It More than Adds Up: Interaction of Antibiotic Mixing and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth and Antibiotic Assays
2.2. Dose–Response, Antibiotic–Temperature and Antibiotic–Antibiotic Interaction
3. Results
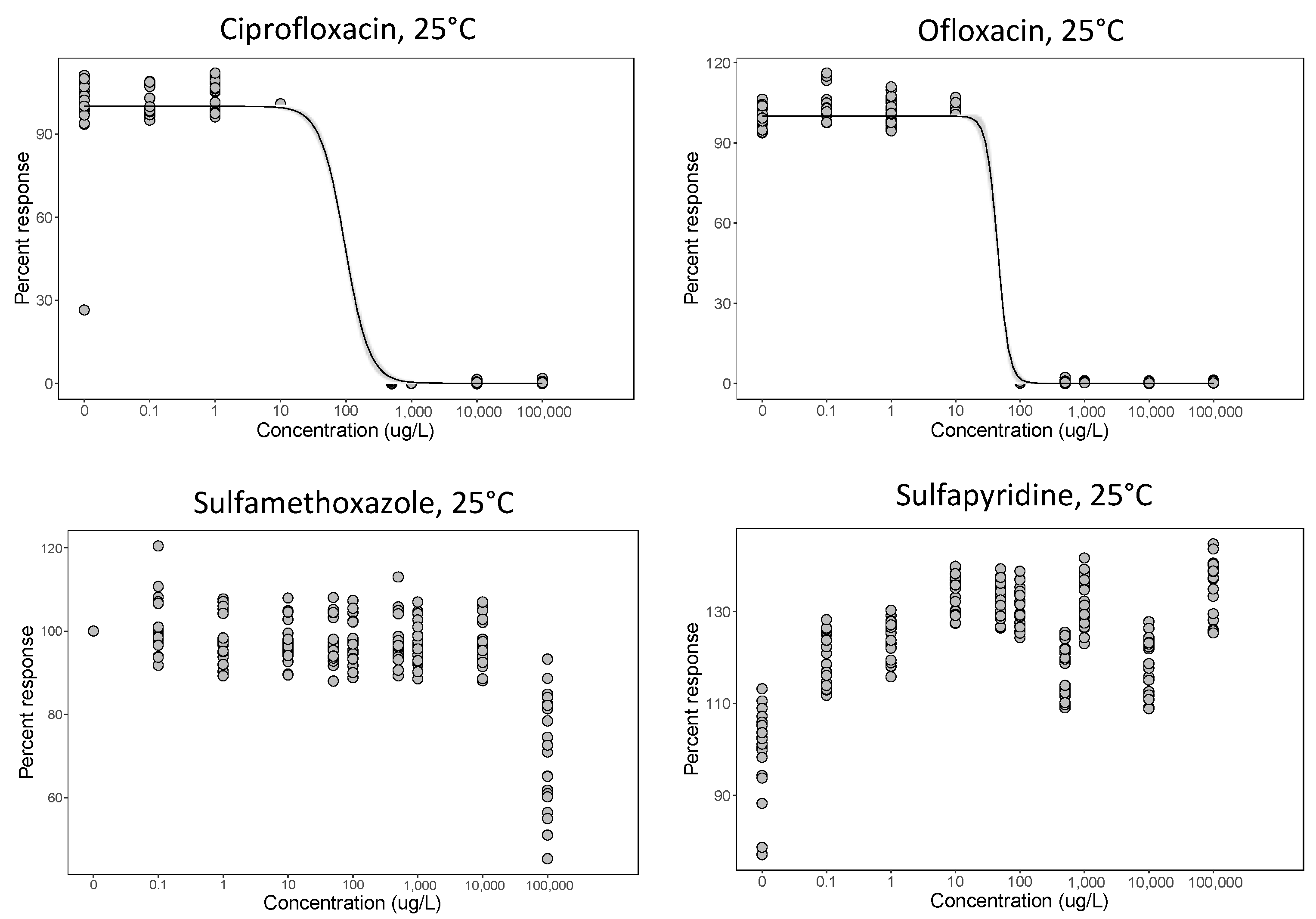
3.1. MIC and Dose–Response (EC50)
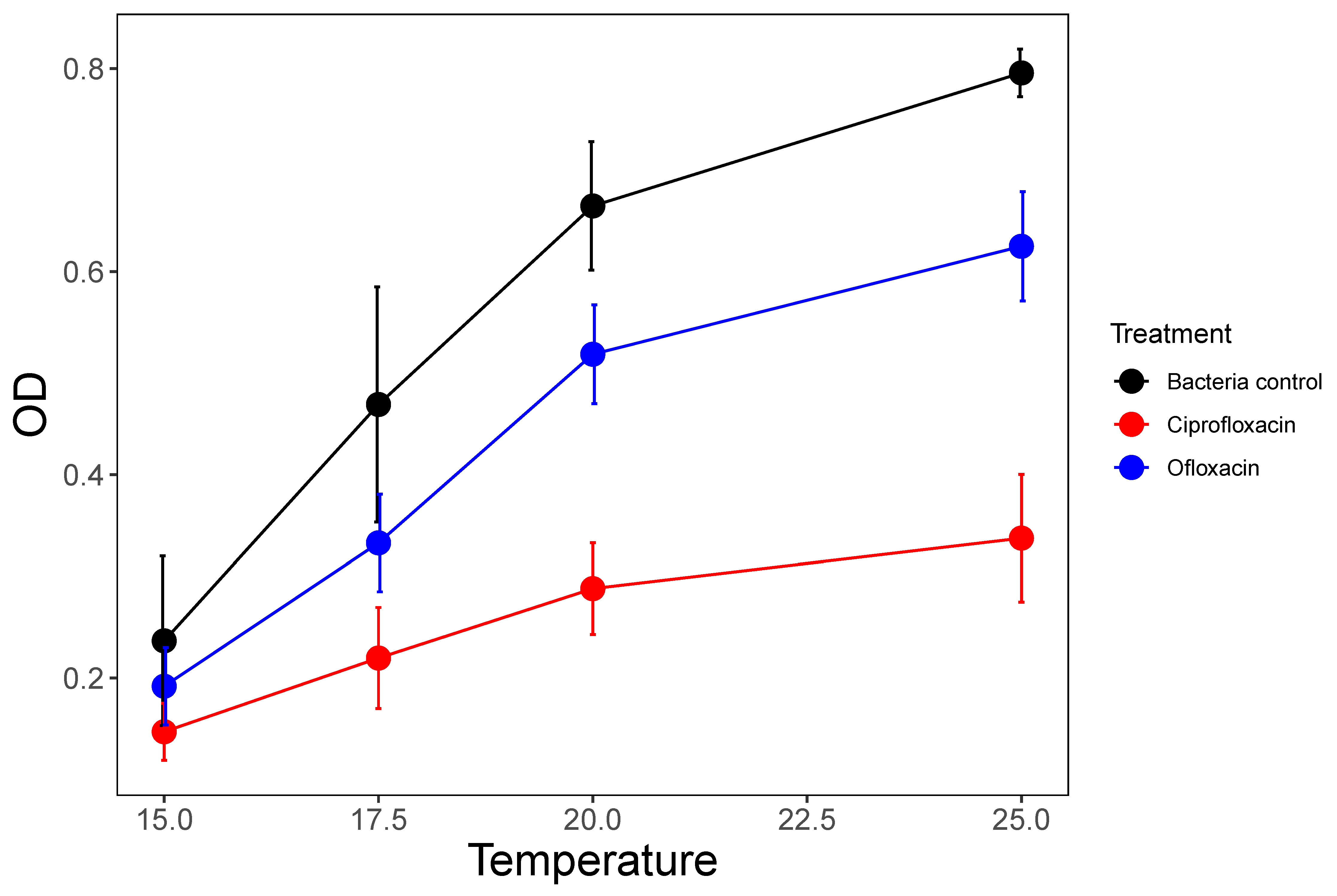
3.2. Temperature Effects
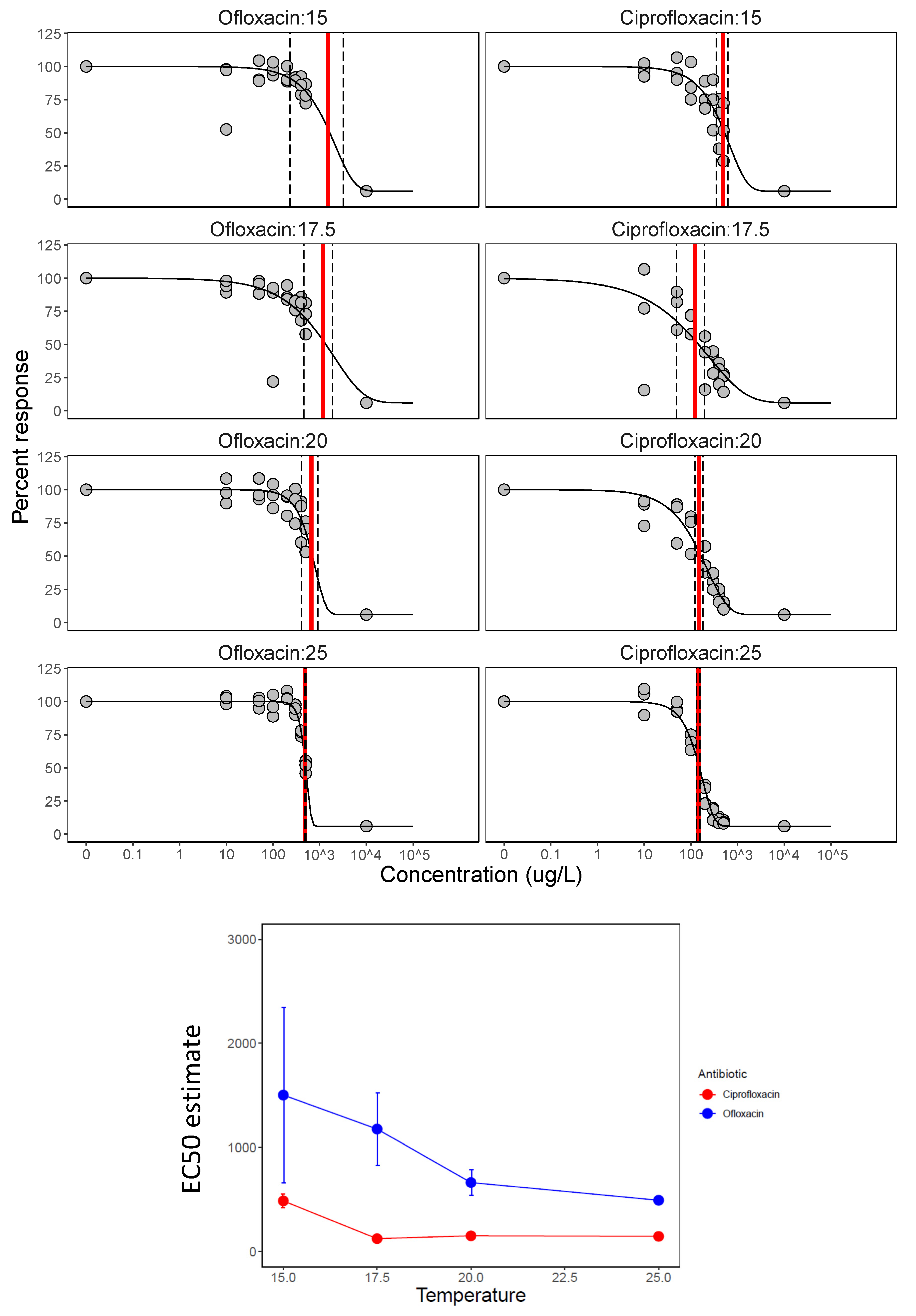
3.3. Antibiotic Mixing and Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Rodríguez-Verdugo, A.; Lozano-Huntelman, N.; Cruz-Loya, M.; Savage, V.; Yeh, P. Compounding effects of climate warming and antibiotic resistance. iScience 2020, 23, 101024. [Google Scholar] [CrossRef]
- López-Roldán, R.; de Alda, M.L.; Gros, M.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography–triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography–time of flight-mass spectrometry. Chemosphere 2010, 80, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updates 2012, 15, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Friman, V.P.; Guzman, L.M.; Reuman, D.C.; Bell, T. Bacterial adaptation to sublethal antibiotic gradients can change the ecological properties of multitrophic microbial communities. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142920. [Google Scholar] [CrossRef]
- Tekin, E.; White, C.; Kang, T.M.; Singh, N.; Cruz-Loya, M.; Damoiseaux, R.; Savage, V.M.; Yeh, P.J. Prevalence and patterns of higher-order drug interactions in Escherichia coli. NPJ Syst. Biol. Appl. 2018, 4, 31. [Google Scholar] [CrossRef]
