Is there a genetic contribution to cultural differences? Collectivism, individualism and genetic markers of social sensitivity. Soc. Cogn. Affect. Neurosci. 2010, 5, 203–211. [Google Scholar] [CrossRef]
- Chiao, J.Y.; Blizinsky, K.D. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc. R. Soc. B 2010, 277, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Gressier, F.; Calati, R.; Serretti, A. 5-HTTLPR and gender in affective disorders: A systematic review. J. Affect. Disord. 2016, 190, 193–207. [Google Scholar] [CrossRef]
- Melas, P.A.; Wei, Y.; Wong, C.C.Y.; Sjoholm, L.K.; Aberg, E.; Mill, J.; Schalling, M.; Forsell, Y.; Lavebratt, C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013, 16, 1513–1528. [Google Scholar] [CrossRef]
- Slavich, G.M.; Tartter, M.A.; Brennan, P.A.; Hammen, C. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology 2014, 49, 141–149. [Google Scholar] [CrossRef]
- Xu, H.-L.; Hsing, A.W.; Koshiol, J.; Chu, L.W.; Cheng, J.-R.; Gao, J.; Tan, Y.-T.; Wang, B.-S.; Shen, M.-C.; Gao, Y.-T. Variants in motilin, somatostatin and their receptor genes and risk of biliary tract cancers and stones in Shanghai, China. Meta Gene 2014, 2, 418–426. [Google Scholar] [CrossRef]
- Rajeevan, H.; Osier, M.V.; Cheug, K.-H.; Deng, H.; Druskin, L.; Heinzen, R.; Kidd, J.R.; Stein, S.; Pakstis, A.J.; Tosches, N.P.; et al. ALFRED: The ALlele FREquency Database. Update. Nucleic Acids Res. 2003, 31, 270–271. [Google Scholar] [CrossRef] [PubMed]
- ALFRED: The ALlele FREquency Database. Available online: http://alfred.med.yale.edu (accessed on 26 July 2021).
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Marqueze, E.C.; Vasconcelos, S.; Garefelt, J.; Skene, D.J.; Moreno, C.R.; Lowden, A. Natural light exposure, sleep and depression among day workers and shiftworkers at arctic and equatorial latitudes. PLoS ONE 2015, 10, e0122078. [Google Scholar]
- GNI per Capita. Atlas Method. Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD (accessed on 29 July 2021).
- Hofstede Insights Country Comparison. Available online: https://www.hofstede-insights.com/country-comparison/ (accessed on 21 August 2021).
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 29 July 2021).
- Parker, G.; Hadzi-Pavlovic, D.; Hickie, I.; Mitchell, P.; Wilhelm, K.; Brodaty, H.; Boyce, P.; Eyers, K.; Pedic, F. Psychotic depression: A review and clinical experience. Aust. N. Z. J. Psychiatry 1991, 25, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, C.; Koschke, M.; Greiner, W.; Peupelmann, J.; Pietsch, U.; Hocke, M.; Yeragani, V.K.; Bar, K.-J. Gastric dysmotility in patients with major depression. J. Affect. Disord. 2008, 110, 185–190. [Google Scholar] [CrossRef]
- Quick, C.; Kliem, A.; Berger, S.; Hocke, M.; Tancer, M.; Juckel, G.; Yeragani, V.K.; Bar, K.-J. Gastric dysmotility in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 92–97. [Google Scholar] [CrossRef]
- Sanger, G.J.; Wang, Y.; Hobson, A.; Broad, J. Motilin: Towards a new understanding of the gastrointestinal neuropharmacology and therapeutic use of motilin receptor agonists. Br. J. Pharmacol. 2013, 170, 1323–1332. [Google Scholar] [CrossRef]
- Esterita, T.; Dewi, S.; Suryatenggara, F.G.; Glenardi, G. Association of functional dyspepsia with depression and anxiety: A systematic review. J. Gastrointest. Liver Dis. 2021, 30, 259–266. [Google Scholar]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome: A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 2016, 46, 3065–3080. [Google Scholar] [CrossRef]
