The Most Common Functional Disorders and Factors Affecting Female Pelvic Floor
Abstract
1. Introduction
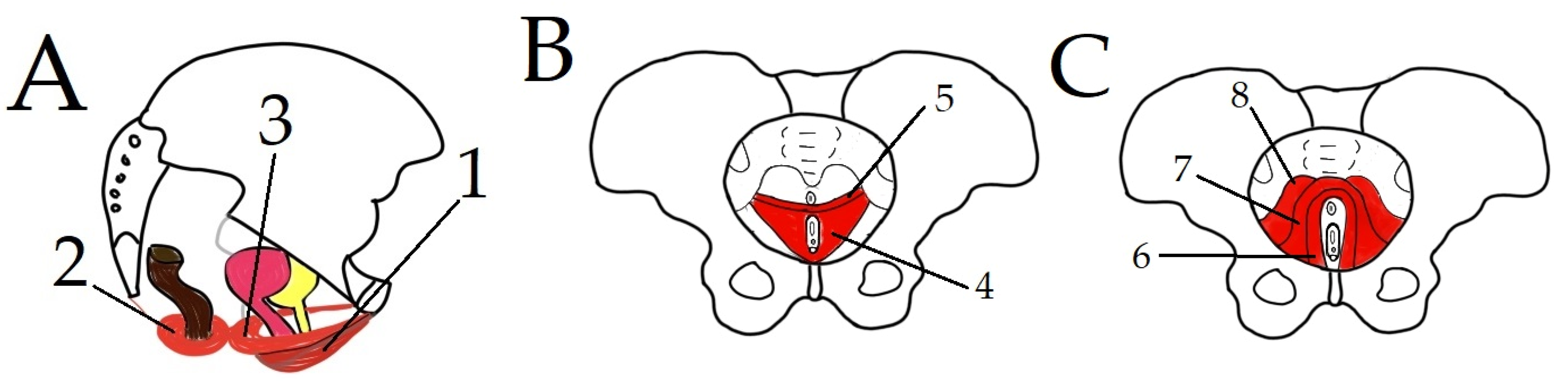
1.1. Anatomy of PF
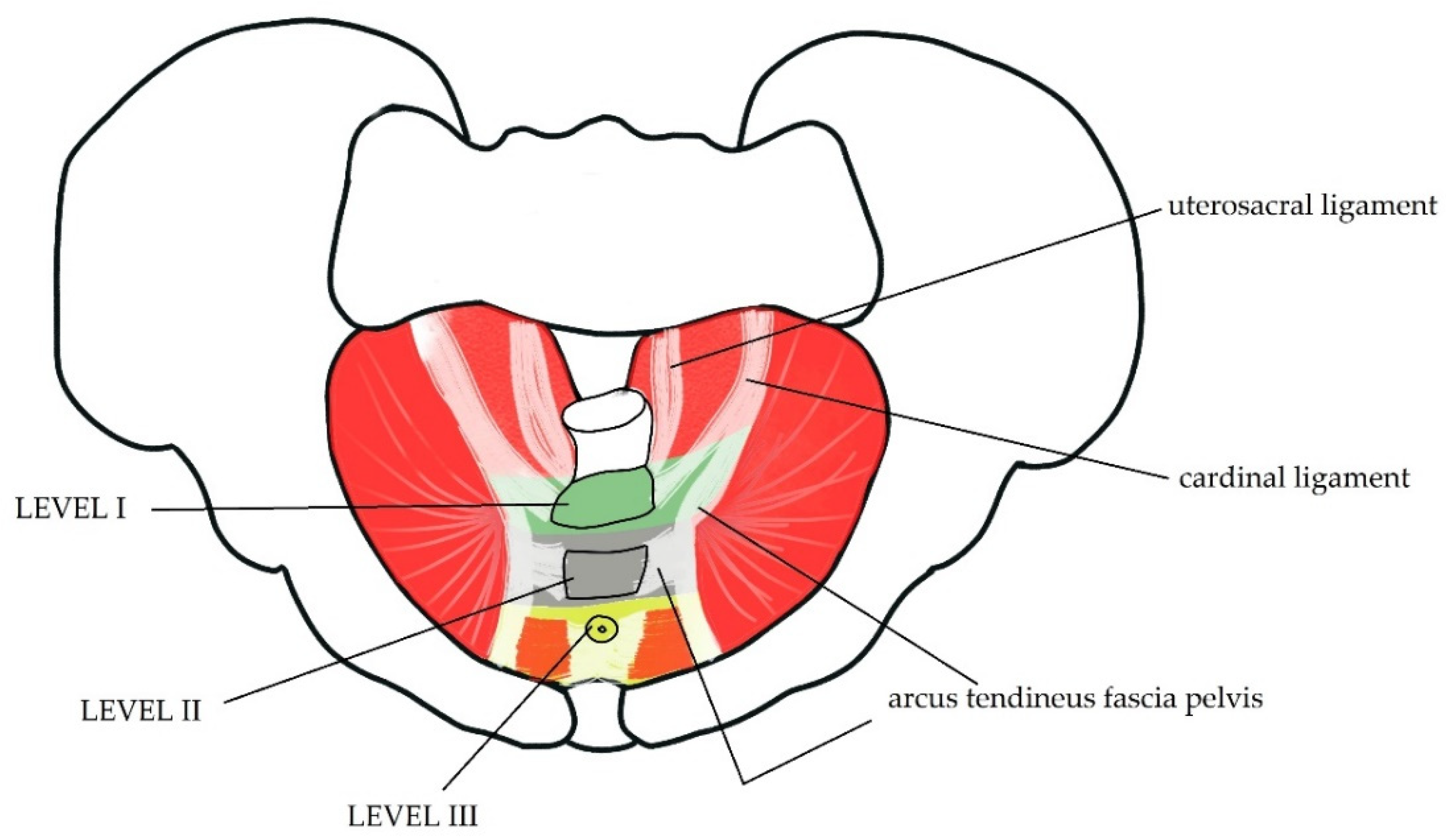
1.2. PF—Blood Supply and Innervation
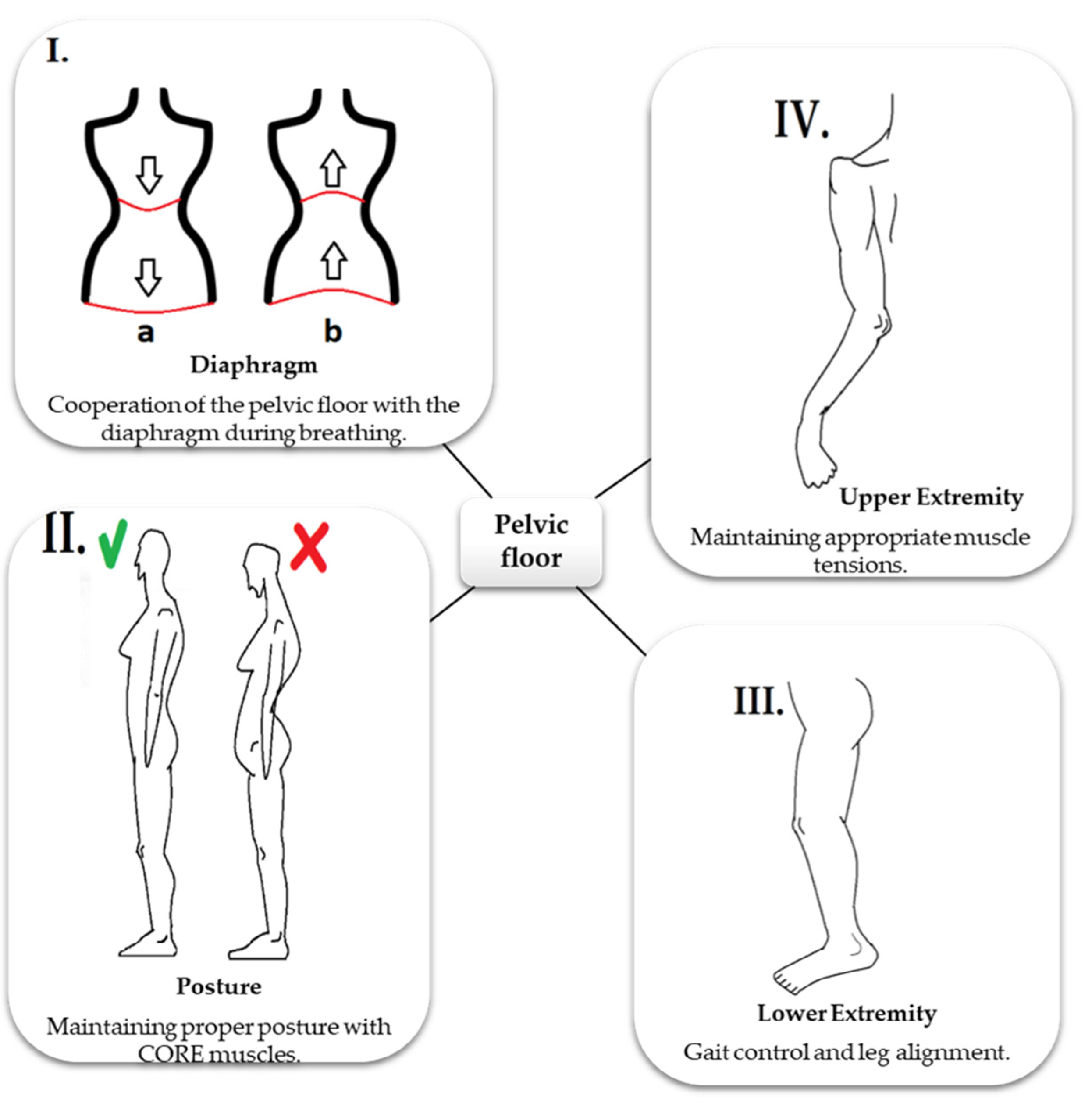
1.3. Role of PF in Stabilization
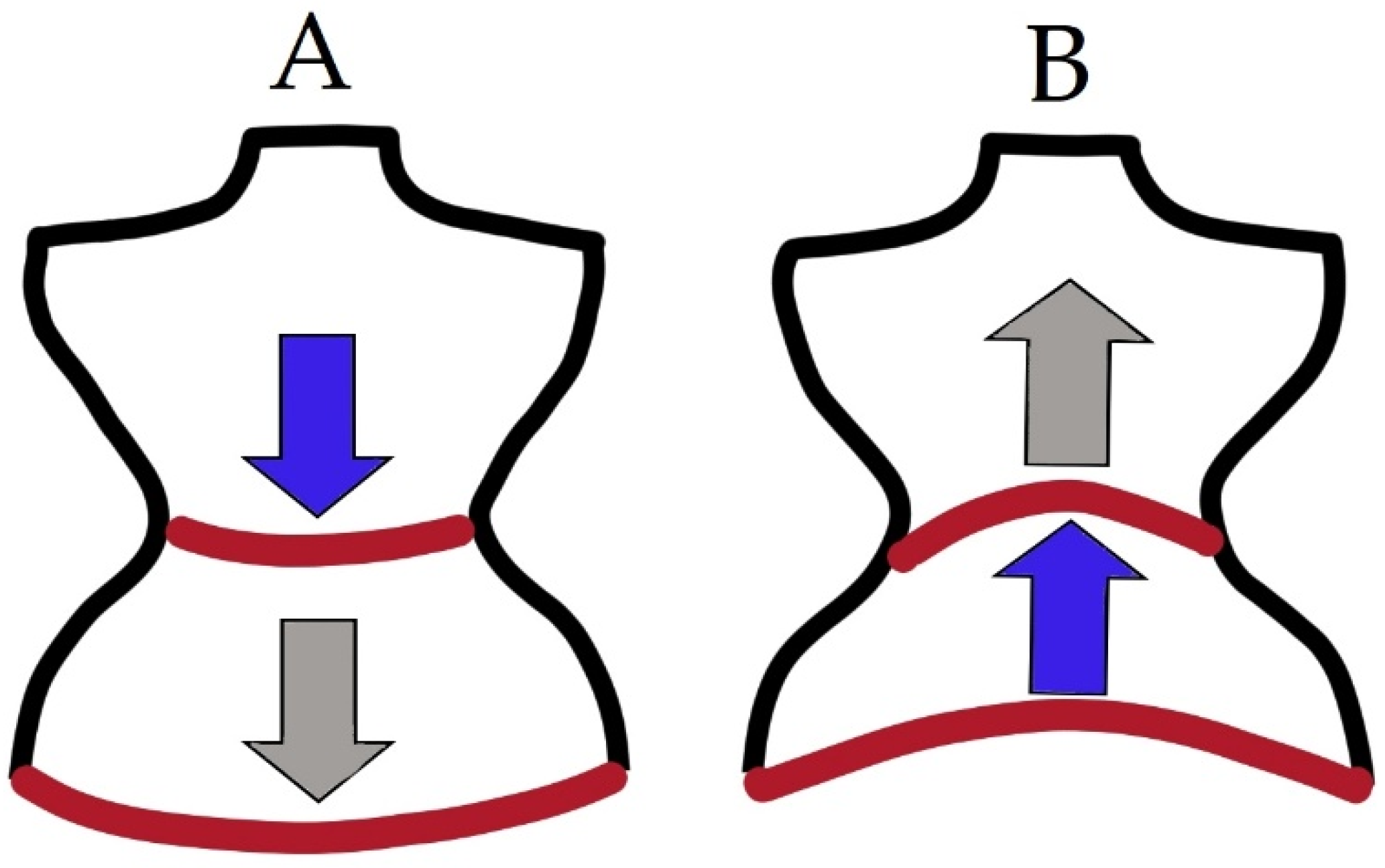
1.4. Role of PF in Respiration
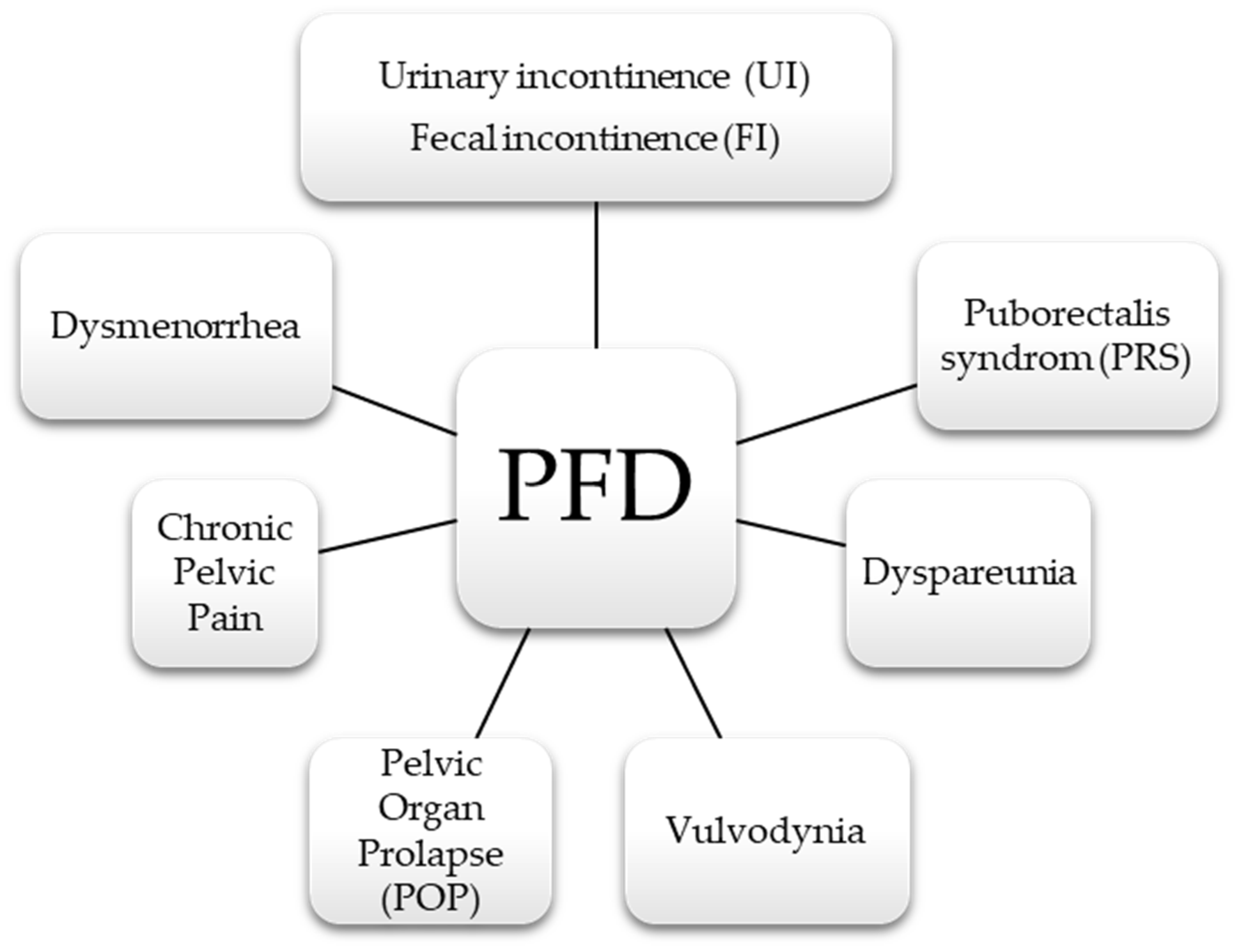
2. Pelvic Floor Dysfunction
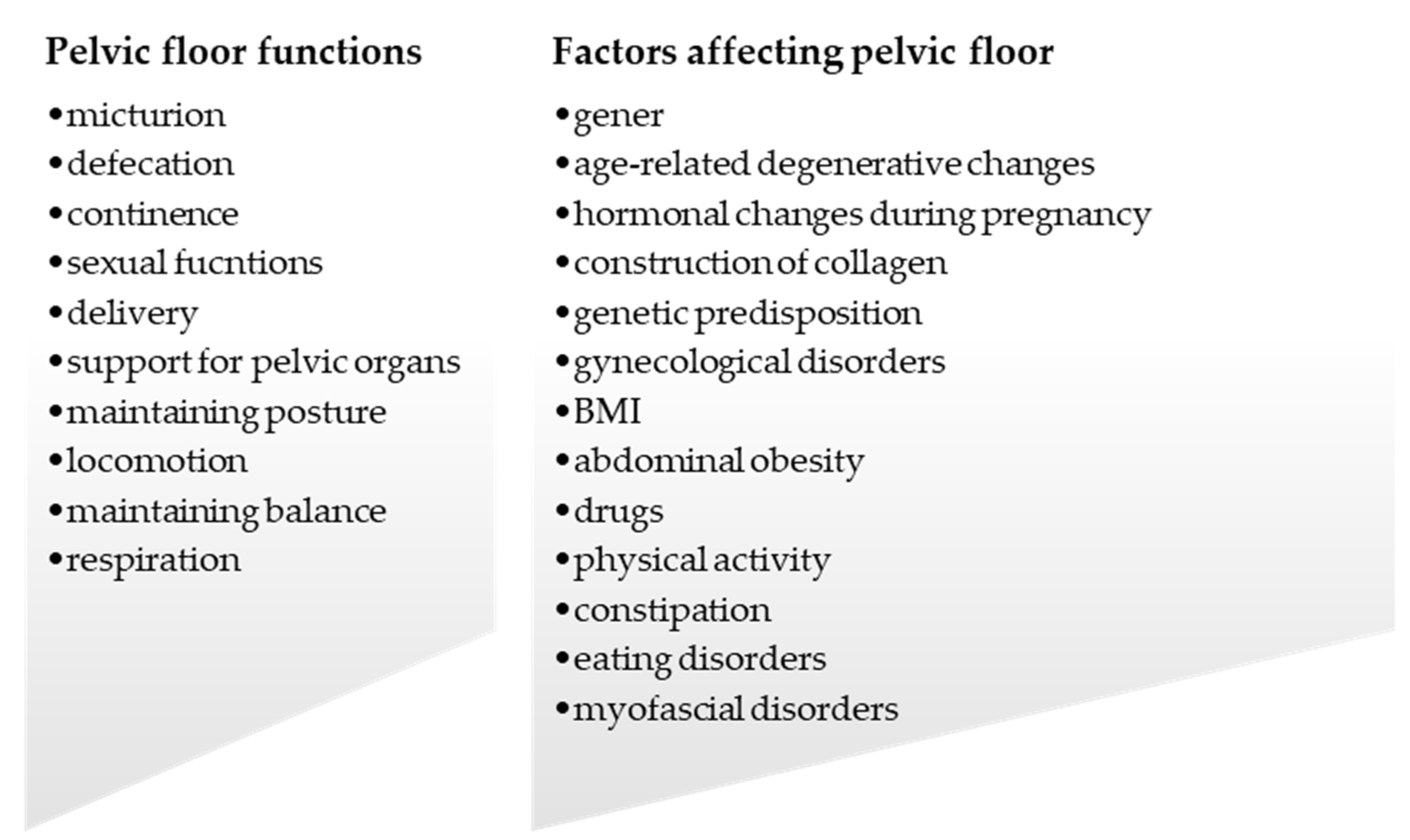
3. Factors Affecting PF
3.1. Pregnancy, Childbirth, and Postpartum
3.2. Aging and Age-Related Hormonal Changes
3.3. Physical Activity
3.4. Obesity and Metabolic Syndrome
3.5. Respiratory System Diseases
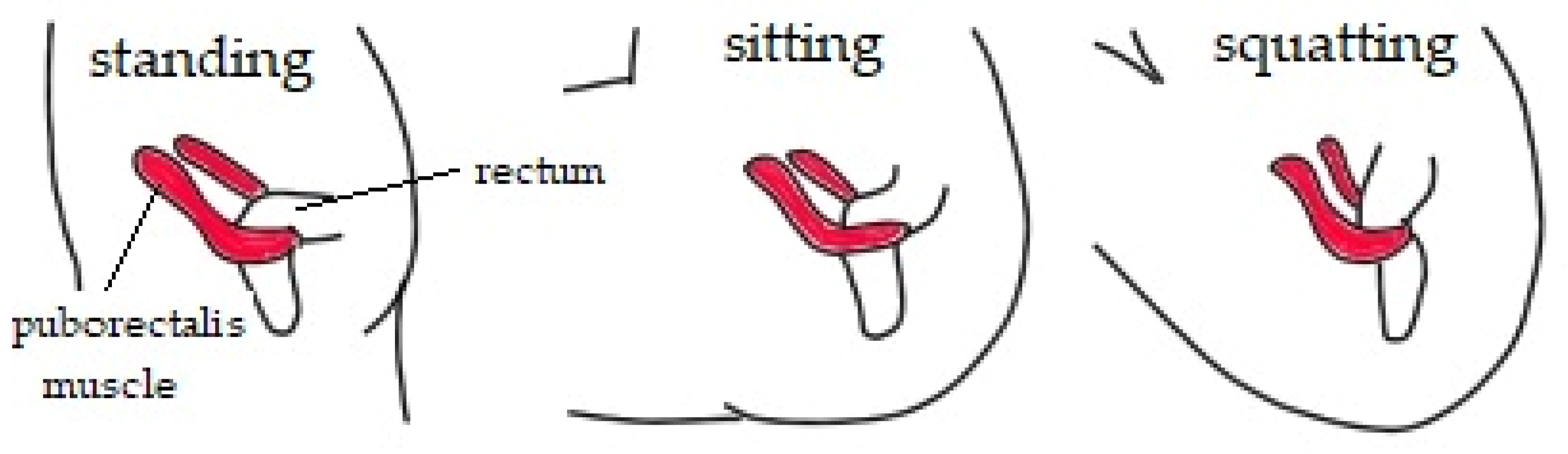
3.6. Chronic Constipation
3.7. Eating Disorders
3.8. Diseases of Connective Tissue
3.9. Gynecological Disorders
3.10. Myofascial Disorders
3.11. Trauma Injuries
4. Physiotherapy and Manual Therapy
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocca Rossetti, S. Functional Anatomy Of Pelvic Floor. Arch. Ital. Urol. Androl. 2016, 88, 28. [Google Scholar] [CrossRef]
- DeSilva, J.; Rosenberg, K. Anatomy, Development, and Function of the Human Pelvis. Anat. Rec. 2017, 300, 628–632. [Google Scholar] [CrossRef]
- D’Ancona, C.; Haylen, B.; Oelke, M.; Abranches-Monteiro, L.; Arnold, E.; Goldman, H.; Hamid, R.; Homma, Y.; Marcelissen, T.; Rademakers, K.; et al. The International Continence Society (ICS) Report on the Terminology for Adult Male Lower Urinary Tract And Pelvic Floor Symptoms And Dysfunction. Neurourol. Urodyn. 2019, 38, 433–477. [Google Scholar] [CrossRef]
- Wu, Y.; Dabhoiwala, N.; Hagoort, J.; Hikspoors, J.; Tan, L.; Mommen, G.; Hu, X.; Zhang, S.; Lamers, W. Architecture Of Structures In The Urogenital Triangle Of Young Adult Males; Comparison With Females. J. Anat. 2018, 233, 447–459. [Google Scholar] [CrossRef]
- Jóźwik, M.; Jóźwik, M.; Adamkiewicz, M.; Szymanowski, P.; Jóźwik, M. Budowa I Czynność Dna Miednicy U Kobiet Uaktualniony Przegląd Z Podkreśleniem Wpływu Porodu Drogami Natury. Dev. Period Med. 2013, XVII, 18–30. [Google Scholar]
- Raizada, V.; Mittal, R. Pelvic Floor Anatomy and Applied Physiology. Gastroenterol. Clin. N. Am. 2008, 37, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Chye Wah, Y.; Heng Hai, C. Obstetrics; IntechOpen: London, UK, 2017; pp. 157–190. [Google Scholar] [CrossRef]
