Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review
Abstract
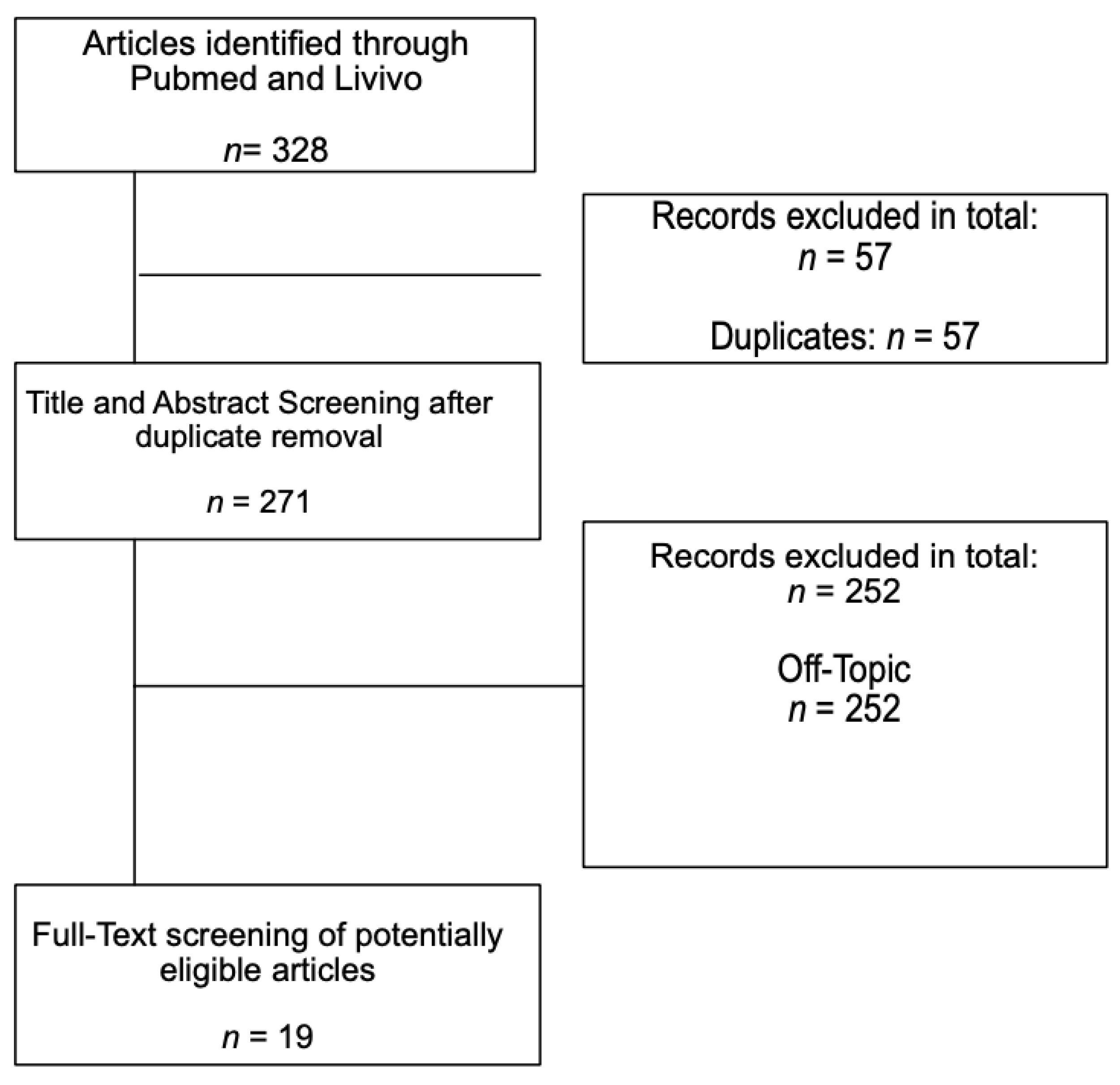
:1. Introduction
2. Results
2.1. Biomechanical Function and Interrelations
2.2. Anatomical Characteristics and Interactions
2.3. Histological Structure
3. Discussion
4. Materials and Methods
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, J.E.; Link, C.L.; Felson, D.; Crincoli, M.G.; Keysor, J.J.; McKinlay, J.B. Prevalence of Foot and Ankle Conditions in a Multiethnic Community Sample of Older Adults. Am. J. Epidemiol. 2004, 159, 491–498. [Google Scholar] [CrossRef]
- Tu, P. Heel Pain: Diagnosis and Management. Am. Fam. Physician 2018, 97, 86–93. [Google Scholar]
- Petraglia, F.; Ramazzina, I.; Costantino, C. Plantar fasciitis in athletes: Diagnostic and treatment strategies. A systematic review. Muscle Ligaments Tendons J. 2019, 7, 107. [Google Scholar] [CrossRef]
- Longo, U.G.; Ronga, M.; Maffulli, N. Achilles Tendinopathy. Sports Med. Arthrosc. Rev. 2018, 26, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Bytomski, J.R. Diagnosis of heel pain. Am. Fam. Physician 2011, 84, 909–916. [Google Scholar]
- Kader, D.; Saxena, A.; Movin, T.; Maffulli, N. Achilles tendinopathy: Some aspects of basic science and clinical management. Br. J. Sports Med. 2002, 36, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Riddle, D.L.; Schappert, S.M. Volume of Ambulatory Care Visits and Patterns of Care for Patients Diagnosed with Plantar Fasciitis: A National Study of Medical Doctors. Foot Ankle Int. 2004, 25, 303–310. [Google Scholar] [CrossRef]
- Vosseller, J.T.; Ellis, S.J.; Levine, D.S.; Kennedy, J.G.; Elliott, A.J.; Deland, J.T.; Roberts, M.M.; O’Malley, M.J. Achilles Tendon Rupture in Women. Foot Ankle Int. 2013, 34, 49–53. [Google Scholar] [CrossRef]
- Bowling, A.; Grundy, E. Activities of daily living: Changes in functional ability in three samples of elderly and very elderly people. Age Ageing 1997, 26, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J. Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef]
- Snow, S.W.; Bohne, W.H. Observations on the Fibrous Retinacula of the Heel Pad. Foot Ankle Int. 2006, 27, 632–635. [Google Scholar] [CrossRef]
- Singh, A.; Zwirner, J.; Templer, F.; Kieser, D.; Klima, S.; Hammer, N. On the morphological relations of the Achilles tendon and plantar fascia via the calcaneus: A cadaveric study. Sci. Rep. 2021, 11, 5986. [Google Scholar] [CrossRef]
- Saladin, K. Anatomy & Physiology: The Unity of Form and Function, 6th ed.; McGraw Hill: New York, NY, USA, 2012; ISBN 978-0-07-337825-1. [Google Scholar]
- Fox, A.J.S.; Wanivenhaus, F.; Rodeo, S.A. The Basic Science of the Patella: Structure, Composition, and Function. J. Knee Surg. 2012, 25, 127–142. [Google Scholar] [CrossRef]
- Gallagher, J.; Tierney, P.; Murray, P.; O’Brien, M. The infrapatellar fat pad: Anatomy and clinical correlations. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 268–272. [Google Scholar] [CrossRef]
- Beckers, A.; Koebke, J. Mechanical strain at the pisotriquetral joint. Clinical Anatomy 1998, 11, 320–326. [Google Scholar] [CrossRef]
- Arandes, R.; Viladot, A. Biomecánica del calcáneo. Med. Clin. 1953, 21, 21–25. [Google Scholar]
- Huerta, J.P. The Effect of the Gastrocnemius on the Plantar Fascia. Foot Ankle Clin. 2014, 19, 701–718. [Google Scholar] [CrossRef]
