The Potential Role of Serotonergic Hallucinogens in Depression Treatment
Abstract
:1. Introduction
1.1. Depression in the General Population
1.2. Current Treatment Methods for Depression and Their Limitations
1.3. Serotonergic Hallucinogens as a Potential Treatment for Depression
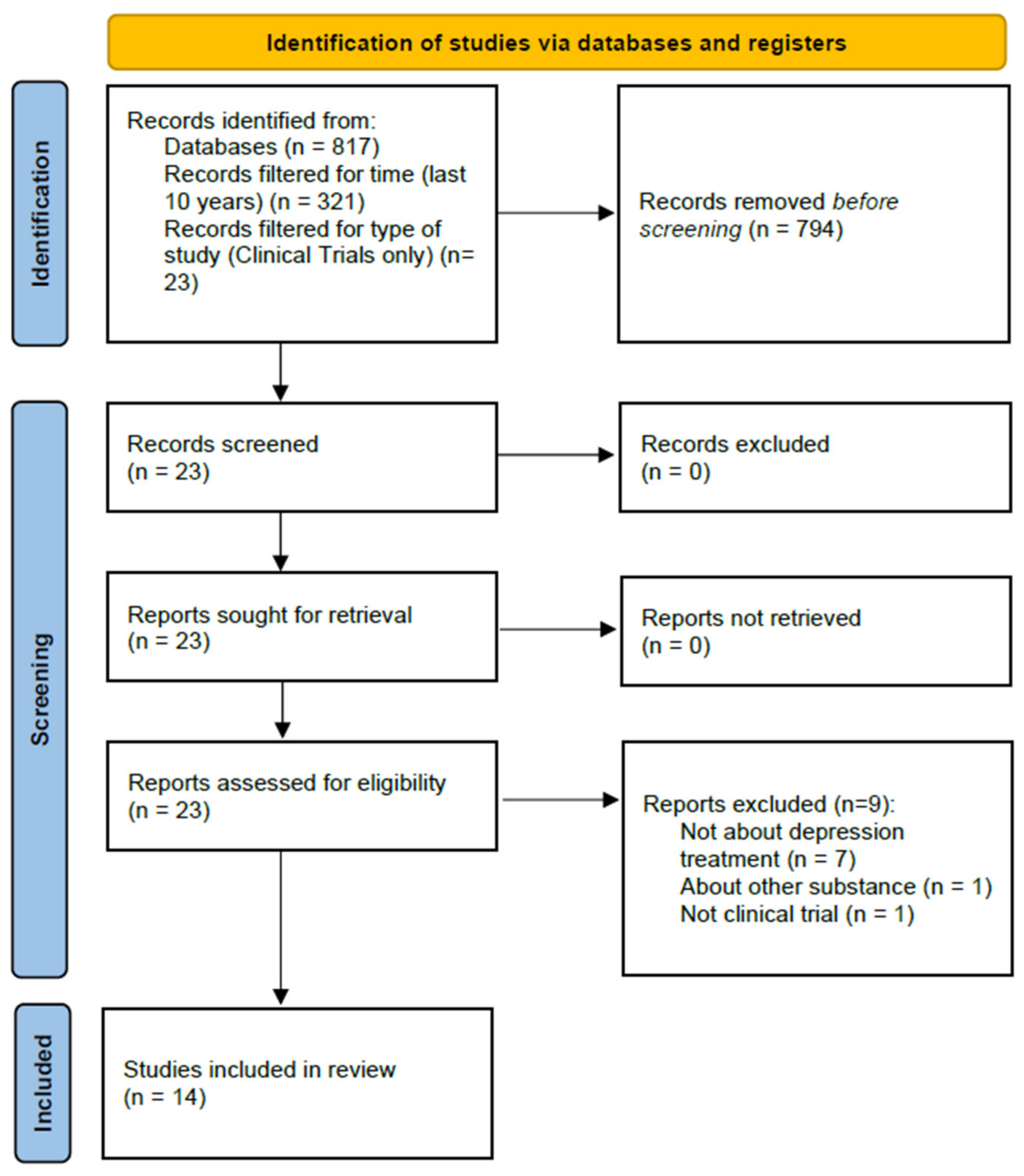
2. Materials and Methods
3. Review
3.1. Psilocybin
3.2. MDMA
3.3. LSD
3.4. Open-Label Studies
3.5. Adverse Events after Hallucinogen Use in the Reviewed Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Depression. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 19 April 2021).