- Bordoni, B.; Sugumar, K.; Leslie, S.W. Anatomy, Abdomen and Pelvis, Pelvic Floor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK482200/ (accessed on 17 November 2021).
- Oh, C.; Won, H.; David Kwon, C.; Chung, I. Morphologic Variations of the Umbilical Ring, Umbilical Ligaments and Ligamentum Teres Hepatis. Yonsei Med. J. 2008, 49, 1004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DeLancey, J. What’s New In The Functional Anatomy Of Pelvic Organ Prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, G.; Delorme, E.; Cosson, M.; Rubod, C. Cystocele and Functional Anatomy of the Pelvic Floor: Review and Update of the Various Theories. Int. Urogynecol. J. 2015, 27, 1297–1305. [Google Scholar] [CrossRef]
- Parks, A.; Porter, N.; Hardcastle, J. The syndrome of the descending perineum. Proc. R. Soc. Med. 1966, 59, 477–482. [Google Scholar] [CrossRef]
- Roch, M.; Gaudreault, N.; Cyr, M.; Venne, G.; Bureau, N.; Morin, M. The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review. Life 2021, 11, 900. [Google Scholar] [CrossRef]
- Ramin, A.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Fascial continuity of the pelvic floor with the abdominal and lumbar region. Pelviperineology 2016, 35, 3–6. [Google Scholar]
- Park, H.; Han, D. The Effect of the Correlation between the Contraction of the Pelvic Floor Muscles and Diaphragmatic Motion during Breathing. J. Phys. Ther. Sci. 2015, 27, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Soljanik, I.; Janssen, U.; May, F.; Fritsch, H.; Stief, C.; Weissenbacher, E.; Friese, K.; Lienemann, A. Functional Interactions Between The Fossa Ischioanalis, Levator Ani And Gluteus Maximus Muscles Of The Female Pelvic Floor: A Prospective Study In Nulliparous Women. Arch. Gynecol. Obstet. 2012, 286, 931–938. [Google Scholar] [CrossRef]
- Halski, T.; Ptaszkowski, K.; Słupska, L.; Dymarek, R.; Paprocka-Borowicz, M. Relationship between Lower Limb Position and Pelvic Floor Muscle Surface Electromyography Activity in Menopausal Women: A Prospective Observational Study. Clin. Interv. Aging 2017, 12, 75–83. [Google Scholar] [CrossRef]
- Shafik, A.; El Sibai, O.; Shafik, A.; Shafik, I. Contraction of Gluteal Maximus Muscle on Increase of Intra-Abdominal Pressure: Role in the Fecal Continence Mechanism. Surg. Innov. 2007, 14, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, D.; Stania, M.; Sobota, G.; Kwaśna, K.; Błaszczak, E.; Taradaj, J.; Juras, G. Impact Of Different Body Positions On Bioelectrical Activity Of The Pelvic Floor Muscles In Nulliparous Continent Women. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, R.; Hodges, P. Contraction of the Pelvic Floor Muscles during Abdominal Maneuvers. Arch. Phys. Med. Rehabil. 2001, 82, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Zanier, E. Anatomic Connections of the Diaphragm: Influence of Respiration on the Body System. J. Multidiscip. Healthc. 2013, 6, 281–291. [Google Scholar] [CrossRef]
- Hodges, P.; Sapsford, R.; Pengel, L. Postural and Respiratory Functions of the Pelvic Floor Muscles. Neurourol. Urodyn. 2007, 26, 362–371. [Google Scholar] [CrossRef]
- Kocjan, J.; Adamek, M.; Gzik-Zroska, B.; Czyżewski, D.; Rydel, M. Network of Breathing. Multifunctional Role of the Diaphragm: A Review. Adv. Respir. Med. 2017, 85, 224–232. [Google Scholar] [CrossRef]
- Krause, F.; Wilke, J.; Vogt, L.; Banzer, W. Intermuscular Force Transmission along Myofascial Chains: A Systematic Review. J. Anat. 2016, 228, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B. The Five Diaphragms In Osteopathic Manipulative Medicine: Neurological Relationships, Part 1. Cureus 2020, 12, e8697. [Google Scholar] [CrossRef]
- Maccioni, F. Functional Disorders Of The Ano-Rectal Compartment Of The Pelvic Floor: Clinical And Diagnostic Value of Dynamic MRI. Abdom. Imaging 2012, 38, 930–951. [Google Scholar] [CrossRef]
- Salvador, J.; Coutinho, M.; Venâncio, J.; Viamonte, B. Dynamic Magnetic Resonance Imaging of the Female Pelvic Floor—A Pictorial Review. Insights Imaging 2019, 10, 4. [Google Scholar] [CrossRef]
- Davis, K.; Kumar, D. Posterior Pelvic Floor Compartment Disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2005, 19, 941–958. [Google Scholar] [CrossRef]
- Padoa, A.; McLean, L.; Morin, M.; Vandyken, C. “The Overactive Pelvic Floor (OPF) and Sexual Dysfunction” Part 1: Pathophysiology of OPF and Its Impact on the Sexual Response. Sex Med. Rev. 2021, 9, 64–75. [Google Scholar] [CrossRef]
- Thibault-Gagnon, S.; Morin, M. Active And Passive Components Of Pelvic Floor Muscle Tone In Women With Provoked Vestibulodynia: A Perspective Based On A Review Of The Literature. J. Sex Med. 2015, 12, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Scoti, F.; Subramaniam, N.; Friedman, T.; Shek, K. Impact of Subsequent Pregnancies on Pelvic Floor Functional Anatomy. Int. Urogynecol. J. 2018, 29, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wen, Y.; Yu, X.; Polan, M. Elastin Metabolism In Pelvic Tissues: Is It Modulated By Reproductive Hormones? Am. J. Obstet. Gynecol. 2005, 192, 1605–1613. [Google Scholar] [CrossRef]