- Cheung, J.T.-M.; Zhang, M.; An, K.-N. Effect of Achilles tendon loading on plantar fascia tension in the standing foot. Clin. Biomech. 2006, 21, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; McGonagle, D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 2001, 199, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Chang, C.W.; Li, C.T.; Chang, C.H.; Lin, C.F. Finite element analysis of plantar fascia during walking: A quasi-static simulation. Foot Ankle Int. 2015, 36, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Evans, E.J. Fibrocartilage. J. Anat. 1990, 171, 1–15. [Google Scholar]
- Liu, C.-L.; Zhou, J.-P.; Sun, P.-T.; Chen, B.-Z.; Zhang, J.; Tang, C.-Z.; Zhang, Z.-J. Influence of different knee and ankle ranges of motion on the elasticity of triceps surae muscles, Achilles tendon, and plantar fascia. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qin, K.; Tang, C.; Zhu, Y.; Klein, C.S.; Zhang, Z.; Liu, C. Assessment of Passive Stiffness of Medial and Lateral Heads of Gastrocnemius Muscle, Achilles Tendon, and Plantar Fascia at Different Ankle and Knee Positions Using the MyotonPRO. Med. Sci. Monit. 2018, 24, 7570–7576. [Google Scholar] [CrossRef]
- Orner, S.; Kratzer, W.; Schmidberger, J.; Grüner, B. Quantitative tissue parameters of Achilles tendon and plantar fascia in healthy subjects using a handheld myotonometer. J. Bodyw. Mov. Ther. 2018, 22, 105–111. [Google Scholar] [CrossRef]
- Blechschmidt, E. The Structure of the Calcaneal Padding. Foot Ankle 1982, 2, 260–283. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.W. Anatomy Trains—Myofascial Meridians for Manual Therapists and Movement Professionals, 4th ed.; Elsevier LTD: Oxford, UK, 2020; ISBN 0702078131. [Google Scholar]
- Snow, S.W.; Bohne, W.H.; Dicarlo, E.; Chang, V.K. Anatomy of the Achilles Tendon and Plantar Fascia in Relation to the Calcaneus in Various Age Groups. Foot Ankle Int. 1995, 16, 418–421. [Google Scholar] [CrossRef]
- Zwirner, J.; Zhang, M.; Ondruschka, B.; Akita, K.; Hammer, N. An ossifying bridge—on the structural continuity between the Achilles tendon and the plantar fascia. Sci. Rep. 2020, 10, 14523. [Google Scholar] [CrossRef]
- Cowin, S.C. Wolff’s Law of Trabecular Architecture at Remodeling Equilibrium. J. Biomech. Eng. 1986, 108, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Milz, S.; Rufai, A.; Buettner, A.; Putz, R.; Ralphs, J.; Benjamin, M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J. Anat. 2002, 200, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Martin, E.; Ballehr, L.; Richey, J.-M.; Steinberg, J.S. Variability of Insertion of the Achilles Tendon on the Calcaneus: An MRI Study of Younger Subjects. J. Foot Ankle Surg. 2011, 50, 41–43. [Google Scholar] [CrossRef]
- Pękala, P.A.; Henry, B.M.; Pękala, J.R.; Piska, K. A Tomaszewski, K. Erratum: The Achilles tendon and the retrocalcaneal bursa: An anatomical and radiological study. Bone Joint Res. 2019, 8, 437. [Google Scholar] [CrossRef]
- Ballal, M.S.; Walker, C.R.; Molloy, A.P. The anatomical footprint of the Achilles tendon: A cadaveric study. Bone Joint J. 2014, 96, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.L. Sistema Aquíleo Calcáneo Plantar. Biomecánica 2011, 18, 35–43. [Google Scholar] [CrossRef]