- Beppler, C.; Tekin, E.; White, C.; Mao, Z.; Miller, J.H.; Damoiseaux, R.; Savage, V.M.; Yeh, P.J. When more is less: Emergent suppressive interactions in three-drug combinations. BMC Microbiol. 2017, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Flaherty, C.M.; Dodson, S.I. Effects of pharmaceuticals on Daphnia survival, growth, and reproduction. Chemosphere 2005, 61, 200–207. [Google Scholar] [CrossRef]
- Nolan, C.; Behrends, V. Sub-inhibitory antibiotic exposure and virulence in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Loya, M.; Kang, T.M.; Lozano, N.A.; Watanabe, R.; Tekin, E.; Damoiseaux, R.; Savage, V.M.; Yeh, P.J. Stressor interaction networks suggest antibiotic resistance co-opted from stress responses to temperature. ISME J. 2019, 13, 12–23. [Google Scholar] [CrossRef]
- Feller, G. Protein folding at extreme temperatures: Current issues. In Proceedings of the Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 84, pp. 129–137. [Google Scholar]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.M.; Bailey, R.A.; Dossena, M.; Gamfeldt, L.; Reiss, J.; Trimmer, M.; Woodward, G. Higher biodiversity is required to sustain multiple ecosystem processes across temperature regimes. Glob. Chang. Biol. 2015, 21, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Tekin, E.; Diamant, E.S.; Cruz-Loya, M.; Enriquez, V.; Singh, N.; Savage, V.M.; Yeh, P.J. Using