Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis
Abstract
:1. Introduction
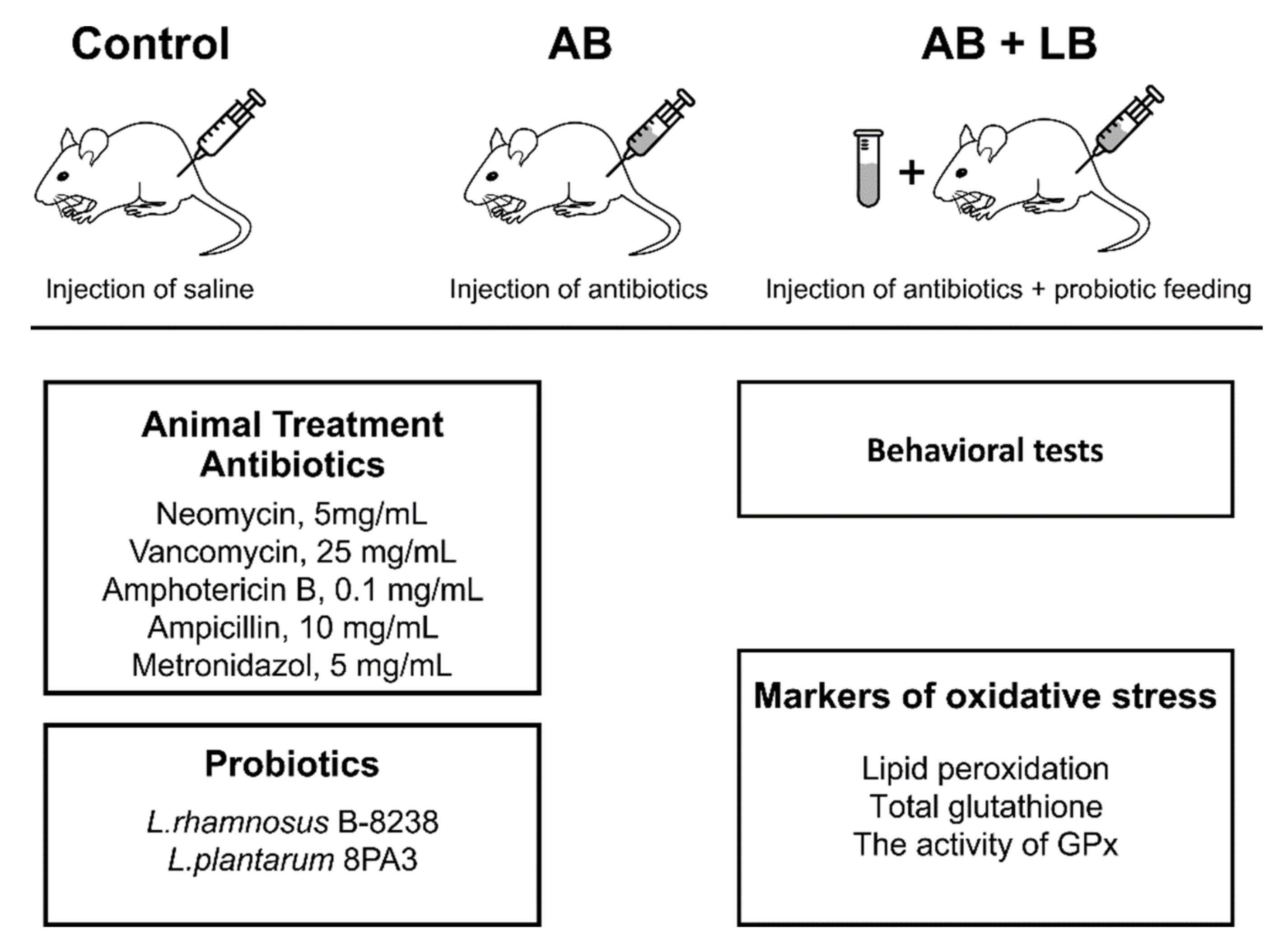
2. Materials and Methods
2.1. Animals
2.2. Probiotics
2.3. Microbial Culture Methods for Fecal Microbiota Assessment
2.4. Metagenomic Analyses
2.4.1. Sample Collection
2.4.2. DNA Extraction
2.4.3. 16S rRNA Gene Amplification and Sequencing
2.4.4. Quantitative Analysis of Microbiome Composition
2.5. Behavior Tests
2.5.1. Open Field Test
2.5.2. The Rotarod Test
2.5.3. Paw Grip Endurance Test (PaGE)
2.5.4. Light–Dark Box Test
2.5.5. The New Object Recognition Test (NOR)
2.5.6. T Maze
2.5.7. Von Frey Test
2.5.8. Visceral Sensitivity
2.6. Lipid Peroxidation and the Activity of Glutathione Peroxidases
2.7. Statistical Analysis
3. Results
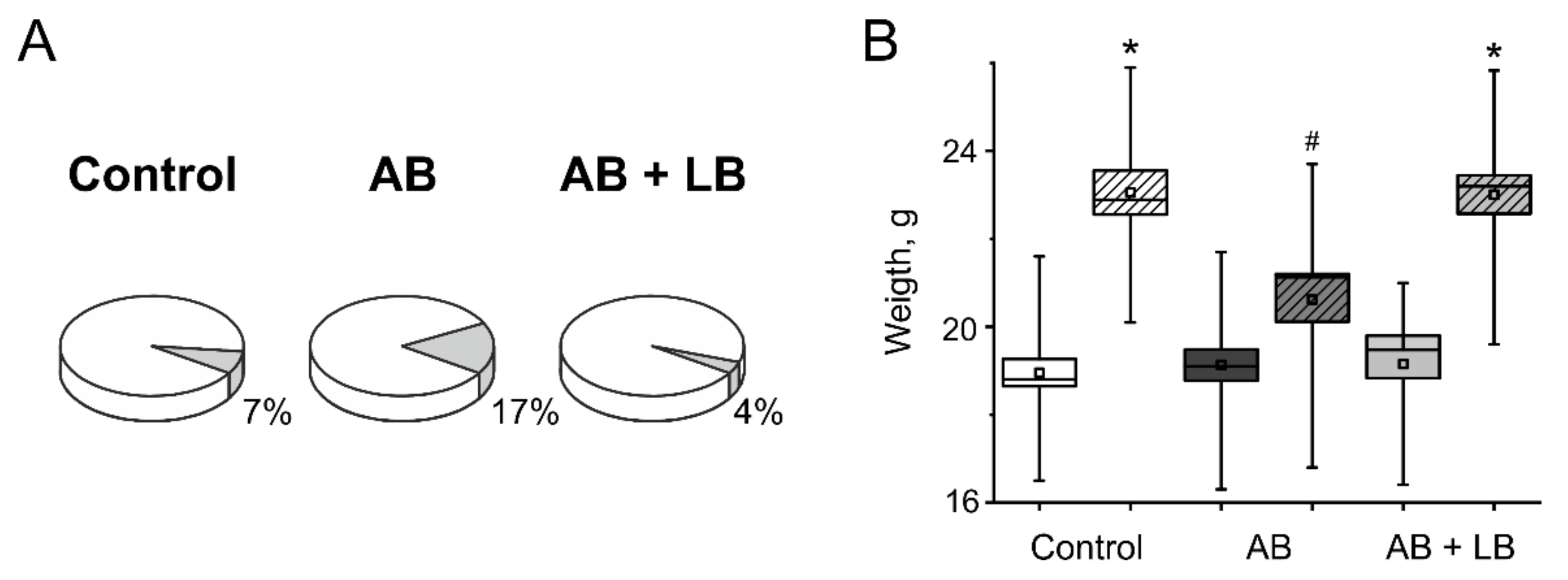
3.1. Weight Gain and Mortality
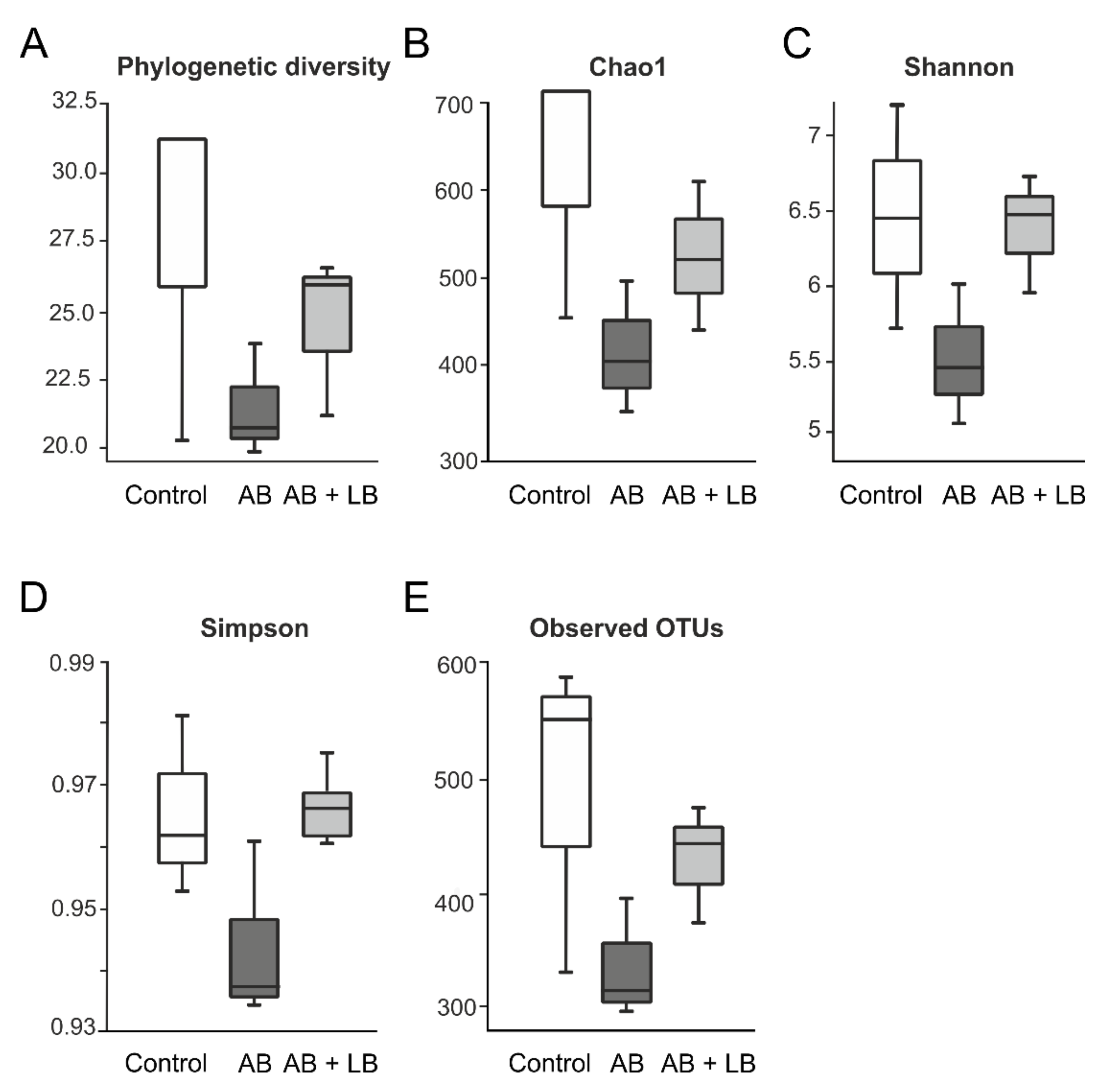
3.2. Modulation of Gut Microbiota by Antibiotics and Lactobacilli
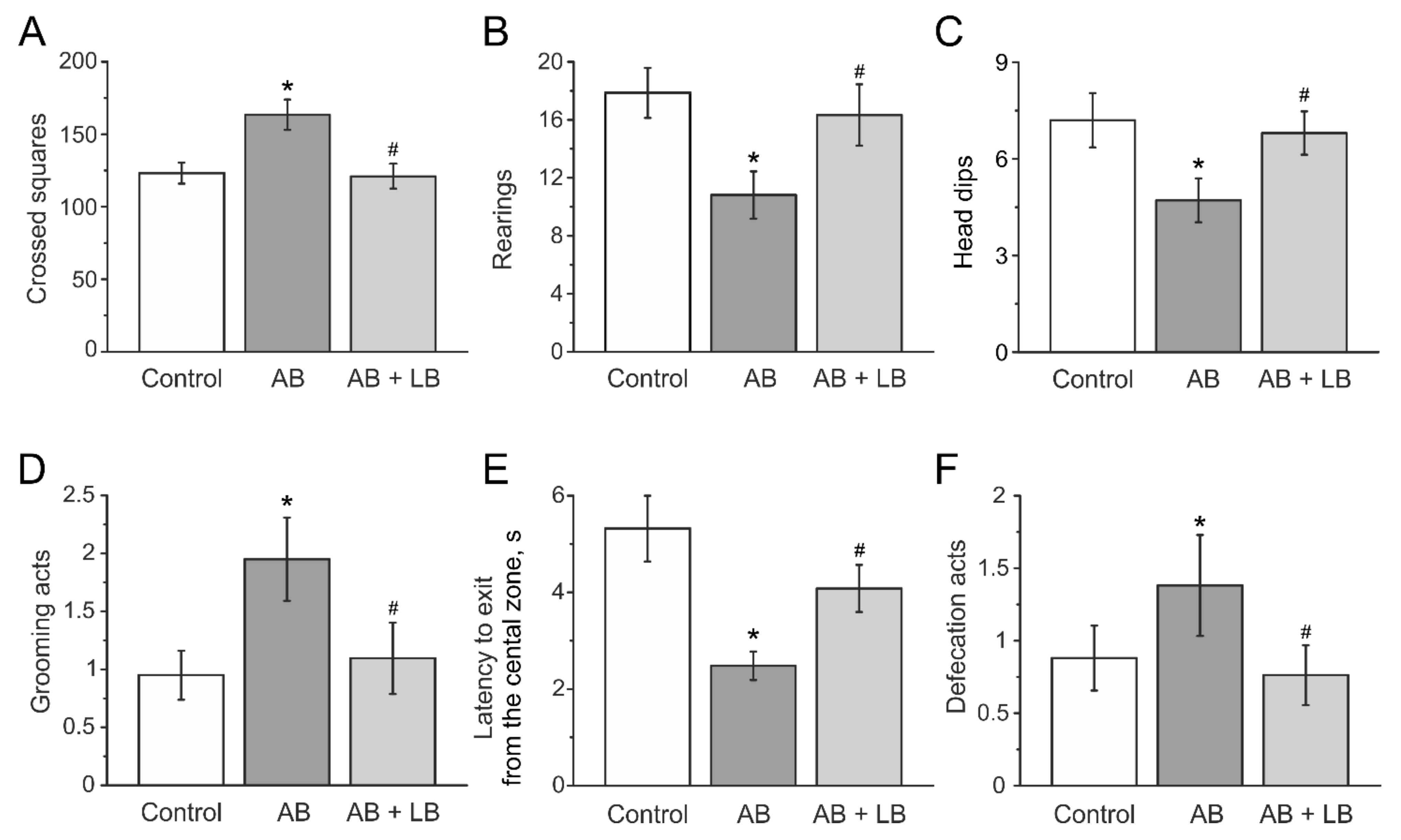
3.3. Behavioral Activity Measured in the Open Field Test
3.4. Lactobacilli Treatments Decreased the Anxiety Level of Mice with Administration of Antibiotics
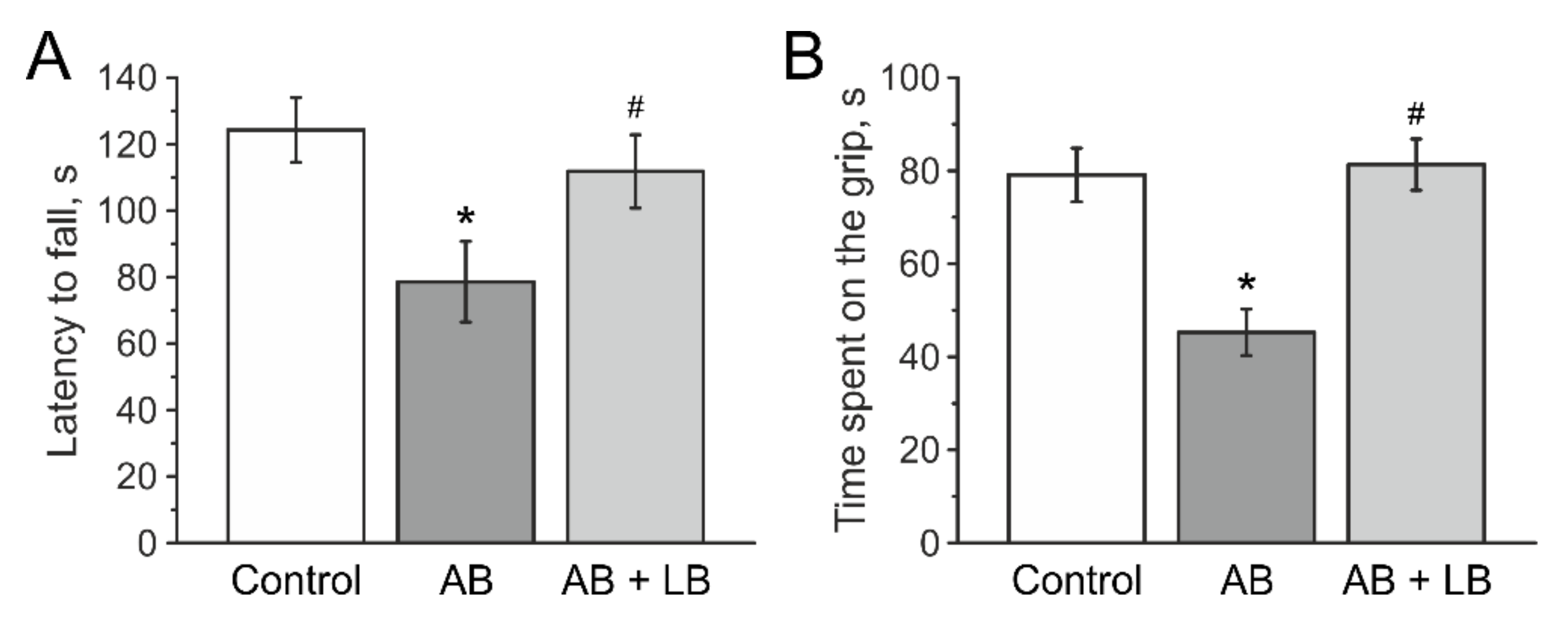
3.5. Lactobacilli Increases Muscle Endurance and Motor Coordination of Mice with Antibiotic Treatment
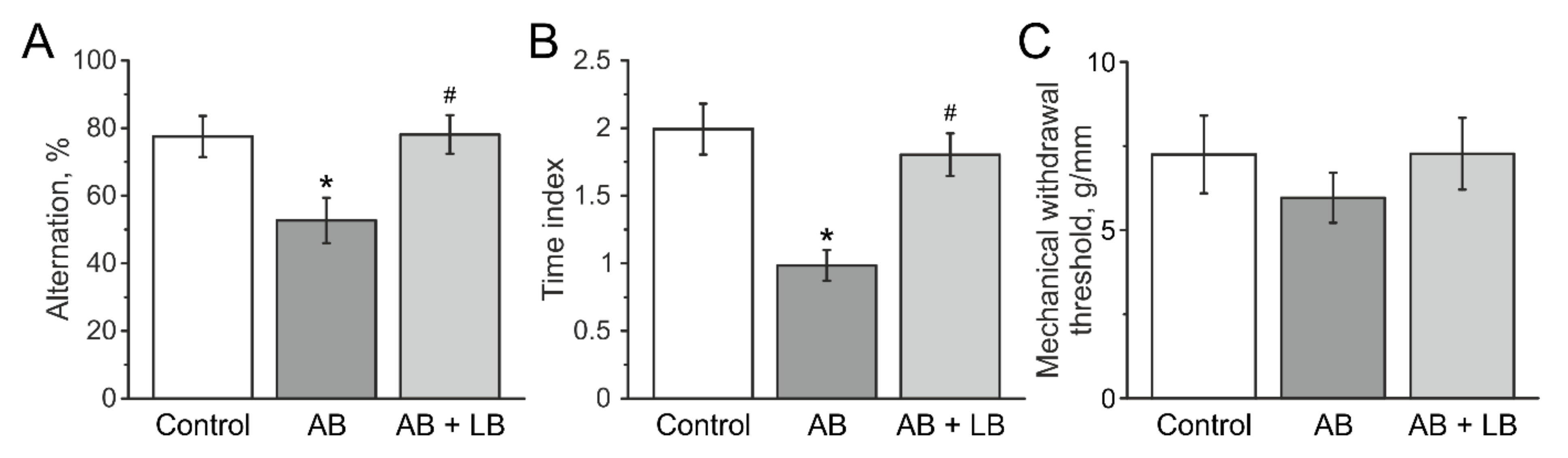
3.6. Lactobacilli Treatment Improved the Cognitive Functions of Mice after Antibiotic Administration
3.7. Mechanical Sensitivity and Visceral Nociception
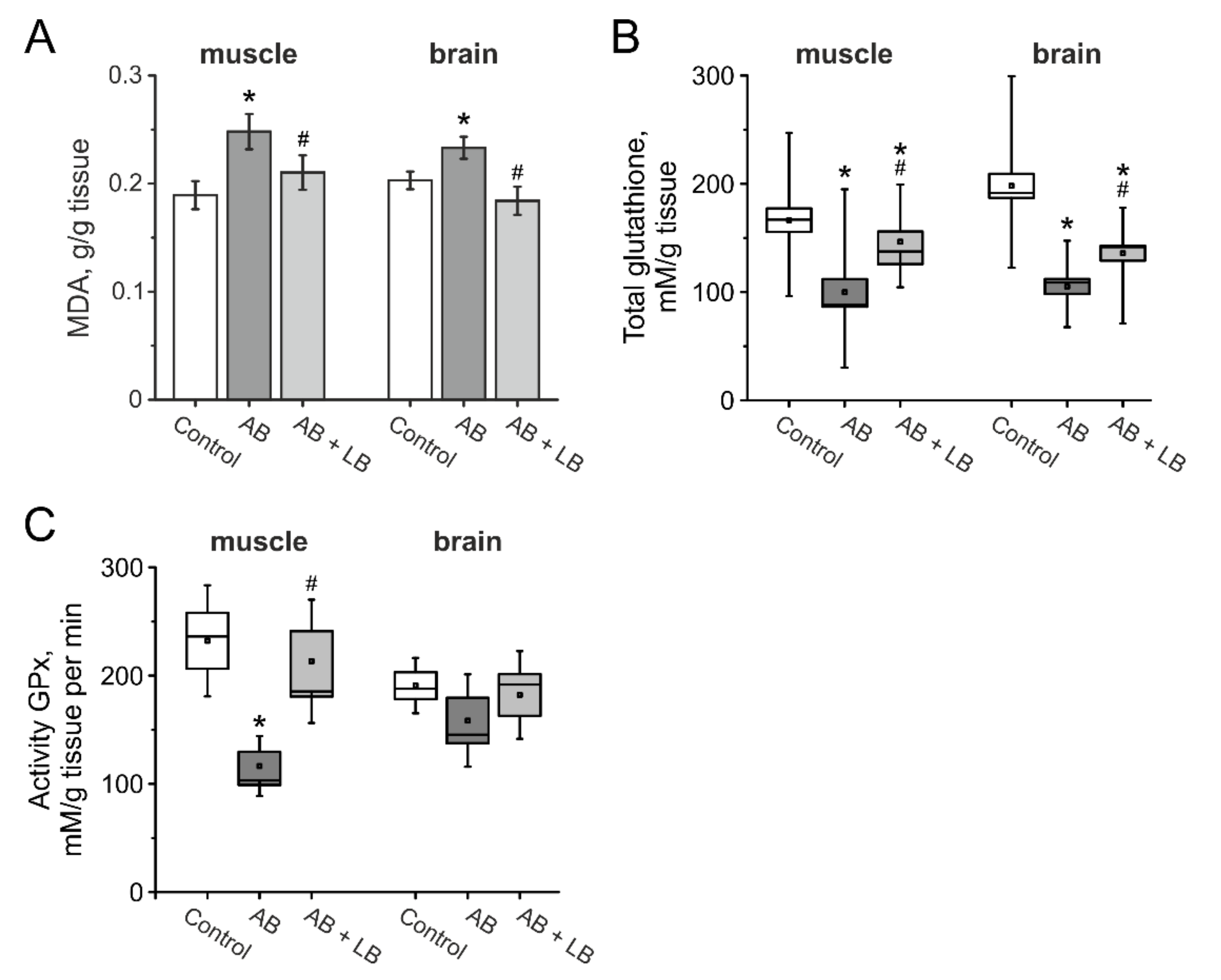
3.8. The Level of Oxidative Stress in the Brain and Muscle Tissues of Mice after Administration of Antibiotics and Lactobacilli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Woting, A.; Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.A.T.E.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef]