- Shaw, H.M.; Vázquez, O.T.; McGonagle, D.; Bydder, G.; Santer, R.M.; Benjamin, M. Development of the human Achilles tendon enthesis organ. J. Anat. 2008, 213, 718–724. [Google Scholar] [CrossRef]
- Benjamin, M.; Moriggl, B.; Brenner, E.; Emery, P.; McGonagle, D.; Redman, S. The ?enthesis organ? concept: Why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004, 50, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.M.; Santer, R.M.; Watson, A.H.D.; Benjamin, M. Adipose tissue at entheses: The innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J. Anat. 2007, 211, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Theobald, P.; Bydder, G.; Dent, C.; Nokes, L.; Pugh, N.; Benjamin, M. The functional anatomy of Kager’s fat pad in relation to retrocalcaneal problems and other hindfoot dis-orders. J. Anat. 2006, 208, 91–97. [Google Scholar] [CrossRef]
- O’Brien, M. The Anatomy of the Achilles Tendon. Foot Ankle Clin. 2005, 10, 225–238. [Google Scholar] [CrossRef]
- Rigozzi, S.; Müller, R.; Snedeker, J. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J. Biomech. 2009, 42, 1547–1552. [Google Scholar] [CrossRef]
- Erik Hedbom, D.H. Binding of Fibromodulin and Decorin to Separate Sites on Fibrillar Collagens. J. Biol. Chem. 1993, 268, 27307–27312. [Google Scholar] [CrossRef]
- Waggett, A.D.; Ralphs, J.; Kwan, A.P.; Woodnutt, D.; Benjamin, M. Characterization of collagens and proteoglycans at the insertion of the human achilles tendon. Matrix Biol. 1998, 16, 457–470. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña, L.; Coto, G. In vitro Anti-Inflammatory Effects of Hyaluronic Acid in Ethanol-Induced Damage in Skin Cells. J. Pharm. Pharm. Sci. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strocchi, R.; De Pasquale, V.; Guizzardi, S.; Govoni, P.; Facchini, A.; Raspanti, M.; Girolami, M.; Giannini, S. Human Achilles Tendon: Morphological and Morphometric Variations as a Function of Age. Foot Ankle 1991, 12, 100–104. [Google Scholar] [CrossRef]
- Schneider, H.P.; Baca, J.M.; Carpenter, B.B.; Dayton, P.D.; Fleischer, A.; Sachs, B.D. American College of Foot and Ankle Surgeons Clinical Consensus Statement: Diagnosis and Treatment of Adult Acquired Infracalcaneal Heel Pain. J. Foot Ankle Surg. 2018, 57, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of Homo. Nat. Cell Biol. 2004, 432, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sellers, W.I.; Pataky, T.C.; Caravaggi, P.; Crompton, R.H. Evolutionary Robotic Approaches in Primate Gait Analysis. Int. J. Primatol. 2010, 31, 321–338. [Google Scholar] [CrossRef]
- Carlson, R.E.; Fleming, L.L.; Hutton, W.C. The Biomechanical Relationship Between The Tendoachilles, Plantar Fascia and Metatarsophalangeal Joint Dorsiflexion Angle. Foot Ankle Int. 2000, 21, 18–25. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Study Setting | Any, | Foreign languages other than German, English, and Spanish; |
| no date restriction | ||
| no journal restrictions, | Digital unavailability | |
| no language restrictions | ||
| Study design | Any, except for other systematic reviews | Systematic review; |
| Reviews | ||
| Study aim | Necessary to analyze or examine the interplay between the three structures “Achilles tendon”, “calcaneus”, and “plantar fascia” or the interplay between at least two structures | Assessing current knowledge; |