- Kim, S.; Harvey, M.; Johnston, S. A Review Of The Epidemiology And Pathophysiology Of Pelvic Floor Dysfunction: Do Racial Differences Matter? J. Obstet. Gynaecol. Can. 2005, 27, 251–259. [Google Scholar] [CrossRef]
- Dehghan, F.; Haerian, B.; Muniandy, S.; Yusof, A.; Dragoo, J.; Salleh, N. The Effect of Relaxin on the Musculoskeletal System. Scand. J. Med. Sci. Sports 2013, 24, e220–e229. [Google Scholar] [CrossRef]
- Morino, S.; Ishihara, M.; Umezaki, F.; Hatanaka, H.; Yamashita, M.; Aoyama, T. Pelvic Alignment Changes during the Perinatal Period. PLoS ONE 2019, 14, e0223776. [Google Scholar] [CrossRef]
- Ashton-Miller, J.; DeLancey, J. On The Biomechanics of Vaginal Birth and Common Sequelae. Annu. Rev. Biomed. Eng. 2009, 11, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Handa, V. Vaginal Childbirth and Pelvic Floor Disorders. Womens Health 2013, 9, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Parente, M.; Calvo, B.; Mascarenhas, T.; Natal Jorge, R. A Holistic View of the Effects of Episiotomy on Pelvic Floor. Int. J. Numer. Method. Biomed. Eng. 2017, 33, e2892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, X.; Chen, G.; Sun, X.; Wang, J. Does Diastasis Recti Abdominis Weaken Pelvic Floor Function? A Cross-Sectional Study. Int. Urogynecol. J. 2019, 31, 277–283. [Google Scholar] [CrossRef]
- Chen, G. Pelvic Floor Dysfunction in Aging Women. Taiwan J. Obstet. Gynecol. 2007, 46, 374–378. [Google Scholar] [CrossRef][Green Version]
- Jozwik, M.; Jozwik, M. The Physiological Basis of Pelvic Floor Exercises in the Treatment of Stress Urinary Incontinence. BJOG 1998, 105, 1046–1051. [Google Scholar] [CrossRef]
- Tinelli, A.; Malvasi, A.; Rahimi, S.; Negro, R.; Vergara, D.; Martignago, R.; Pellegrino, M.; Cavallotti, C. Age-Related Pelvic Floor Modifications And Prolapse Risk Factors In Postmenopausal Women. Menopause 2010, 17, 204–212. [Google Scholar] [CrossRef]
- Moalli, P.; Talarico, L.; Sung, V.; Klingensmith, W.; Shand, S.; Meyn, L.; Watkins, S. Impact of Menopause on Collagen Subtypes in the Arcus Tendineous Fasciae Pelvis. Am. J. Obstet. Gynecol. 2004, 190, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Micussi, M.; Freitas, R.; Angelo, P.; Soares, E.; Lemos, T.; Maranhão, T. Is There A Difference In The Electromyographic Activity Of The Pelvic Floor Muscles Across The Phases Of The Menstrual Cycle? J. Phys. Ther. Sci. 2015, 27, 2233–2237. [Google Scholar] [CrossRef][Green Version]