- Pokrovskaya, E.V.; Shamkhalova, M.S.; Shestakova, M.V. The new views on the state of the gut microbiota in obesity and diabetes mellitus type 2. Review. Diabetes Mellit. 2019, 22, 253–262. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Moos, W.H.; Faller, D.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Powers, W.R.; Steliou, K. Microbiota and Neurological Disorders: A Gut Feeling. Biores. Open Access 2016, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Stefano, G.B.; Pilonis, N.; Ptacek, R.; Raboch, J.; Vnukova, M.; Kream, R.M. Gut, Microbiome, and Brain Regulatory Axis: Relevance to Neurodegenerative and Psychiatric Disorders. Cell. Mol. Neurobiol. 2018, 38, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keightley, P.C.; Koloski, N.A.; Talley, N.J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust. N. Z. J. Psychiatry 2015, 49, 207–214. [Google Scholar] [CrossRef]
- Skolnick, S.D.; Greig, N.H. Microbes and Monoamines: Potential Neuropsychiatric Consequences of Dysbiosis. Trends Neurosci. 2019, 42, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.L.; Cooke, A.C.; Johnson, C.N.; Quinn, J.L. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [Green Version]
- Bruch, J.D. Intestinal infection associated with future onset of an anxiety disorder: Results of a nationally representative study. Brain Behav. Immun. 2016, 57, 222–226. [Google Scholar] [CrossRef]
- Goehler, L.E.; Park, S.M.; Opitz, N.; Lyte, M.; Gaykema, R.P.A. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 2008, 22, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Lyte, M.; Varcoe, J.J.; Bailey, M.T. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 1998, 65, 63–68. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Logan, A.C.; Jacka, F.N.; Prescott, S.L. Immune Deficiency and Dysregulation. Curr. Allergy Asthma Rep. 2016, 16, 13. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R. Allergies and Asthma: Do Atopic Disorders Result from Inadequate Immune Homeostasis arising from Infant Gut Dysbiosis? Expert Rev. Clin. Immunol. 2016, 12, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef]