| No analysis of interconnectivity of the named three structures | ||
| Research Approach | Study | Sample Size | Focus of Examination | Conclusion Concerning Connection of AT and PF |
|---|---|---|---|---|
| Anatomical combined Functional | Arandes and Viladot, 1953 | - | Ontogenic (histological) and phylogenic anatomic (animal) observations. | Theoretical model: AS-calcaneus-PF system with anatomic and functional continuity. |
| Functional | Cheung et al., 2006 | 6 feet of middle-aged male cadavers | 3D finite element model of human foot and ankle using fresh cadaveric ankle–foot specimens investigating loading response in the standing foot of AT and PF. | Increase in AT load leads to PF increase in PF tension (by arch flattening). |
| Shaw et al., 2008 | 17 feet of spontaneously aborted fetuses, 7–32 weeks and of 6 adults | Histological sections of fetuses’ feet and MRI of adult feet. | Continuing fibers between AT and PF as a thickened perichondrium in the posterior part of the main calcaneal body. | |
| Chen et al., 2014 | 1 foot of a 30-year-old cadaver | 3D finite element model of a human foot and ankle was developed using computed tomography images. | Higher AT force during gait correlate with higher PF tension during gait. | |
| Huerta et al., 2014 | - | Theoretical model of force transmission within gastrocnemius, AT, calcaneus, and PF. | Gastrocnemius tightness leads to higher AT tension, finally increasing higher plantar tension and stiffness of the PF. | |
| Huang et al., 2018 | 15 female and 15 male healthy participants, average are 22.9 ± 3.8 years | Correlation of frequency, decrement, stiffness, creep, and relaxation between AT and PF using Myoton Pro device. | Increase in AT and PF stiffness during higher ankle dorsiflexion, even higher during knee Extension. | |
| Orner et al., 2018 | 150 healthy males and females, mean age 33 years | Correlation of frequency, decrement, stiffness, creep, and relaxation between AT and PF using Myoton Pro device. | Significant positive correlation of AT and PF. | |
| Liu et al., 2020 | 20 males, 19–23 years | Passive elastic properties of triceps surae, AT and PF measurement with shear wave elastography in different knee and ankle angles. | During ankle dorsiflexion, AT and PF show an increase in stiffness, while 90° knee flexion and stiffness of AT and PF seem to be correlated oppositely according to knee flexion and extension. | |
| Anatomical and histological | Snow et al., 1995 | 15 feet from human cadavers | Histologic dissection and observation of human cadaveric feet. | In fetuses, there is a continuous collagen layer within the AT wrapping around the calcaneus into the PF. In older feet, the continuity is not visible anymore. |
| Milz et al., 2002 | 4 feet of male and female cadavers, 32 to 73 years old | 3D model of the AT insertion of 4 cadavers. | Alignment of the calcaneal trabeculae along the direction of the AT fascicles in orientation towards the proximal attachment of the PF. | |
| Shaw et al., 2008 | Fetuses of 17 different crown rump lengths | Histologic investigation of sectioned fetuses. | AT and PF merging into the thickened perichondrium in the posterior part of 152 the main calcaneal body, thus AT and PF initially attached to the perichondrium of the calcaneus. | |