- Van den Houte, K.; Scarpellini, E.; Verbeure, W.; Mori, H.; Schol, J.; Masuy, I.; Carbone, F.; Tack, J. The role of GI peptides in functional dyspepsia and gastroparesis: A systematic review. Front. Psychiatry 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Sjolund, K.; Ekman, R.; Lindgren, S.; Rehfeld, J.F. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand. J. Gastroenterol. 1996, 31, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veek, P.P.J.; Biemond, I.; Masclee, A.A.M. Proximal and distal gut hormone secretion in irritable bowel syndrome. Scand. J. Gastroenterol. 2006, 41, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sjolund, K.; Ekman, R.; Wierup, N. Covariation of plasma ghrelin and motilin in irritable bowel syndrome. Peptides 2010, 31, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Suzuki, J. Colonic motility, autonomic function, and gastrointestinal hormones under psychological stress on irritable bowel syndrome. Tohoko J. Exp. Med. 1987, 151, 373–385. [Google Scholar] [CrossRef]
- Jonsson, B.H.; Hellstrom, P.M. Motilin- and neuropeptide Y-like immunoreactivity in a psychophysiological stress experiment on patients with functional dyspepsia. Integr. Physiol. Behav. Sci. 2000, 35, 256–265. [Google Scholar] [CrossRef]
- Jiang, S.-M.; Jia, L.; Liu, J.; Shi, M.-M.; Xu, M.-Z. Beneficial effects of antidepressant mirtazapine in functional dyspepsia patients with weight loss. World J. Gastroenterol. 2016, 22, 5260–5266. [Google Scholar] [CrossRef]
- Du, H.-G.; Ming, L.; Chen, S.-J.; Li, C.-D. Xiaoyao pill for treatment of functional dyspepsia in perimenopausal women with depression. World J. Gastroenterol. 2014, 20, 16739–16744. [Google Scholar] [CrossRef]
- Ko, S.-J.; Park, J.; Kim, M.-J.; Kim, J.; Park, J.-W. Effects of the herbal medicine Rikkunshito, for functional dyspepsia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 64–74. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, M.; Cheng, G.-Y.; Chen, Y.-P.; Li, D.-G. Prevalence and associated factors of functional gastrointestinal disorders and bowel habits in Chinese adolescents: A school-based study. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, E.; Arslan, N.; Kume, T.; Ulgenalp, A.; Cirah, C.; Bozkaya, O.; Ercal, D. Serum motilin levels and motilin gene polymorphisms in children with functional constipation. Minerva Pediatr. 2016. Available online: https://minervamedica.it/en/journals/minerva-pediatrics/article.php?cod=R15Y9999N00A1610050 (accessed on 29 July 2021).
- Sakkas, P.N.; Soldatos, C.R.; Bergiannaki, J.D.; Paparrigopoulous, T.J.; Stefanis, C.N. Growth hormone secretion during sleep in male depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 1998, 22, 467–483. [Google Scholar] [CrossRef]
- Naughton, M.; Dinan, T.G.; Scott, L.V. Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014, 124, 69–91. [Google Scholar]
- Bueno, L.; Fargeas, M.J.; Gue, M.; Peeters, T.L.; Bormans, V.; Fioramonti, J. Effects of cotricotropin-releasing factor on plasma motilin and somatostatin levels and gastrointestinal motility in dogs. Gastroenterology 1986, 91, 884–889. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, J.T.; Song, Q.H.; Xu, G.H.; Cai, L.; Tang, X.D.; Zhang, H.F.; Liu, F.-E.; Jia, Z.S.; Zhang, H.W. Melatonin attenuates noise stress-induced gastrointestinal motility disorder and gastric stress ulcer: Role of gastrointestinal hormones and oxidative stress in rats. J. Neurogastroenterol. Motil. 2015, 21, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xu, L.; Sun, X.; Gao, S.; Zhu, H. The paraventricular nucleus modulates thyroidal motilin release and rat gastric motility. J. Neuroendocrinol. 2011, 23, 767–777. [Google Scholar] [CrossRef]
- Cosci, F.; Fava, G.A.; Sonino, N. Mood and anxiety disorders as early manifestations of medical illness: A systematic review. Psychother. Psychosom. 2015, 84, 22–29. [Google Scholar] [CrossRef]
- Aoyagi, K.; Mishima, Y.; Murakami, S.; Ito, K. Serum gastrin and motilin in treated and untreated hypothyroidism. Bull. Tokyo Med. Dent. Univ. 1982, 29, 153–159. [Google Scholar]