- Roy Sarkar, S.; Mitra Mazumder, P.; Banerjee, S. Probiotics protect against gut dysbiosis associated decline in learning and memory. J. Neuroimmunol. 2020, 348, 577390. [Google Scholar] [CrossRef]
- Wang, P.; Tu, K.; Cao, P.; Yang, Y.; Zhang, H.; Qiu, X.T.; Zhang, M.M.; Wu, X.J.; Yang, H.; Chen, T. Antibiotics-induced intestinal dysbacteriosis caused behavioral alternations and neuronal activation in different brain regions in mice. Mol. Brain 2021, 14, 49. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Yarullina, D.R.; Il’inskaya, O.N.; Aganov, A.V.; Silkin, N.I.; Zverev, D.G. Alternative pathways of nitric oxide formation in Lactobacilli: Evidence for nitric oxide synthase activity by EPR. Microbiology 2006, 75, 634–638. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- 16S Metagenomic Sequencing Library Preparation. Available online: https://emea.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 21 June 2021).
- Watt, E.; Gemmell, M.R.; Berry, S.; Glaire, M.; Farquharson, F.; Louis, P.; Murray, G.I.; El-Omar, E.; Hold, G.L. Extending colonic mucosal microbiome analysis-assessment of colonic lavage as a proxy for endoscopic colonic biopsies. Microbiome 2016, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula Vieira, A.; de Passillé, A.M.; Weary, D.M. Effects of the early social environment on behavioral responses of dairy calves to novel events. J. Dairy Sci. 2012, 95, 5149–5155. [Google Scholar] [CrossRef] [Green Version]
- Rodina, V.I.; Krupina, N.A.; Kryzhanovskiǐ, G.N.; Oknina, N.B. Mnogoparametrovyǐ metod kompleksnoǐ otsenki trevozhno-fobicheskikh sostoianiǐ u krys. Zhurnal Vysshei Nervnoi Deiatelnosti im. Pavlova 1993, 43, 1006–1017. [Google Scholar]
- Karl, T.; Pabst, R.; Von Hörsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Weydt, P.; Hong, S.Y.; Kliot, M.; Möller, T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport 2003, 14, 1051–1054. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 2017, e55718. [Google Scholar] [CrossRef]