- Schmaal, L.; Pozzi, E.; Ho, T.C.; Van Velzen, L.S.; Veer, I.M.; Opel, N.; Van Someren, E.J.W.; Han, L.K.M.; Aftanas, L.; Aleman, A.; et al. ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl. Psychiatry 2020, 10, 172. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penninx, B.W.J.H.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Zeng, L.-N.; Lu, L.; Li, X.-H.; Ungvari, G.S.; Ng, C.H.; Chow, I.H.I.; Zhang, L.; Zhou, Y.; Xiang, Y.-T. Prevalence of suicide attempt in individuals with major depressive disorder: A meta-analysis of observational surveys. Psychol. Med. 2018, 49, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Berking, M.; Andersson, G.; Quigley, L.; Kleiboer, A.; Dobson, K. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can. J. Psychiatry 2013, 58, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Furukawa, T.; Salanti, G.; Chaimani, A.; Atkinson, L.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Buckman, J.; Underwood, A.; Clarke, K.; Saunders, R.; Hollon, S.; Fearon, P.; Pilling, S. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin. Psychol. Rev. 2018, 64, 13–38. [Google Scholar] [CrossRef]
- Thase, M.E.; Mahableshwarkar, A.R.; Dragheim, M.; Loft, H.; Vieta, E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur. Neuropsychopharmacol. 2016, 26, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Read, J. Adverse effects of antidepressants reported by a large international cohort: Emotional blunting, suicidality, and withdrawal effects. Curr. Drug Saf. 2018, 13, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S.; et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Suicide Prevention. 2021. Available online: https://www.who.int/health-topics/suicide#tab=tab_1 (accessed on 4 June 2021).
- Farah, W.H.; Alsawas, M.; Mainou, M.; Alahdab, F.; Farah, M.; Ahmed, A.T.; Mohamed, E.A.; Almasri, J.; Gionfriddo, M.R.; Castaneda-Guarderas, A.; et al. Non-pharmacological treatment of depression: A systematic review and evidence map. Evid. Based Med. 2016, 21, 214–221. [Google Scholar] [CrossRef]
- Hermida, A.P.; Glass, O.M.; Shafi, H.; McDonald, W.M. Electroconvulsive therapy in depression. Psychiatr. Clin. N. Am. 2018, 41, 341–353. [Google Scholar] [CrossRef]
- Price, L.; Briley, J.; Haltiwanger, S.; Hitching, R. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J. Psychiatr. Res. 2021, 135, 119–134. [Google Scholar] [CrossRef]
- Nauphal, M.; Mischoulon, D.; Uebelacker, L.; Streeter, C.; Nyer, M. Yoga for the treatment of depression: Five questions to move the evidence-base forward. Complement. Ther. Med. 2019, 46, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Mansur, R.B.; Brietzke, E.; Carmona, N.E.; Subramaniapillai, M.; Pan, Z.; Shekotikhina, M.; Rosenblat, J.D.; Suppes, T.; Cosgrove, V.E.; et al. Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav. Immun. 2020, 88, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.L.; Nichols, F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr. Clin. N. Am. 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Health Quality Ontario. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis of randomized controlled trials. Ont. Health Technol. Assess. Ser. 2016, 16, 1–66. [Google Scholar]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry 2018, 79, 35–48. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Prisinzano, T. Novel psychotherapeutics—A cautiously optimistic focus on hallucinogens. Expert Rev. Clin. Pharmacol. 2017, 11, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L. How do psychedelics work? Curr. Opin. Psychiatry 2019, 32, 16–21. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Richards, W.A. Current perspectives on psychedelic therapy: Use of serotonergic hallucinogens in clinical interventions. Int. Rev. Psychiatry 2018, 30, 291–316. [Google Scholar] [CrossRef]
- Johnson, M.W.; Hendricks, P.S.; Barrett, F.; Griffiths, R.R. Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 2019, 197, 83–102. [Google Scholar] [CrossRef]