- Krysiak, R.; Kowalcze, K.; Okopien, B. Sexual functioning and depressive symptoms in young women with overt hypothyroidism. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 43–48. [Google Scholar] [CrossRef]
- Krysiak, R.; Marek, B.; Okopien, B. Sexual function and depressive symptoms in men with overt hyperthyroidism. Endokrynol. Pol. 2019, 70, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tsukamura, H.; Tsukahara, S.; Maekawa, T.; Moriyama, R.; Reyes, B.A.S.; Sakai, T.; Niwa, Y.; Foster, D.L.; Maeda, K.-I. Peripheral or central administration of motilin suppresses LH release in female rats: A novel role for motilin. J. Neuroendocrinol. 2000, 12, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Holst, N.; Jennsen, T.G.; Burhol, P.G.; Haug, E.; Forsdahl, F. Plasma gastrointestinal hormones during spontaneous and induced menstrual cycles. J. Clin. Endocrin. Metab. 1989, 68, 1160–1166. [Google Scholar] [CrossRef]
- Rapkin, A.J.; Akopians, A.L. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. 2012, 18, 52–59. [Google Scholar] [CrossRef]
- Kornstein, S.G.; Harvey, A.T.; Rush, A.J.; Wisniewski, S.R.; Trivedi, M.H.; Svikis, D.S.; McKenzie, N.D.; Bryan, C.; Harley, R. Self-reported premenstrual exacerbation of depressive symptoms in patients seeking treatment for major depression. Psychol. Med. 2005, 35, 683–692. [Google Scholar] [CrossRef]
- Dennis, C.-L.; Ross, L.E.; Herxheimer, A. Oestrogens and progestins for preventing and treating postpartum depression. Cochrane Database Syst. Rev. 2008, 2008, CD001690. [Google Scholar] [CrossRef]
- Pae, C.-U.; Mandelli, L.; Kim, T.-S.; Han, C.; Masand, P.S.; Marks, D.M.; Patkar, A.A.; Steffens, D.C.; De Ronchi, D.; Serretti, A. Effectiveness of antidepressant treatments in pre-menopausal versus post-menopausal women: A pilot study on differential effects of sex hormones on antidepressant effects. Biomed. Pharmacother. 2009, 63, 228–235. [Google Scholar] [CrossRef]
- Pillai, R.R.; Sharon, L.; Premkumar, N.C.; Kattimani, S.; Sagili, H.; Rajendiran, S. Luteinizing hormone-follicle stimulating hormone ratio as biological predictor of post-partum depression. Compr. Psychiatry 2017, 72, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ansseau, M.; Von Frenckell, R.; Cerfontaine, J.L.; Papart, P.; Franck, G.; Timsit-Berthier, M.; Geenen, V.; Legros, J.J. Blunted response of growth hormone to clonidine and apomorphine in endogenous depression. Br. J. Psychiatry 1988, 153, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Krogh, J.; Nordentoft, M.; Mohammad-Nezhad, M.; Westrin, A. Growth hormone, prolactin and cortisol response to exercise in patients with depression. J. Affect. Disord. 2010, 125, 189–197. [Google Scholar] [CrossRef]
- Birmaher, B.; Heydl, P. Biological studies in depressed children and adolescents. Int. J. Neuropsychopharmacol. 2001, 4, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.K.; Lumpkin, M.D.; McCann, S.M. Motilin stimulates growth hormone release in vitro. Brain Res. Bull. 1982, 8, 117–121. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Hafizi, S.; Cowen, P.J. Cortisol modulation of 5-HT-mediated growth hormone release in recovered depressed patients. J. Affect. Disord. 2002, 72, 249–255. [Google Scholar] [CrossRef]
- Thakore, J.H.; Dinan, T.G. Effect of fluoxetine on dexamethasone-induced growth hormone release in depression: A double-blind, placebo-controlled study. Am. J. Psychiatry 1995, 152, 616–618. [Google Scholar]
- Kluge, M.; Schussler, P.; Dresler, M.; Schmidt, D.; Yassouridis, A.; Uhr, M.; Steiger, A. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J. Psychiatry Res. 2011, 45, 421–426. [Google Scholar] [CrossRef]