- Shaw, J.; Nygaard, I. Role of Chronic Exercise on Pelvic Floor Support and Function. Curr. Opin. Urol. 2017, 27, 257–261. [Google Scholar] [CrossRef][Green Version]
- Bø, K.; Nygaard, I. Is Physical Activity Good or Bad For the Female Pelvic Floor? A Narrative Review. Sports Med. 2019, 50, 471–484. [Google Scholar] [CrossRef]
- Eliasson, K.; Larsson, T.; Mattsson, E. Prevalence of Stress Incontinence in Nulliparous Elite Trampolinists. Scand. J. Med. Sci. Sports 2002, 12, 106–110. [Google Scholar] [CrossRef]
- Oliviera, M.; Varella, L.; Angelo, P.; Albuquerque, M.; Micussi, B. The Relationship between the Presence of Lower Urinary Tract Symptoms and Waist Circumference. Diabetes Metab. Syndr. Obes. 2016, 9, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Kim, S.; Choi, Y.; Jeon, M. Association between Metabolic Syndrome and Pelvic Floor Dysfunction in Middle-Aged To Older Korean Women. Am. J. Obstet. Gynecol. 2011, 205, e1–e71. [Google Scholar] [CrossRef] [PubMed]
- Micussi, M.; Freitas, R.; Angelo, P.; Soares, E.; Lemos, T.; Maranhão, T. Evaluation of the Relationship between the Pelvic Floor Muscles and Insulin Resistance. Diabetes Metab. Syndr. Obes. 2015, 8, 49–413. [Google Scholar] [CrossRef]
- Arikan, H.; Savci, S.; Calik-Kutukcu, E.; Vardar-Yagli, N.; Saglam, M.; Inal-Ince, D.; Coplu, L. The Relationship between Cough-Specific Quality Of Life and Abdominal Muscle Endurance, Fatigue, and Depression in Patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1829–1835. [Google Scholar] [PubMed]
- Mobley, D.; Baum, N. Smoking: It’s Impact on Urologic Health. Rev. Urol. 2015, 17, 220–225. [Google Scholar] [CrossRef]
- Alnaif, B.; Drutz, H. The Association of Smoking with Vaginal Flora, Urinary Tract Infection, Pelvic Floor Prolapse, and Post-Void Residual Volumes. J. Low. Genit. Tract. Dis. 2001, 5, 7–11. [Google Scholar] [CrossRef]
- Andiya, B.; Masoud, A.; Bijan, K.; Shabnam, S.; Mojgan, F. Systematic Review: The Role of Pelvic Floor Muscles Dysfunction in Constipation. J. Phys. Ther. 2015, 4, 177–182. [Google Scholar]
- Takano, S.; Sands, D. Influence Of Body Posture on Defecation: A Prospective Study of “The Thinker” Position. Tech. Coloproctol. 2015, 20, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. Dyssynergic Defecation and Biofeedback Therapy. Gastroenterol. Clin. N. Am. 2008, 37, 569–586. [Google Scholar] [CrossRef]