- Calvey, T.; Howells, F. An introduction to psychedelic neuroscience. Prog. Brain Res. 2018, 242, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Majić, T.; Schmidt, T.T.; Gallinat, J. Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J. Psychopharmacol. 2015, 29, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraehenmann, R.; Preller, K.H.; Scheidegger, M.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol. Psychiatry 2015, 78, 572–581. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Ot’alora, G.M.; Grigsby, J.; Poulter, B.; Van DerVeer, I.J.W.; Giron, S.G.; Jerome, L.; Feduccia, A.A.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. J. Psychopharmacol. 2018, 32, 1295–1307. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, M.C.; Feduccia, A.A.; Jerome, L.; Mithoefer, A.; Wagner, M.; Walsh, Z.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Doblin, R. MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology 2019, 236, 2735–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfson, P.E.; Andries, J.; Feduccia, A.A.; Jerome, L.; Wang, J.B.; Williams, E.; Carlin, S.C.; Sola, E.; Hamilton, S.; Yazar-Klosinski, B.; et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: A randomized pilot study. Sci. Rep. 2020, 10, 20442. [Google Scholar] [CrossRef] [PubMed]
- Bershad, A.K.; Schepers, S.T.; Bremmer, M.P.; Lee, R.; de Wit, H. Acute subjective and behavioral effects of micro-doses of lysergic acid diethylamide in healthy human volunteers. Biol. Psychiatry 2019, 86, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Doblin, R. The safety and efficacy of ± 3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J. Psychopharmacol. 2010, 25, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Martin, S.F.; Yazar-Klosinski, B.; Michel, Y.; Brewerton, T.D.; Doblin, R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. J. Psychopharmacol. 2012, 27, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Oehen, P.; Traber, R.; Widmer, V.; Schnyder, U. A randomized, controlled pilot study of MDMA (±3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic post-traumatic stress disorder (PTSD). J. Psychopharmacol. 2012, 27, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; Mcgonigle, J.; Murphy, K.; et al. Psilocybin for treatment-resistant depression: FMRI-measured brain mechanisms. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2017, 235, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Roseman, L.; Demetriou, L.; Wall, M.B.; Nutt, D.J.; Carhart-Harris, R.L. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology 2018, 142, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.J.; Wall, M.B.; Roseman, L.; Demetriou, L.; Nutt, D.J.; Carhart-Harris, R.L. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J. Psychopharmacol. 2020, 34, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Uthaug, M.V.; Lancelotta, R.; Szabo, A.; Davis, A.K.; Riba, J.; Ramaekers, J.G. Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: Effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology 2019, 237, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.R.; Richards, W.A.; McCann, U.; Jesse, R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 2006, 187, 268–283. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Behav. Neurobiol. Psychedelic Drugs 2017, 36, 45–73. [Google Scholar] [CrossRef]
- Muttoni, S.; Ardissino, M.; John, C. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J. Affect. Disord. 2019, 258, 11–24. [Google Scholar] [CrossRef]
- Preller, K.H.; Vollenweider, F.X. Phenomenology, structure, and dynamic of psychedelic states. Behav. Neurobiol. Psychedelic Drugs 2016, 36, 221–256. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic serotonergic psychedelics for mood and depressive symptoms: A meta-analysis of mood disorder patients and healthy participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef]