- Liang, C.; Luo, H.; Liu, Y.; Cao, J.; Xia, H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS ONE 2012, 7, e31774. [Google Scholar] [CrossRef]
- Cai, G.-X.; Liu, B.-Y.; Yi, J.; Chen, X.-M.; Liu, F.-L. Simotang enhances gastrointestinal motility, motilin and cholecystokin expression in chronically stressed mice. World J. Gastroenterol. 2011, 17, 1594–1599. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef]
- Zhu, G.; Kang, Z.; Chen, Y.; Zeng, J.; Su, C.; Li, S. Ultrasound-guided stellate ganglion block alleviates stress responses and promotes recovery of gastrointestinal function in patients. Dig. Liver Dis. 2021, 53, 581–586. [Google Scholar] [CrossRef]
- Mu, D.-Z.; Xue, M.; Xu, J.-J.; Hu, Y.; Chen, Y.; Ren, P.; Huang, X. Antidepression and prokinetic effects of paeoniflorin on rats in the forced swimming test via polypharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 2153571. [Google Scholar] [CrossRef]
- Marzio, L.; Neri, M.; Pieramico, O.; Delle Donne, M.; Peeters, T.L.; Cuccurullo, F. Dopamine interrupts gastrointestinal fed motility patterns in humans. Effect on motilin and somatostatin blood levels. Dig. Dis Sci. 1990, 35, 327–332. [Google Scholar] [CrossRef]
- Funakoshi, A.; Matsumoto, M.; Sekiya, K.; Nakano, I.; Shinozaki, H.; Ibayashi, H. Cholinergic independent dopaminergic regulation of motilin release in man. Gastroenterol. Jpn. 1983, 18, 525–529. [Google Scholar] [CrossRef]
- Itoh, H.; Katagiri, F.; Ikawa, K.; Takeyama, M. Effects of domperidone on human plasma levels of motilin, somatostatin, gastrin, adrenocorticotropic hormone and cortisol. Biol. Pharm. Bull. 2005, 28, 1752–1756. [Google Scholar] [CrossRef][Green Version]
- Takahashi, T. Mechanism of interdigestive migrating motor complex. J. Neurogastroenterol. Motil. 2012, 18, 246–257. [Google Scholar] [CrossRef]
- Koutsoumbi, P.; Epanomeritakis, E.; Tsiaoussis, J.; Athanasakis, H.; Chrysos, E.; Zoras, O.; Vassilakis, J.S.; Xynos, E. The effect of erythromycin on human esophageal motility is mediated by serotonin receptors. Am. J. Gastroenterol. 2000, 95, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Tack, J. Georges Brohee Prize 1994. Motilin and the enteric nervous system in the control of interdigestive and postprandial gastric motility. Acta Gastroenterol. Belg. 1995, 58, 21–30. [Google Scholar] [PubMed]
- Chan-Palay, V.; Ito, M.; Tongroach, P.; Sakurai, M.; Palay, S. Inhibitory effects of motilin, somatostatin, [leu]enkephalin, [met]enkephalin, and taurine on neurons of the lateral vestibular nucleus: Interactions with γ-aminobutyric acid. Proc. Natl. Acad. Sci. USA 1982, 79, 3355–3359. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Liu, J.-C.; Zhang, J.; Ozaki, K.-I.; Guo, Y.-Y.; Yi, D.-H.; Li, X.-Q.; Zhao, M.-G. Anxiolytic actions of motilin in the basolateral amygdala. Mol. Neurobiol. 2013, 47, 892–902. [Google Scholar] [CrossRef]
- Yao, Z.; Fu, Y.; Wu, J.; Zhang, W.; Yu, Y.; Zhang, Z.; Wu, X.; Wang, Y.; Hu, B. Morphological changes in subregions of hippocampus and amygdala in major depressive disorder patients. Brain Imaging Behav. 2020, 14, 653–667. [Google Scholar] [CrossRef]
- Tang, S.; Li, H.; Lu, L.; Wang, Y.; Zhang, L.; Hu, X.; Bu, X.; Hu, X.; Gao, Y.; Gong, Q.; et al. Anomalous functional connectivity of amygdala subregional networks in major depressive disorder. Depress. Anxiety 2019, 36, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Tang, M.; Jiang, Z.; Peeters, T.L. Excitatory effects of motilin in the hippocampus on gastric motility in rats. Brain Res. 2003, 984, 33–41. [Google Scholar] [CrossRef]
- Wu, M.; Tang, M.; Adriaensen, D.; Depoortere, I.; Peeters, T.L.; Timmermans, J.-P. Central, but not peripheral application of motilin increases c-Fos expression in hypothalamic nuclei in the rat brain. Histochem. Cell Biol. 2005, 123, 139–145. [Google Scholar] [CrossRef]