- Wenk, G.L. Assessment of Spatial Memory Using the T Maze. Curr. Protoc. Neurosci. 1998, 4, 8.5B.1–8.5A.7. [Google Scholar] [CrossRef] [PubMed]
- Gaifullina, A.S.; Lazniewska, J.; Gerasimova, E.V.; Burkhanova, G.F.; Rzhepetskyy, Y.; Tomin, A.; Rivas-Ramirez, P.; Huang, J.; Cmarko, L.; Zamponi, G.W.; et al. A potential role for T-type calcium channels in homocysteinemia-induced peripheral neuropathy. Pain 2019, 160, 2798–2810. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Liu, X.-G.; Wang, H.-H.; Li, J.-X.; Li, Y.-X. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz. J. Med. Biol. Res. 2012, 45, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhou, X.C.; Lan, C. Impact of the alterations in the interstitial cells of Cajal on intestinal motility in post-infection irritable bowel syndrome. Mol. Med. Rep. 2015, 11, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Shaidullov, I.F.; Sorokina, D.M.; Sitdikov, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 37. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Razygraev, A.V.; Yushina, A.D.; Titovich, I.A. A Method of Measuring Glutathione Peroxidase Activity in Murine Brain in Pharmacological Experiments. Bull. Exp. Biol. Med. 2018, 165, 292–295. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, G.; Lagier, J.C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.X.; Wang, Y.P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbǎ, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative stress and the microbiota-gut-brain axis. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, S.M.; Felice, V.D.; Nally, K.; Savignac, H.M.; Claesson, M.J.; Scully, P.; Woznicki, J.; Hyland, N.P.; Shanahan, F.; Quigley, E.M.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Ceylani, T.; Jakubowska-Doğru, E.; Gurbanov, R.; Teker, H.T.; Gozen, A.G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 2018, 4, e00644. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [Green Version]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Dhotre, D.P.; Marathe, N.P.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, F.; Moitinho-Silva, L.; Blasche, S.; Jahn, M.T.; Gossmann, T.I.; Huerta-Cepas, J.; Hercog, R.; Luetge, M.; Bahram, M.; Pryszlak, A.; et al. Antibiotics-induced monodominance of a novel gut bacterial order. Gut 2019, 68, 1781–1790. [Google Scholar] [CrossRef]
- Lach, G.; Fülling, C.; Bastiaanssen, T.F.S.; Fouhy, F.; Donovan, A.N.O.; Ventura-Silva, A.P.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry 2020, 10, 382. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef] [Green Version]
- Yarullina, D.R.; Shafigullin, M.U.; Sakulin, K.A.; Arzamastseva, A.A.; Shaidullov, I.F.; Markelova, M.I.; Grigoryeva, T.V.; Karpukhin, O.Y.; Sitdikova, G.F. Characterization of gut contractility and microbiota in patients with severe chronic constipation. PLoS ONE 2020, 15, e0235985. [Google Scholar] [CrossRef]
- Hegde, S.; Lin, Y.M.; Golovko, G.; Khanipov, K.; Cong, Y.; Savidge, T.; Fofanov, Y.; Shi, X.Z. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci. Rep. 2018, 8, 13044. [Google Scholar] [CrossRef] [Green Version]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Anisimova, E.A.; Yarullina, D.R. Antibiotic Resistance of Lactobacillus Strains. Curr. Microbiol. 2019, 76, 1407–1416. [Google Scholar] [CrossRef]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Gou, Y.K.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Kellingray, L.; Zhai, Q.; Gall, G.L.; Narbad, A.; Zhao, J.; Zhang, H.; Chen, W. Structural and Functional Alterations in the Microbial Community and Immunological Consequences in a Mouse Model of Antibiotic-Induced Dysbiosis. Front. Microbiol. 2018, 9, 1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Singhi, S.C.; Kumar, S. Probiotics in critically ill children. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [Green Version]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.S.; et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A novel prebiotic blend product prevents irritable bowel syndrome in mice by improving gut microbiota and modulating immune response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Hayashi, H.; Abe, K. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997, 179, 3362–3364. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef]
- Su, M.S.; Schlicht, S.; Gänzle, M.G. Contribution of glutamate decarboxylase in Lactobacillus reuteri to acid resistance and persistence in sourdough fermentation. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of Lactobocillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, 5662. [Google Scholar] [CrossRef] [Green Version]
- Yakovleva, O.; Bogatova, K.; Mukhtarova, R.; Yakovlev, A.; Shakhmatova, V.; Gerasimova, E.; Ziyatdinova, G.; Hermann, A.; Sitdikova, G. Hydrogen sulfide alleviates anxiety, motor, and cognitive dysfunctions in rats with maternal hyperhomocysteinemia via mitigation of oxidative stress. Biomolecules 2020, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Olejar, K.J.; On, S.L.W.; Chelikani, V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef]
- Salami, M. Interplay of Good Bacteria and Central Nervous System: Cognitive Aspects and Mechanistic Considerations. Front. Neurosci. 2021, 15, 25. [Google Scholar] [CrossRef]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, J.; Collins, S. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Psycho-neuroimmunology and the intestinal microbiota: Clinical observations and basic mechanisms. Clin. Exp. Immunol. 2010, 160, 85–91. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]


| Control | AB | AB + LB | |
|---|---|---|---|
| Total bacterial growth | |||
| Input data | 15.1 ± 0.1 | 15.6 ± 0.1 | 15.4 ± 0.1 |
| 1 week | 15.1 ± 0.1 | 15.6 ± 0.1 | 15.0 ± 0.1 |