- Romeo, B.; Karila, L.; Martelli, C.; Benyamina, A. Efficacy of psychedelic treatments on depressive symptoms: A meta-analysis. J. Psychopharmacol. 2020, 34, 1079–1085. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Santos, R.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2018, 49, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uthaug, M.V.; Mason, N.L.; Toennes, S.W.; Reckweg, J.T.; Perna, E.B.D.S.F.; Kuypers, K.P.C.; van Oorsouw, K.; Riba, J.; Ramaekers, J.G. A placebo-controlled study of the effects of ayahuasca, set and setting on mental health of participants in ayahuasca group retreats. Psychopharmacology 2021, 238, 1899–1910. [Google Scholar] [CrossRef]
- González, D.; Cantillo, J.; Pérez, I.; Farré, M.; Feilding, A.; Obiols, J.E.; Bouso, J.C. Therapeutic potential of ayahuasca in grief: A prospective, observational study. Psychopharmacology 2020, 237, 1171–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locher, C.; Koechlin, H.; Zion, S.R.; Werner, C.P.; Pine, D.S.; Kirsch, I.; Kessler, R.C.; Kossowsky, J. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents. JAMA Psychiatry 2017, 74, 1011–1020. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, Z.; Xue, M.; Zhang, J.; Leng, L. Application of antidepressants in depression: A systematic review and meta-analysis. J. Clin. Neurosci. 2020, 80, 169–181. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can. J. Psychiatry 2016, 61, 540–560. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and body weight. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Serretti, A.; Chiesa, A. Treatment-emergent sexual dysfunction related to antidepressants. J. Clin. Psychopharmacol. 2009, 29, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. SSRI antidepressant medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Masand, P.S.; Gupta, S. Long-term side effects of newer-generation antidepressants: SSRIS, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann. Clin. Psychiatry 2002, 14, 175–182. [Google Scholar] [CrossRef]
- Bet, P.M.; Hugtenburg, J.G.; Penninx, B.W.; Hoogendijk, W.J. Side effects of antidepressants during long-term use in a naturalistic setting. Eur. Neuropsychopharmacol. 2013, 23, 1443–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, S.H. A review of antidepressant therapy in primary care. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Nutt, D. Psychedelic drugs—A new era in psychiatry? Dialog. Clin. Neurosci. 2019, 21, 139–147. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Miuli, A.; Corkery, J.M.; Scherbaum, N.; Napoletano, F.; Arillotta, D.; Zangani, C.; Catalani, V.; Vento, A.; et al. New psychoactive substances (NPS) and serotonin syndrome onset: A systematic review. Exp. Neurol. 2021, 339, 113638. [Google Scholar] [CrossRef]
- Litjens, R.P.; Brunt, T.M.; Alderliefste, G.-J.; Westerink, R.H. Hallucinogen persisting perception disorder and the serotonergic system: A comprehensive review including new MDMA-related clinical cases. Eur. Neuropsychopharmacol. 2014, 24, 1309–1323. [Google Scholar] [CrossRef]
- Martinotti, G.; De Risio, L.; Vannini, C.; Schifano, F.; Pettorruso, M.; Di Giannantonio, M. Substance-related exogenous psychosis: A postmodern syndrome. CNS Spectr. 2020, 26, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.; Green, A. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br. J. Pharmacol. 2010, 160, 1029–1044. [Google Scholar] [CrossRef] [Green Version]
- Bickel, M.; Ditting, T.; Watz, H.; Roesler, A.; Weidauer, S.; Jacobi, V.; Gueller, S.; Betz, C.; Fichtlscherer, S.; Stein, J. Severe rhabdomyolysis, acute renal failure and posterior encephalopathy after “magic mushroom” abuse. Eur. J. Emerg. Med. 2005, 12, 306–308. [Google Scholar] [CrossRef]
- Kim, J.; Fan, B.; Liu, X.; Kerner, N.; Wu, P. Ecstasy use and suicidal behavior among adolescents: Findings from a national survey. Suicide Life Threat. Behav. 2011, 41, 435–444. [Google Scholar] [CrossRef]
- Reiff, C.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and psychedelic-assisted psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Hartogsohn, I. Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law 2017, 3. [Google Scholar] [CrossRef]