| Myers, 2009 | - | Anatomical investigation as macroscopic preparation of human cadavers. | Superficial back line flowing into anatomical connection of AT and PF. | |
| Kim et al., 2010 | 60 human cadaveric limbs, of 40 cadavers, 43 to 98 years old | Macroscopic visualization and palpation of cadaveric feet. | Eight percent had a lower calcaneal insertion of the Achilles tendon and retained a connection between AT and PF in younger specimen (43 and 48 years old). None of the specimen showed a complete continuation. | |
| Kim et al., 2011 | 69 MRIs, 10 to 40 years old | MRI review from database by radiology group. | In two young subjects, 12 and 16 years of age, a contiguous relationship between AT and PF was seen. AT’s insertion location seems to migrate proximally by 0.63% each year of life. | |
| Stecco et al., 2013 | 12 feet from unembalmed human cadavers, 67 to 92 years old, and 52 MRIs, mean age 44.2 years | Histologic dissection and observation of human cadaveric feet, also microscopically, and MRI investigation of patients with heel pain. | The paratenon of the AT is in continuity with the periosteum of the calcaneus and that is in continuity with part of the PF. Addoitionally, cellular and extracellular components seem similar. | |
| Ballal et al., 2014 | 12 fresh frozen specimens and 10 embalmed specimens, 57 to 95 years old | Macroscopic visualization of cadaveric feet. | Only three of the specimens showed a continuation of AT fascicles with the PF through the periosteum. | |
| Pekala et al., 2019 | 202 MRIs | MRI review from two observers. | Insertion of the PF does not change in comparison to the changing insertion location of the AT. | |
| Zwirner et al., 2020 | 9 feet of human cadavers, 28 to 93 years old | Macroscopic and histologic investigations. | At the calcaneal AT and PF insertion, the fibers from both sides seem to continue into the posterior calcaneal trabecular bone meshwork, appearing in the fiber tension direction. | |
| Singh et al., 2021 | 18 male and female specimen, 38 to 94 years old | Histological and radiological investigation of cadaveric feet. | Partial continuation of AT fibers in the trabecular meshwork in stress direction of the AT. AT paratenon continues into the calcaneal periosteum, merging with the PF. AT thickness has a positive correlation with the insertional length of the AT. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinrich, L.; Paraskevaidis, M.; Schleip, R.; Agres, A.N.; Tsitsilonis, S. Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review. Life 2021, 11, 745. https://doi.org/10.3390/life11080745
Weinrich L, Paraskevaidis M, Schleip R, Agres AN, Tsitsilonis S. Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review. Life. 2021; 11(8):745. https://doi.org/10.3390/life11080745
Chicago/Turabian StyleWeinrich, Luise, Melissa Paraskevaidis, Robert Schleip, Alison N. Agres, and Serafeim Tsitsilonis. 2021. "Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review" Life 11, no. 8: 745. https://doi.org/10.3390/life11080745
APA StyleWeinrich, L., Paraskevaidis, M., Schleip, R., Agres, A. N., & Tsitsilonis, S. (2021). Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review. Life, 11(8), 745. https://doi.org/10.3390/life11080745