| 2 weeks | 15.2 ± 0.1 | 15.3 ± 0.1 | 15.8 ± 0.1 |
| Lactic acid bacteria | |||
| Input data | 15.1 ± 0.1 | 13.4 ± 0.2 | 13.5 ± 0.2 |
| 1 week | 15.1 ± 0.2 | 15.2 ± 0.3 | 15.4 ± 0.1 |
| 2 weeks | 15.7 ± 0.1 | 15.6 ± 0.1 | 15.6 ± 0.1 |
| Lactobacillusspp. | |||
| Input data | 15.1 ± 0.1 | 15.7 ± 0.1 | 15.6 ± 0.1 |
| 1 week | 15.1 ± 0.1 | 15.3 ± 0.1 | 15.0 ± 0.1 |
| 2 weeks | 15.8 ± 0.0 | 15.4 ± 0.1 | 15.7 ± 0.1 |
| Lactose-positiveEnterobacteriaceae | |||
| Input data | 14.6 ± 0.1 | 14.0 ± 0.2 | 13.6 ± 0.3 |
| 1 week | 15.4 ± 0.1 | 14.3 ± 0.3 | 14.9 ± 0.1 |
| 2 weeks | 15.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Lactose-negativeEnterobacteriaceae | |||
| Input data | 14.2 ± 0.2 | 14.5 ± 0.1 | 14.4 ± 0.1 |
| 1 week | 13.4 ± 0.1 | 15.7 ± 0.1 | 14.1 ± 0.1 |
| 2 weeks | 0.0 ± 0.0 | 15.1 ± 0.1 | 15.6 ± 0.1 |
| Parameters | Before Injections | After Injections | Before Injections | After Injections | Before Injections | After Injections |
|---|---|---|---|---|---|---|
| Control Group n = 25 | Control Group n = 23 | AB Group n = 25 | AB Group n = 21 | AB + LB Group n = 25 | AB + LB Group n = 24 | |
| Test Open field | ||||||
| Crossed squares, number | 130.5 ± 13.9 | 123.2 ± 7.2 | 128.0 ± 12.4 | 163.5 ± 10.4 &* | 128.5 ± 10.0 | 120.4 ± 8.3 # |
| Rearing, number | 13.4 ± 1.8 | 17.8 ± 1.7 & | 13.5 ± 2.5 | 10.9 ± 1.7 &* | 12.9 ± 2.2 | 16.3 ± 2.1 &# |
| Head dips, number | 4.1 ± 0.9 | 7.2 ± 0.8 & | 5.9 ± 0.5 | 4.8 ± 0.7 * | 4.4 ± 0.7 | 6.8 ± 0.6 &# |
| Grooming act, number | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 2.19 ± 0.4 &* | 1.1 ± 0.2 | 1.1 ± 0.3 # |
| Defecation act, number | 0.9 ± 0.4 | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.4 ± 0.3 &* | 0.9 ± 0.3 | 0.8 ± 0.2 # |
| Latency to exit from central zone, s | 4.3 ± 0.4 | 5.3 ± 0.7 | 4.3 ± 0.3 | 2.5 ± 0.3 &* | 3.6 ± 0.4 | 4.1 ± 0.5 # |
| Rotarod test | ||||||
| Latency to falls, s | 80.1 ± 15.4 | 124.2 ± 9.7 & | 82.6 ± 7.9 | 78.6 ± 12.1 * | 82.8 ± 14.5 | 111.7±11.0 &# |