- Nutt, D. Drugs without the Hot Air: Making Sense of Legal and Illegal Drugs; UIT Cambridge Ltd.: Cambridge, UK, 2012; p. 254. [Google Scholar]
| No. | Studies’ Authors, Year | Substance/Test Group | Substance/Control Group | Comorbid Condition | No. of Participants | Used Questionnaires to Measure Depressive Symptoms |
|---|---|---|---|---|---|---|
| 1 | Kraehenmann R et al., 2015 [29] | Psilocybin, 0.16 mg/kg | Lactose | Healthy volunteers | 25 | PANAS, STAI |
| 2 | Ross S et al., 2016 [30] | Psilocybin, 0.3 mg/kg | Niacin, 250 mg | Advanced cancer | 29 | HADS, BDI, STAI, MEQ 30 |
| 3 | Griffiths RR et al., 2016 [31] | Psilocybin, 22 or 30 mg/70 kg | Psilocybin, 1 or 3 mg/70 kg | Advanced cancer | 51 | GRID-HAMD-17, BDI, HADS, STAI |
| 4 | Carhart-Harris R et al., 2021 [32] | Psilocybin, 25 mg versus Escitalopram, 10–25 mg | Psilocybin 1 mg, placebo | Major depressive disorder | 66 | QIDS-SR-16 |
| 5 | Ot’alora G M et al., 2018 [33] | MDMA, 125 or 100 mg | MDMA, 40 mg | PTSD | 28 | BDI |
| 6 | Mithoefer MC et al., 2019 [34] | MDMA, 75–125 mg | MDMA, 0–40 mg | PTSD | 103 | BDI |
| 7 | Wolfson PE et al., 2020 [35] | MDMA, 125 mg | Lactose, 125 mg | Life-threatening illness | 18 | BDI, MADRS |
| 8 | Bershad AK et al., 2019 [36] | LSD, 6.5–26 μg | Lactose | Healthy volunteers | 20 | POMS |
| No. | Studies’ Authors, Year | Substance | No. of Participants | Comorbid Condition | Results |
|---|---|---|---|---|---|
| 1. | Carhart RL et al., 2016 [41] | Psilocybin, 1. dose: 10 mg, 2. dose: 25 mg | 12 | Major depression disorder (MDD) (moderate to severe degree, treatment-resistant) | A significant reduction in depressive symptoms lasting up to 3 months after 2. dose, relative to the baseline, measured by: QIDS (10.0 vs. 19.2) BDI (15.2 vs. 33.7) STAI-T (54.8 vs. 70.1) SHAPS (2.8 vs. 7.5) |
| 2. | Carhart RL et al., 2017 [42] | Psilocybin, 1. dose: 10 mg, 2. dose: 25 mg | 19 | MDD (treatment-resistant) | A significant reduction in depressive symptoms lasting up to 5 weeks, measured by QIDS-SR16, were observed in 18 patients (95%) (mean change: −9.2); 9 patients (47%) met criteria for response (≤50% reductions). Reduced depressive symptoms were correlated with decreased amygdala CBF in fMRI. |
| 3. | Carhart RL et al., 2018 [43] | Psilocybin, 1. dose: 10 mg, 2. dose: 25 mg | 19 | MDD (severe, treatment-resistant) | A significant reduction in depressive symptoms lasting up to 6 months, relative to the baseline, measured by: QIDS-SR16 (p = 0.0035) BDI (19.5 vs. 34.5) STAI (53.8 vs. 68.6) |
| 4. | Roseman L et al., 2018 [44] | Psilocybin, 1. dose: 10 mg, 2. dose: 25 mg | 20 | MDD (moderate to severe, treatment-resistant) | Post-treatment increased amygdala responses to emotional stimuli in fMRI suggest that psilocybin allows to confront and work through negative emotions (induced by showing fearful and happy faces). |
| 5. | Mertens LJ et al., 2020 [45] | Psilocybin, 1. dose: 10 mg, 2. dose: 25 mg | 19 | MDD (moderate to severe, treatment-resistant) | Post-treatment increase in functional connectivity between the amygdala and ventromedial prefrontal cortex to occipital-parietal cortices in fMRI during face processing. |
| 6. | Uthaug MV et al., 2020 [46] | DMT, 17–61 mg | 10 | -(Healthy volunteers) | Significant reduction of depressive symptoms, sustained at 7-day follow-up, measured in DASS-21. |
| Clinical Trial | Adverse Events (AEs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Study | No. of Participants | Substance | Dose | Psychological | Neurological | Cardiovascular | Gastroenterological | General |
| 1. | Ross S et al., 2016 [30] | 29 | psilocybin | 0.3 mg/kg | transient anxiety: 17%, transient psychotic-like symptoms: 7% | headache/migraine: 28% | elevation in blood pressure (BP) and heart rate (HR): 76% | nausea: 14% | - |
| 2. | Griffiths RR et al., 2016 [31] | 51 | psilocybin | 22 or 30 mg/70 kg | psychological discomfort: 32%, anxiety: 26%, paranoid ideation: 2% | headache: 2% | elevation in systolic BP: 34%, elevation in diastolic BP: 12% | nausea/vomiting: 15% | physical discomfort: 21% |