a newly introduced framework to measure ecological stressor interactions. Ecol. Lett. 2020, 23, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://intro2r.com/citing-r.html (accessed on 11 December 2021).
- Backhaus, T.; Karlsson, M. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res. 2014, 49, 157–165. [Google Scholar] [CrossRef]
- Tekin, E.; Beppler, C.; White, C.; Mao, Z.; Savage, V.M.; Yeh, P.J. Enhanced identification of synergistic and antagonistic emergent interactions among three or more drugs. J. R. Soc. Interface 2016, 13, 20160332. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhang, J.; Li, X.; Gao, B. Stimulation effects of ciprofloxacin and sulphamethoxazole in Microcystis aeruginosa and isobaric tag for relative and absolute quantitation-based screening of antibiotic targets. Mol. Ecol. 2017, 26, 689–701. [Google Scholar] [CrossRef]
- Cruz-Loya, M.; Tekin, E.; Kang, T.M.; Cardona, N.; Lozano-Huntelman, N.; Rodriguez-Verdugo, A.; Savage, V.M.; Yeh, P.J. Antibiotics shift the temperature response curve of Escherichia coli growth. Msystems 2021, 6, e00228-21. [Google Scholar] [CrossRef] [PubMed]
- Loughman, K.; Hall, J.; Knowlton, S.; Sindeldecker, D.; Gilson, T.; Schmitt, D.M.; Birch, J.W.-M.; Gajtka, T.; Kobe, B.N.; Florjanczyk, A. Temperature-dependent gentamicin resistance of Francisella tularensis is mediated by uptake modulation. Front. Microbiol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Raychaudhuri, S.; Hayer-Hartl, M.; Hartl, F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010, 2, a004390. [Google Scholar] [CrossRef]