- Todaka, H.; Tatsukawa, T.; Hashikawa, T.; Yanagawa, Y.; Shibuki, K.; Nagao, S. Heterotrimeric guanosine triphosphate-binding protein-coupled modulatory actions of motilin on K+ channels and postsynaptic γ-aminobutyric acid receptors in mouse medial vestibular nuclear neurons. Eur. J. Neurosci. 2013, 37, 339–350. [Google Scholar] [CrossRef]
- Lupo, M.; Siciliano, L.; Leggio, M. From cerebellar alterations to mood disorders: A systematic review. Neurosci. Biobehav. Rev. 2019, 103, 21–28. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Janssen, P.; Van der Bergh, O.; Van Oudenhove, L.; Depoortere, I.; Tack, J. The motilin receptor agonist erythromycin stimulates hunger and food intake through a cholinergic pathway. Am. J. Clin. Nutr. 2016, 103, 730–737. [Google Scholar] [CrossRef]
- Mochiki, E.; Yanai, M.; Ohno, T.; Kuwano, H. The effect of traditional Japanese medicine (Kampo) on gastrointestinal function. Surg. Today. 2010, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Sanger, G.J. Motilin receptor neuropharmacology: Revised understanding. Curr. Opin. Pharmacol. 2012, 12, 641–646. [Google Scholar] [CrossRef]
- Suzuki, H.; Mochiki, E.; Haga, N.; Satoh, M.; Mizumoto, A.; Itoh, Z. Motilin controls cyclic release of insulin through vagal cholinergic muscarinic pathways in fasted dogs. Am. J. Physiol. 1998, 274, G87–G95. [Google Scholar] [CrossRef] [PubMed]
- Mochiki, E.; Inui, A.; Satoh, M.; Mizumoto, A.; Itoh, Z. Motilin is a biosignal controlling cyclic release of pancreatic polypeptide via the vagus in fasted dogs. Am. J. Physiol. 1997, 272, G224–G232. [Google Scholar] [CrossRef]
- Bottomley, J.M.; LeReun, C.; Diamantopoulos, A.; Mitchell, S.; Gaynes, B.N. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: A systematic review and meta-analysis. Compr. Psychiatry 2019, 98, 152156. [Google Scholar] [CrossRef]
- Zhao, D.; Meyer-Gerspach, A.C.; Deloose, E.; Iven, J.; Weltens, N.; Depoortere, I.; O’Daly, O.; Tack, J.; Van Oudenhove, L. The motilin agonist erythromycin increases hunger by modulating homeostatic and hedonic brain circuits in healthy women: A randomized, placebo-controlled study. Sci. Rep. 2018, 8, 1819. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, I.; Thijs, T.; Keith, J., Jr.; Peeters, T.L. Treatment with interleukin-11 affects plasma leptin levels in inflamed and non-inflamed rabbits. Regul. Pept. 2004, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Smith, R.G.; Hackinger, S.; Schalkwyk, L.C.; Uher, R.; McGuffin, P.; Mill, J.; Tansey, K.E. DNA methylation in interleukin-11 predicts clinical response to antidepressants in GENDEP. Transl. Psychiatry 2013, 3, e300. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, L.; Yang, J.; Han, D.; Fang, D.; Qiu, X.; Yang, X.; Qiao, Z.; Ma, J.; Wang, L.; et al. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J. Affect. Disord. 2018, 227, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analysis on 179 associations (n = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Slattery, D.A. GABAB receptors and depression. Current status. Adv. Pharmacol. 2010, 58, 427–451. [Google Scholar]
- Westover, A.N.; Marangell, L.B. A cross-national relationship between sugar consumption and major depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Cross-national variations in COVID-19 mortality: The role of diet, obesity and depression. Diseases 2021, 9, 36. [Google Scholar] [CrossRef]
- Wu, H.; Xia, F.-Z.; Xu, H.; Zhai, H.-L.; Zhang, M.-F.; Zhang, H.-X.; Li, Y.-X.; Li, Y.; Gu, T.; Ma, L.-M.; et al. Acute effects of different glycemic index diets on serum motilin, orexin and neuropeptide Y concentrations in healthy individuals. Neuropeptides 2012, 46, 113–118. [Google Scholar] [CrossRef]