| 3. | Carhart RL et al., 2016 [41] | 12 | psilocybin | 10 and 25 mg | transient anxiety: 100%, thought disorder: 75% | headache: 33% | - | nausea: 33% | - |
| 4. | Carhart RL et al., 2018 [42] | 19 | psilocybin | 10 and 25 mg | transient anxiety: 79%, transient paranoia: 16% | headache: 42% | - | nausea: 26% | - |
| 5. | Carhart RL et al., 2021 [32] | 30 | psilocybin | 25 mg | feeling jittery: 7%, sleep disorder: 3% | headache: 67%, migraine: 10% | palpitations: 3% | nausea: 27%, vomiting: 7%, diarrhea: 3% | fatigue: 7% |
| 6. | Ot’alora GM et al., 2018 [33] | 28 | MDMA | 125 mg | During experimental session anxiety: 54% | During experimental session dizziness: 54%, headache: 23%, jaw clenching: 62%, muscle tension: 54% | - | - | During experimental session fatigue: 31% |
| During 7 days’ observation anxiety: 77%, depressed mood: 15%, insomnia: 46% | During 7 days’ observation headache: 39%, muscle tension: 46% | During 7 days’ observation nausea: 62% | During 7 days’ observation fatigue: 69%, lack of appetite: 62% | ||||||
| 100 mg | During experimental session anxiety: 67% | During experimental session dizziness: 22%, headache: 44%, jaw clenching: 56%, muscle tension: 44% | - | - | During experimental session fatigue: 44% | ||||
| During 7 days’ observation anxiety: 89%, depressed mood: 22%, insomnia: 78% | During 7 days’ observation headache: 33%, muscle tension: 11% | During 7 days’ observation nausea: 33% | During 7 days’ observation fatigue: 78%, lack of appetite: 11% | ||||||
| 40 mg | During experimental session anxiety: 33% | During experimental session dizziness: 17%, headache: 67%, jaw clenching: 33%, muscle tension: 33% | - | - | During experimental session fatigue: 33% | ||||
| During 7 days’ observation anxiety: 33%, depressed mood: 0%, insomnia: 50% | During 7 days’ observation headache: 67%, muscle tension: 33% | During 7 days’ observation nausea: 17% | During 7 days’ observation fatigue: 33%, lack of appetite: 17% | ||||||
| 7. | Mithoefer MC et al., 2019 [34] | 103 | MDMA | 75–125 mg | anxiety: 72% | dizziness: 40%, headache: 53%, jaw clenching: 64% | - | nausea: 40% | fatigue: 49%, lack of appetite: 49% |
| 0–40 mg | anxiety: 48% | dizziness: 19%, headache: 71%, jaw clenching: 19% | - | nausea: 19% | fatigue: 58%, lack of appetite: 23% | ||||
| 8. | Wolfson PE et al., 2020 [35] | 18 | MDMA | 125 mg | During experimental session anxiety: 23%, insomnia: 15% | During experimental session headache: 62%, jaw clenching: 85% | - | During experimental session nausea: 23% | During experimental session dry mouth: 69%, perspiration: 69%, thirst: 85%, lack of appetite: 31% |
| During 7 days’ observation anxiety: 62%, insomnia: 69%, low mood: 62% | During 7 days’ observation jaw clenching: 62% | During 7 days’ observation nausea: 46% | During 7 days’ observation dry mouth: 23%, fatigue: 92%, lack of appetite: 31% | ||||||
| 9. | Uthaug MV et al., 2020 [46] | 10 | DMT | 17–61 mg | anxiety: 20%, insomnia: 10% | muscle tension: 20% | - | vomiting: 10% | physical distress: 10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psiuk, D.; Nowak, E.; Cholewa, K.; Łopuszańska, U.; Samardakiewicz, M. The Potential Role of Serotonergic Hallucinogens in Depression Treatment. Life 2021, 11, 765. https://doi.org/10.3390/life11080765
Psiuk D, Nowak E, Cholewa K, Łopuszańska U, Samardakiewicz M. The Potential Role of Serotonergic Hallucinogens in Depression Treatment. Life. 2021; 11(8):765. https://doi.org/10.3390/life11080765
Chicago/Turabian StylePsiuk, Dominika, Emilia Nowak, Krystian Cholewa, Urszula Łopuszańska, and Marzena Samardakiewicz. 2021. "The Potential Role of Serotonergic Hallucinogens in Depression Treatment" Life 11, no. 8: 765. https://doi.org/10.3390/life11080765
APA StylePsiuk, D., Nowak, E., Cholewa, K., Łopuszańska, U., & Samardakiewicz, M. (2021). The Potential Role of Serotonergic Hallucinogens in Depression Treatment. Life, 11(8), 765. https://doi.org/10.3390/life11080765