- Soligo, M.; Salvatore, S.; Emmanuel, A.; De Ponti, E.; Zoccatelli, M.; Cortese, M.; Milani, R. Patterns Of Constipation In Urogynecology: Clinical Importance And Pathophysiologic Insights. Am. J. Obstet. Gynecol. 2006, 195, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Barros-Neto, J.; Santos, T.; Cortes, M.; Jesus, R.; Freitas, M.; Kraychete, D. Constipation in Patients with Myofascial Pain Syndrome as Important Aspect for Clinical and Nutritional Treatment: A Case-Control Study. Rev. Nutr. 2017, 30, 567–581. [Google Scholar] [CrossRef]
- Carvalhais, A.; Araújo, J.; Natal Jorge, R.; Bø, K. Urinary Incontinence and Disordered Eating In Female Elite Athletes. J. Sci. Med. Sport 2019, 22, 140–144. [Google Scholar] [CrossRef]
- Abraham, S.; Luscombe, G.; Kellow, J. Pelvic Floor Dysfunction Predicts Abdominal Bloating and Distension in Eating Disorder Patients. Scand. J. Gastroenterol. 2012, 47, 625–631. [Google Scholar] [CrossRef]
- Skowron, K.; Kurnik-Łucka, M.; Dadański, E.; Bętkowska-Korpała, B.; Gil, K. Backstage Of Eating Disorder—About The Biological Mechanisms Behind The Symptoms Of Anorexia Nervosa. Nutrients 2020, 12, 2604. [Google Scholar] [CrossRef]
- Malik, M.; Stratton, J.; Sweeney, B. Rectal Prolapse Associated With Bulimia Nervosa. Dis. Colon. Rectum. 1997, 40, 1382–1385. [Google Scholar] [CrossRef]
- Norton, P. Pelvic Floor Disorders: The Role of Fascia and Ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef]
- Hastings, J.; Forster, J.; Witzeman, K. Joint Hypermobility among Female Patients Presenting With Chronic Myofascial Pelvic Pain. PM&R 2019, 11, 1193–1199. [Google Scholar] [CrossRef]
- Campeau, L.; Gorbachinsky, I.; Badlani, G.; Andersson, K. Pelvic Floor Disorders: Linking Genetic Risk Factors to Biochemical Changes. BJU Int. 2011, 108, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.; Heckman, S.; Qualls, C.; Muller, C.; Rogers, R. Pelvic Floor Disorders and Sexual Function in Gynecologic Cancer Survivors: A Cohort Study. Am. J. Obstet. Gynecol. 2010, 203, e1–e514. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Naik, R. Pelvic Floor Dysfunction and Radical Hysterectomy. Int. J. Gynecol. Cancer 2006, 16, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Crafoord, K.; Sydsjö, A.; Johansson, T.; Brynhildsen, J.; Kjølhede, P. Factors Associated With Symptoms of Pelvic Floor Dysfunction Six Years after Primary Operation of Genital Prolapse. Acta. Obstet. Gynecol. Scand. 2008, 87, 910–915. [Google Scholar] [CrossRef]