- Mondal, S.; Pathak, B.K.; Ray, S.; Barat, C. Impact of P-Site tRNA and antibiotics on ribosome mediated protein folding: Studies using the Escherichia coli ribosome. PLoS ONE 2014, 9, e101293. [Google Scholar] [CrossRef] [PubMed]
- van Houte, S.; Padfield, D.; Gómez, P.; Luján, A.M.; Brockhurst, M.A.; Paterson, S.; Buckling, A. Compost spatial heterogeneity promotes evolutionary diversification of a bacterium. J. Evol. Biol. 2021, 34, 246–255. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.G.; Vinebrooke, R.D.; Chimimba, C.T. Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.M.; Reiss, J.; Yvon-Durocher, G.; Woodward, G. Global change and food webs in running waters. Hydrobiologia 2010, 657, 181–198. [Google Scholar] [CrossRef]
- Atkinson, D.; Ciotti, B.J.; Montagnes, D.J.S. Protists decrease in size linearly with temperature: Ca. 2.5% C−1. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 2605–2611. [Google Scholar] [CrossRef]
- DeLong, J.P.; Okie, J.G.; Moses, M.E.; Sibly, R.M.; Brown, J.H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc. Natl. Acad. Sci. USA 2010, 107, 12941–12945. [Google Scholar] [CrossRef] [PubMed]
- Padfield, D.; Yvon-Durocher, G.; Buckling, A.; Jennings, S.; Yvon-Durocher, G. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 2016, 19, 133–142. [Google Scholar] [CrossRef] [PubMed]