| Paw Grip Endurance | ||||||
| Time to spent on the grip, s | 80.9 ± 11.9 | 73.7 ± 5.7 | 80.0 ± 12.5 | 45.1 ± 5.5 &* | 80.9 ± 12.2 | 78.6 ± 6.3 # |
| T maze | ||||||
| Alternation, % | 68.0 ± 5.4 | 77.4 ± 6.3 | 73.3 ± 6.1 | 52.8 ± 7.1 &* | 73.2 ± 4.8 | 78.7 ± 6.4 # |
| Novel Object Recognition | ||||||
| Time index | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.1 ± 0.9 | 1.2 ± 0.1 &* | 1.9 ± 0.3 | 1.7 ± 0.1 # |
| Group | n | L | ||||
|---|---|---|---|---|---|---|
| 0.1 | 0.25 | 0.35 | 0.5 | 0.65 | ||
| Control | 10 | 0.29 ± 0.11 | 0.85 ± 0.15 | 1.77 ± 0.18 | 2.8 ± 0.09 | 3.68 ± 0.04 |
| AB | 8 | 0.5 ± 0.07 | 1.33 ± 0.11 | 2.67 ± 0.21 * | 3.51 ± 0.22 * | 3.83 ± 0.16 |
| AB + LB | 12 | 0.33 ± 0.11 | 0.83 ± 0.16 | 1.83 ± 0.22 # | 2.91 ± 0.12 # | 3.67 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslanova, A.; Tarasova, A.; Alexandrova, A.; Novoselova, V.; Shaidullov, I.; Khusnutdinova, D.; Grigoryeva, T.; Yarullina, D.; Yakovleva, O.; Sitdikova, G. Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis. Life 2021, 11, 764. https://doi.org/10.3390/life11080764
Arslanova A, Tarasova A, Alexandrova A, Novoselova V, Shaidullov I, Khusnutdinova D, Grigoryeva T, Yarullina D, Yakovleva O, Sitdikova G. Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis. Life. 2021; 11(8):764. https://doi.org/10.3390/life11080764
Chicago/Turabian StyleArslanova, Alisa, Aksiniya Tarasova, Anastasia Alexandrova, Vera Novoselova, Ilnar Shaidullov, Dilyara Khusnutdinova, Tatiana Grigoryeva, Dina Yarullina, Olga Yakovleva, and Guzel Sitdikova. 2021. "Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis" Life 11, no. 8: 764. https://doi.org/10.3390/life11080764
APA StyleArslanova, A., Tarasova, A., Alexandrova, A., Novoselova, V., Shaidullov, I., Khusnutdinova, D., Grigoryeva, T., Yarullina, D., Yakovleva, O., & Sitdikova, G. (2021). Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis. Life, 11(8), 764. https://doi.org/10.3390/life11080764