- Awad, E.; Ahmed, H.; Yousef, A.; Abbas, R. Efficacy Of Exercise On Pelvic Pain And Posture Associated With Endometriosis: Within Subject Design. J. Phys. Ther. Sci. 2017, 29, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Aredo, J.; Heyrana, K.; Karp, B.; Shah, J.; Stratton, P. Relating Chronic Pelvic Pain And Endometriosis To Signs Of Sensitization And Myofascial Pain And Dysfunction. Semin. Reprod. Med. 2017, 35, 088–097. [Google Scholar] [CrossRef]
- Taghavi, S.; Bazarganipour, F.; Allan, H.; Khashavi, Z.; Reisi, N.; Dosha, N.; Aghili, F.; Keramati, M.; Zahedi, S.; Aji-Ramkani, A. Pelvic Floor Dysfunction And Polycystic Ovary Syndrome. Hum. Fertil. 2017, 20, 262–267. [Google Scholar] [CrossRef]
- de Abreu, D.; Rodrigues, P.; Amaral Corrêa, L.; Lacombe, A.; Andreotti, D.; Nogueira, L. The Relationship between Urinary Incontinence, Pelvic Floor Muscle Strength and Lower Abdominal Muscle Activation among Women with Low Back Pain. Eur. J. Physiother. 2018, 21, 2–7. [Google Scholar] [CrossRef]
- Oleksy, Ł.; Mika, A.; Kielnar, R.; Grzegorczyk, J.; Marchewka, A.; Stolarczyk, A. The Influence Of Pelvis Reposition Exercises On Pelvic Floor Muscles Asymmetry A Randomized Prospective Study. Medicine 2019, 98, e13988. [Google Scholar] [CrossRef]
- Tamaki, T.; Oinuma, K.; Shiratsuchi, H.; Akita, K.; Iida, S. Hip Dysfunction-Related Urinary Incontinence: A Prospective Analysis of 189 Female Patients Undergoing Total Hip Arthroplasty. Int. J. Urol. 2014, 21, 729–731. [Google Scholar] [CrossRef]
- Stein, A.; Sauder, S.; Reale, J. The Role of Physical Therapy in Sexual Health in Men and Women: Evaluation And Treatment. Sex Med. Rev. 2019, 7, 46–56. [Google Scholar] [CrossRef]
- Saito, E.; Akashi, P.; Sacco, I. Global Body Posture Evaluation in Patients with Temporomandibular Joint Disorder. Clinics 2009, 64, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Vandyken, B.; Forget, M.; Vandyken, C. Association between Lumbopelvic Pain and Pelvic Floor Dysfunction in Women: A Cross Sectional Study. Musculoskelet. Sci. Pract. 2018, 34, 47–53. [Google Scholar] [CrossRef]
- López-Liria, R.; Varverde-Martínez, M.; Padilla-Góngora, D.; Rocamora-Pérez, P. Effectiveness of Physiotherapy Treatment for Urinary Incontinence in Women: A Systematic Review. J. Womens Health (Larchmt) 2019, 28, 490–501. [Google Scholar] [CrossRef]