- Kay, R.G.; Foreman, R.E.; Roberts, G.P.; Hardwick, R.; Reimann, F.; Gribble, F.M. Mass spectrometric characterisation of the circulating peptidome following oral glucose ingestion in control and gastrectomised patients. Rapid Commun. Mass Spectrom. 2020, 34, e8849. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef]
- Kunugi, H. Gut microbiota and pathophysiology of depressive disorder. Ann. Nutr. Metab. 2021. [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.-J.; Xie, J.; Chen, J.-J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X.; et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, M.; Zhong, Y.; Gao, T.; Zhang, Y.; Yan, F.; Huang, D.; Wu, Y.; Weng, Z. Partially hydrolyzed guar gum modulates gut microbiota, regulates the levels of neurotransmitters, and prevents CUMS-induced depressive-like behavior in mice. Mol. Nutr. Food Res. 2021, 65, e2100146. [Google Scholar] [CrossRef] [PubMed]
- Sileikiene, V.; Mosenthin, R.; Bauer, E.; Piepho, H.-P.; Tafaj, M.; Kruszewska, D.; Westrom, B.; Erlanson-Albertsson, C.; Pierzynowski, S.G. Effect of ileal infusion of short-chain fatty acids on pancreatic prandial secretion and gastrointestinal hormones in pigs. Pancreas 2008, 37, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, S.-M.; Jia, L.; You, L.-Q.; Huang, Y.-X.; Gong, Y.-M.; Wang, G.-Q. Effect of amitryptiline on gastrointestinal function and brain-gut peptides: A double-blind trial. World J. Gastroenterol. 2013, 19, 4214–4220. [Google Scholar] [CrossRef]
- Allen, J.M.; Christofides, N.D.; Cramer, P.A.; Steinert, J.; Bloom, S.R. Elevated motilin levels in patients treated with antidepressant and neuroleptic drugs. Br. J. Psychiatry 1982, 141, 27–29. [Google Scholar] [CrossRef]
- Wang, T.; Yan, Y.-F.; Yang, L.; Huang, Y.-Z.; Duan, X.-H.; Su, K.-H.; Liu, W.-L. Effects of Zuojin pill on depressive behavior and gastrointestinal function in rats with chronic unpredictable mild stress: Role of the brain-gut axis. J. Ethnopharmacol. 2020, 254, 112713. [Google Scholar] [CrossRef]
- Abouesh, A.; Hobbs, W.R. Clarithromycin-induced mania. Am. J. Psychiatry 1998, 155, 1626. [Google Scholar] [CrossRef]
- Kwan, B.Y.M.; Rabheru, K. Manic episode associated with clarithromycin in a patient with medically treated depression. Prim. Care Companion CNS Disord. 2012, 14, e01285. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Truman, C.J. Antidepressant-induced mania: An overview of current controversies. Bipolar Disord. 2003, 5, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Kuang, Y.; Jing, Y.; Li, Y.; Zhao, H.; Ouyang, H. Pediatric allergic rhinitis with functional gastrointestinal disease: Associations with the intestinal microbiota and gastrointestinal peptides and therapeutic effects of interventions. Hum. Exp. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Domin, H. Neuropeptide Y Y2 and Y5 receptors as potential targets for neuroprotective and antidepressant therapies: Evidence from preclinical studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110349. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 2013, 18, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Zhang, Y.; Chardoul, S.; Ghimire, D.J.; Smoller, J.W.; Axinn, W.G. Resilience to mental disorders in a low-income, non-Westernized setting. Psychol. Med. 2020, 1–10. [Google Scholar] [CrossRef]
- Miller, R.; Kirschbaum, C. Cultures under stress: A cross-national meta-analysis of cortisol responses to the Trier Social Stress Test and their association with anxiety-related value orientations and internalizing mental disorders. Psychoneuroendocrinology 2019, 105, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wum, C.; Wei, J.; Xian, M.; Wang, T.; Yang, B.; Chen, M. An orally administered magnosolide A ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota. Life Sci. 2019, 233, 116749. [Google Scholar] [CrossRef]