| Experiment | Antibiotic | Temp. | Antibiotic Concentration | Antibiotic Mixtures | Analysis |
|---|---|---|---|---|---|
| 1 P. fluorescens 105 cells/mL initial% | Ciprofloxacin, ofloxacin, sulfamethoxazole and sulfapyridine | 30 | Nine concentrations: 125 to 128,000 µg/L for single antibiotics and the mixtures were run from 1/32× to 4× MIC | Antibiotics on their own and all possible two-way combinations | MIC, checkerboard for antibiotic–antibiotic interaction |
| 2 P. fluorescens 106 cells/mL initial% | Ciprofloxacin, ofloxacin, sulfamethoxazole and sulfapyridine | 25 | Eleven concentrations: 0.1 to 100,000 µg/L | Antibiotics on their own only | Dose–response and EC50 |
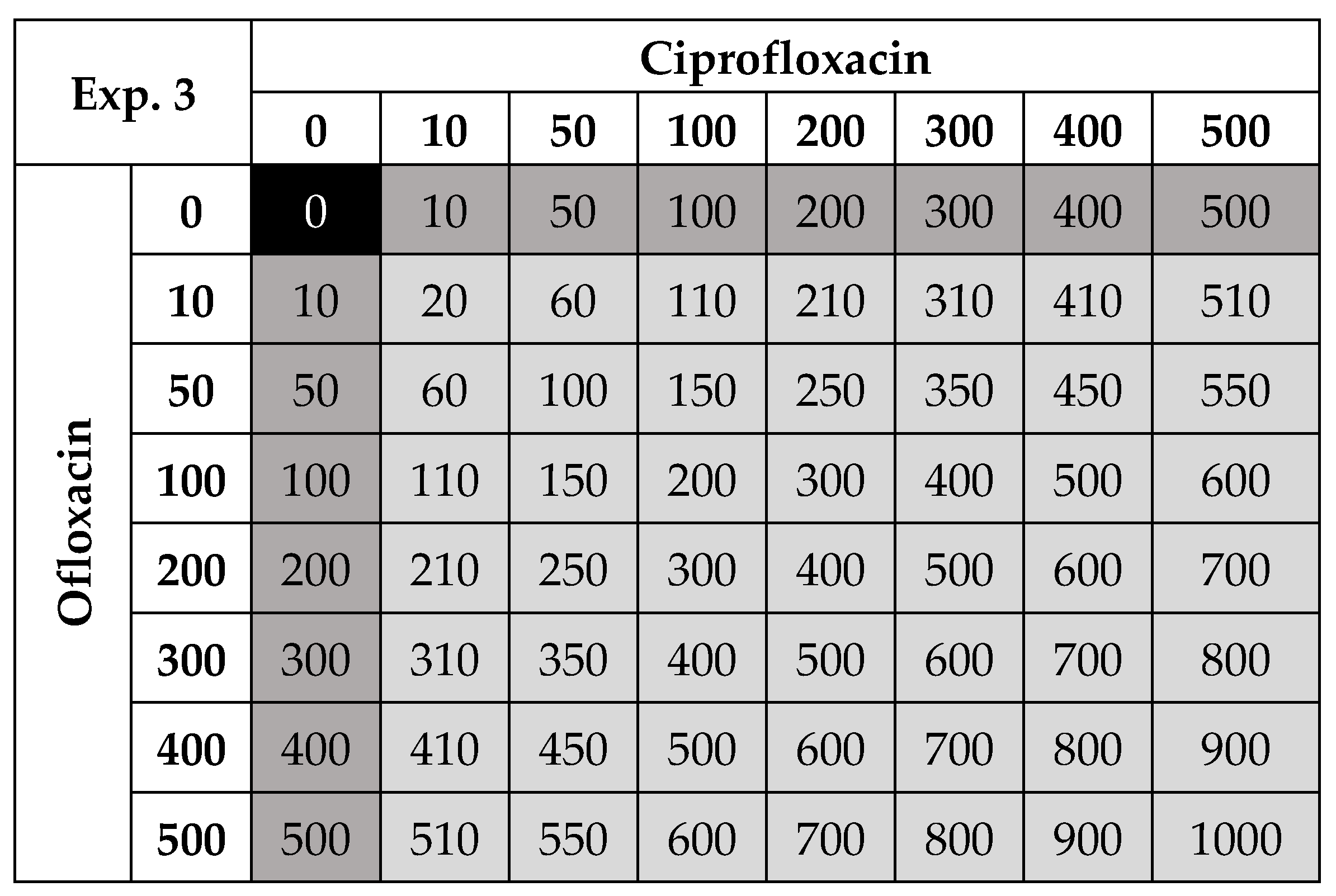
| 3 P. fluorescens 105 cells/mL initial% | Ciprofloxacin and ofloxacin | 15, 17.5, 20, 25 | Control and nine ‘field-realistic’ concentrations: 10 to 500 µg/L | Antibiotics on their own and all possible two-way combinations of ciprofloxacin and ofloxacin | Dose–response for C and O, temperature effects on growth and antibiotic–antibiotic interactions |
| MIC µg/L | Effect in Mixture | ||||
|---|---|---|---|---|---|
| Alone | |||||
| Ciprofloxacin | Ofloxacin | Sulfamethoxazole | Sulfapyridine | ||
| Ciprofloxacin | 500 | na | S | I | I |
| Ofloxacin | 125 | S | na | I | I |
| Sulfamethoxazole | 32,000 | I | I | na | I |