- Mattox, T.; Lucente, V.; McIntyre, P.; Miklos, J.; Tomezsko, J. Abnormal Spinal Curvature and Its Relationship to Pelvic Organ Prolapse. Am. J. Obstet. Gynecol. 2000, 183, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- van Reijn-Baggen, D.; Han-Geurts, I.; Voorham-van der Zalm, P.; Pelger, R.; Hagenaars-van Miert, C.; Laan, E. Pelvic Floor Physical Therapy for Pelvic Floor Hypertonicity: A Systematic Review of Treatment Efficacy. Sex Med. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tullington, J.E.; Blecker, N. Pelvic Trauma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; Available online: https://www.ncbi.nlm.nih.gov/books/NBK556070/ (accessed on 7 December 2021).
- Piccione, F.; Maccarone, M.; Cortese, A.; Rocca, G.; Sansubrino, U.; Piran, G.; Masiero, S. Rehabilitative Management of Pelvic Fractures: A Literature-Based Update. Eur. J. Transl. Myol. 2021, 31, 9933. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.; Kołomańska-Bogucka, D.; Nowakowski, C.; Tim, S. Urinary Incontinence in Women: Modern Methods of Physiotherapy as a Support for Surgical Treatment or Independent Therapy. J. Clin. Med. 2020, 9, 1211. [Google Scholar] [CrossRef]
- Berghmans, B. Physiotherapy for pelvic pain and female sexual dysfunction: An untapped resource. Int. J. Urol. 2018, 29, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Torres, J.; McGeary, D.; Nagpal, A. Complementary and Alternative (CAM) Treatment Options for Women with Pelvic Pain. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zhang, N.; Carolan, A.; Tay, K.; Wolter, C. Evaluating The Prevalence Of Pelvic Floor Disorders In Women In Nonmetropolitan Communities. Female Pelvic. Med. Reconstr. Surg. 2020, 27, e295–e300. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tim, S.; Mazur-Bialy, A.I. The Most Common Functional Disorders and Factors Affecting Female Pelvic Floor. Life 2021, 11, 1397. https://doi.org/10.3390/life11121397
Tim S, Mazur-Bialy AI. The Most Common Functional Disorders and Factors Affecting Female Pelvic Floor. Life. 2021; 11(12):1397. https://doi.org/10.3390/life11121397
Chicago/Turabian StyleTim, Sabina, and Agnieszka I. Mazur-Bialy. 2021. "The Most Common Functional Disorders and Factors Affecting Female Pelvic Floor" Life 11, no. 12: 1397. https://doi.org/10.3390/life11121397
APA StyleTim, S., & Mazur-Bialy, A. I. (2021). The Most Common Functional Disorders and Factors Affecting Female Pelvic Floor. Life, 11(12), 1397. https://doi.org/10.3390/life11121397