- Raison, C.L.; Lowry, C.A.; Rook, G.A.W. Inflammation, sanitation and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry 2010, 67, 1211–1224. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Yates, K.P.; Nguyen, L.; Clarke, J.; Abell, T.L.; Farrugia, G.; Hasler, W.L.; Koch, K.L.; Snape, W.J.; McCallum, R.W.; et al. Outcome and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology 2015, 149, 1762–1774. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Sang, Y.; Liu, K.; Zhu, Y.; Yang, L.; Wang, S.; Sheng, J.; Wang, Q.; Zhang, D.; et al. Antibiotic exposure and potential risk of depression in the Chinese elderly: A biomonitoring-based population study. Environ. Sci. Pollut. Res. Int. 2021, 28, 26794–26806. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chen, M.-H.; Wang, W.-P.; Liu, P.-Y.; Hou, M.-C.; Lee, F.-Y.; Lu, C.-L. Increased risk of short-term depressive disorder after Helicobacter pylori eradication: A population-based nested cohort study. Helicobacter 2021, 26, e12824. [Google Scholar] [CrossRef]
- Doan, T.; Hinterwirth, A.; Worden, L.; Arzika, A.M.; Maliki, R.; Abdou, A.; Kane, S.; Zhong, L.; Cummings, S.L.; Sakar, S.; et al. Gut microbiome alteration in MORDOR I: A community-randomized trial of mass azithromycin distribution. Nat. Med. 2019, 25, 1370–1376. [Google Scholar] [CrossRef]
- Brown, E.S.; Varghese, F.P.; McEwen, B.S. Association of depression with medical illness: Does cortisol play a role? Biol. Psychiatry 2004, 55, 1–9. [Google Scholar] [CrossRef]
- Neufeld, N.H.; Mohamed, N.S.; Grujich, N.; Shulman, K. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobacter pylori infections: A review. J. Psychiatry Pract. 2017, 23, 25–35. [Google Scholar] [CrossRef]
- Lally, L.; Mannion, L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013, 2013, bcr2013009659. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Uemoto, M.; Komatsu, Y.; Sato, Y.; Inui, A. A motilin agonist, erythromycin, decreases circulating growth hormone levels in normal subjects but not in diabetic subjects. J. Diabetes Complicat. 2006, 20, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Qi, Q.; Hao, H.; Wang, G.; Chen, Y.; Liang, Y.; Xie, L. The pharmacokinetic-pharmacodynamic model of azithromycin for lipopolysaccharide-induced depressive-like behavior in mice. PLoS ONE 2013, 8, e54981. [Google Scholar] [CrossRef]
- Heightman, T.D.; Conway, E.; Corbett, D.F.; Macdonald, G.J.; Stemp, G.; Westaway, S.M.; Celestini, P.; Gagliardi, S.; Riccaboni, M.; Ronzoni, S.; et al. Identification of small molecule agonists of the motilin receptor. Bioorgan. Med. Chem. Lett. 2008, 18, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Xie, Z.; Miyano, Y.; Tsutsui, C.; Sakata, I.; Kawamoto, Y.; Aizawa, S.; Tanaka, T.; Oda, S.-I.; Sakai, T. Coordination of motilin and ghrelin regulates the migrating motor complex of gastrointestinal motility in Suncus murinus. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1207–G1215. [Google Scholar] [CrossRef][Green Version]
- Ozaki, K.-I.; Onoma, M.; Muramatsu, H.; Sudo, H.; Yoshida, S.; Shiokawa, R.; Yogo, K.; Kamel, K.; Cynshi, O.; Kuromaru, O.; et al. An orally active motilin receptor antagonist, MA-2029, inhibits motilin-induced gastrointestinal motility, increase in fundic tone and diarrhea in conscious dogs without affecting gastric emptying. Eur. J. Pharmacol. 2009, 615, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A. Sleep and endocrinology. J. Intern. Med. 2003, 254, 13–22. [Google Scholar] [CrossRef]