| Sulfapyridine | 8000 | I | I | I | na |
| Treatment | Estimate_EC50 | Std. Error | Lower | Upper |
|---|---|---|---|---|
| Ciprofloxacin: 15 °C | 486 | 65 | 352 | 621 |
| Ciprofloxacin: 17.5 °C | 123 | 36 | 49 | 197 |
| Ciprofloxacin: 20 °C | 150 | 14 | 121 | 180 |
| Ciprofloxacin: 25 °C | 145 | 6 | 133 | 156 |
| Ofloxacin: 15 °C | 1502 | 843 | 233 | 3237 |
| Ofloxacin: 17.5 °C | 1176 | 348 | 459 | 1893 |
| Ofloxacin: 20 °C | 663 | 124 | 407 | 919 |
| Ofloxacin: 25 °C | 492 | 7 | 477 | 506 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danner, M.-C.; Azams, S.O.; Robertson, A.; Perkins, D.; Behrends, V.; Reiss, J. It More than Adds Up: Interaction of Antibiotic Mixing and Temperature. Life 2021, 11, 1435. https://doi.org/10.3390/life11121435
Danner M-C, Azams SO, Robertson A, Perkins D, Behrends V, Reiss J. It More than Adds Up: Interaction of Antibiotic Mixing and Temperature. Life. 2021; 11(12):1435. https://doi.org/10.3390/life11121435
Chicago/Turabian StyleDanner, Marie-Claire, Sharon Omonor Azams, Anne Robertson, Daniel Perkins, Volker Behrends, and Julia Reiss. 2021. "It More than Adds Up: Interaction of Antibiotic Mixing and Temperature" Life 11, no. 12: 1435. https://doi.org/10.3390/life11121435
APA StyleDanner, M.-C., Azams, S. O., Robertson, A., Perkins, D., Behrends, V., & Reiss, J. (2021). It More than Adds Up: Interaction of Antibiotic Mixing and Temperature. Life, 11(12), 1435. https://doi.org/10.3390/life11121435