- Shafton, A.D.; Sanger, G.J.; Witherington, J.; Brown, J.D.; Muir, A.; Butler, S.; Abberley, L.; Shimizu, Y.; Furness, J.B. Oral administration of a centrally acting ghrelin receptor agonist to conscious rats triggers defecation. Neurogastroenterol. Motil. 2009, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, I. Can small non-peptide motilin agonists force a breakthrough in gastroprokinetic drugs? Br. J. Pharmacol. 2012, 167, 760–762. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Hayes, M.G. Testing the null hypothesis: Comments on ‘Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene’. Proc. Biol. Sci. 2011, 278, 329–332. [Google Scholar] [CrossRef]
| Mechanistic Pathway and Supporting References | Search Terms Used |
|---|---|
| Neuroendocrine axes [14,26,43] | “cortisol”, “corticotropin-releasing hormone”, “corticotropin-releasing factor”, “CRH”, “CRF”, “hypothalamic-pituitary-adrenal axis”, “growth hormone”, “thyroid”, “thyroxine”, “thyroid-stimulating hormone”, “thyrotropin-releasing hormone”, “luteinzing hormone”, “follicle-stimulating hormone”, “estrogen”, “estradiol”, “progresterone”, “progestin” and “testosterone” |
| Stress and stress responses [26,35] | “stress”, “stressor”, “stress response”, “stress sensitivity” and “resilience” |
| Monoamine neurotransmitters [10] | “monoamine”, “serotonin”, “dopamine”, “noradrenaline” or “norepinephrine”, with and without “receptor *” |
| Other relevant neurotransmitters [15] | “gamma–aminobutyric acid”, “GABA”, “glutamate”, “acetylcholine”, “cholinergic”, “neuropeptide” and “neuropeptides” with and without “receptor *” |
| Immune and inflammatory pathways [13] | “immune”, “inflammation”, “inflammatory”, “cytokine *” and “chemokine *” |
| Neurotrophic factors [12] | “brain-derived neurotrophic factor”, “BDNF”, “neural plasticity” and “neuroplasticity” |
| Diet [44] | “diet *”, “sugar”, “refined sugar”, “probiotic*”, “prebiotic *”, “short-chain fatty acids” and “SCFA” |
| Studies of antidepressants [25] | “antidepressant *” paired with “tricyclic”, “serotonin reuptake inhibitor” and “selective serotonin reuptake inhibitor” |
| Variable | 1 Depression, Prevalence | 2 MLN rs2281820, C Allele Frequency (ln) | 3 Gross National Income (ln) | 4 Individualism–collectivism (ln) | 5 Distance from the Equator (ln) | 6 Per Capita Sugar Consumption |
|---|---|---|---|---|---|---|
| 1 | - | −0.41 * (0.037) | 0.38 (0.053) | 0.34 (0.116) | 0.49 * (0.012) | 0.38 (0.053) |
| 2 | - | −0.19 (0.365) | −0.28 (0.190) | 0.07 (0.754) | −0.34 (0.091) | |
| 3 | - | 0.75 * (<0.001) | 0.47 * (0.016) | 0.53 * (0.005) | ||
| 4 | - | 0.56 * (0.005) | 0.36 (0.091) | |||
| 5 | - | 0.15 (0.452) |
| Variable | Regression Coefficient (β) | Significance Level | Part Correlation | Variance Inflation Factor |
|---|---|---|---|---|
| MLN rs2281820, C allele frequency (ln) | −0.38 | −0.037 * | −0.36 | 1.15 |
| Distance from the equator (ln) | 0.48 | 0.015 * | 0.42 | 1.33 |
| Gross national income (ln) | −0.01 | 0.950 | −0.01 | 1.78 |
| Per capita sugar consumption | 0.19 | 0.359 | 0.15 | 1.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, R.P. Gut Hormones as Potential Therapeutic Targets or Biomarkers of Response in Depression: The Case of Motilin. Life 2021, 11, 892. https://doi.org/10.3390/life11090892
Rajkumar RP. Gut Hormones as Potential Therapeutic Targets or Biomarkers of Response in Depression: The Case of Motilin. Life. 2021; 11(9):892. https://doi.org/10.3390/life11090892
Chicago/Turabian StyleRajkumar, Ravi Philip. 2021. "Gut Hormones as Potential Therapeutic Targets or Biomarkers of Response in Depression: The Case of Motilin" Life 11, no. 9: 892. https://doi.org/10.3390/life11090892
APA StyleRajkumar, R. P. (2021). Gut Hormones as Potential Therapeutic Targets or Biomarkers of Response in Depression: The Case of Motilin. Life, 11(9), 892. https://doi.org/10.3390/life11090892






