Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma-Generating Device
2.2. Assessment of Virucidal Activity in Dulbecco’s Modified Eagle’s Medium
2.2.1. Virus Isolates
2.2.2. Virus Detection
2.2.3. Determination of Ozone in DMEM
2.2.4. Experimental Setup for Viral Reduction Using CAP
2.3. Effect of CAP on Liquid Matrices
- Tap water (unbuffered control) was tested for pH and temperature.
- DMEM (nutrient-rich, buffered medium) was tested for pH, temperature, and glucose content (D-Glucose/D-Fructose Enzymatic test Kit no. 10139106035, R-Biopharm, Darmstadt, Germany).
- Salt water (the typical oyster-holding medium) was prepared by adding 38 g sea salt (Premium Reef Salt, ARKA Biotechnologie, Röthenbach, Germany) to 1 L distilled water. The average pH was 8.1, with a density of 1.022 g/cm3 at 25 °C. Typical mineral contents were potassium (430 mg/L) and magnesium (1300 mg/L). Nitrates and phosphates were not detectable. Salt water was tested for pH and temperature.
2.4. Effect of CAP on Oyster Slurry
2.5. Statistical Processing of Results
3. Results and Discussion
3.1. Setup for Generating CAP
3.2. Virus Reduction by Exposure to CAP
3.3. Changes in DMEM after CAP Exposure
3.4. Changes in Tap Water and Salt Water after CAP Exposure
3.5. Changes in Oyster Slurry after CAP Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Puligundla, P.; Mok, C. Corona discharge plasma jet for inactivation of Escherichia coli O157:H157 and Listeria monocytogenes on inoculated pork and its impact on meat quality attributes. Ann. Microbiol. 2016, 66, 685–694. [Google Scholar] [CrossRef]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Int. Food Res. J. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azharonok, V.; Krat’ko, L.; Nekrashevich, Y.I.; Filatova, I.; Mel’nikova, L.; Dudchik, N.; Yanetskaya, S.; Bologa, M. Bactericidal action of the plasma of high-frequency capacitive and barrier discharges on microorganisms. J. Eng. Phys. Thermophys. 2009, 82, 419–426. [Google Scholar] [CrossRef]
- Liu, F.; Sun, P.; Bai, N.; Tian, Y.; Zhou, H.; Wei, S.; Zhou, Y.; Zhang, J.; Zhu, W.; Becker, K. Inactivation of bacteria in an aqueous environment by a direct current, cold atmospheric pressure air plasma micro jet. Plasma Process. Polym. 2010, 7, 231–236. [Google Scholar] [CrossRef]
- Ahlfeld, B.; Li, Y.; Boulaaba, A.; Binder, A.; Schotte, U.; Zimmermann, J.L.; Morfill, G.; Klein, G. Inactivation of a Foodborne Norovirus Outbreak Strain with Nonthermal Atmospheric Pressure Plasma. mBio 2015, 6, e02300-14. [Google Scholar] [CrossRef] [Green Version]
- Bunz, O.; Mese, K.; Zhang, W.; Piwowarczyk, A.; Ehrhardt, A. Effect of cold atmospheric plasma (CAP) on human adenoviruses is adenovirus type-dependent. PLoS ONE 2018, 13, e0202352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Tian, Y.; Zhou, H.; Li, Y.; Zhang, Z.; Jiang, B.; Yang, B.; Zhang, J.; Fang, J. Inactivation efficacy of non-thermal plasma activated solutions against Newcastle disease virus. Appl. Environ. Microbiol. 2018, 84, e02836-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muranyi, P.; Wunderlich, J.; Heise, M. Sterilization efficiency of a cascade dielectric barrier discharge. J. Appl. Microbiol. 2007, 103, 1535–1544. [Google Scholar] [CrossRef]
- Yun, H.; Kim, B.; Jung, S.; Kruk, Z.A.; Kim, D.B.; Choe, W.; Jo, C. Inactivation of Listeria monocytogenes inoculated on disposable plastic tray, aluminium foil and paper cup by atmospheric pressure plasma. Food Control 2010, 21, 1182–1186. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankay, S.K.; Segat, A.; Ishikawa, K. Cold Plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Bauer, A.; Ni, Y.; Bauer, S.; Paulsen, P.; Modic, M.; Walsh, J.L.; Smulders, F.J.M. The effects of atmospheric pressure cold plasma treatment on microbiological, physical-chemical and sensory characteristics of vacuum packaged beef loin. Meat Sci. 2017, 128, 77–87. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonςa, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology. Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ge, L.; Huang, Y.; Wang, Y.; Wang, Y.; Lai, H.; Wang, Y.; Zhu, Y.; Zhang, J. Impact of cold plasma processing on quality parameters of packaged fermented vegetable (radish paocai) in comparison with pasteurization processing: Insight into safety and storage stability of products. Innov. Food Sci. Emerg. Technol. 2020, 60, 102300. [Google Scholar] [CrossRef]
- Bak, K.H.; Csadek, I.; Paulsen, P.; Smulders, F.J.M. Application of atmospheric pressure cold plasma (ACP) on meat and meat products II. Effects on the sensory quality with special focus on meat colour and lipid oxidation—Part 2. Fleischwirtschaft 2021, 101, 100–105. [Google Scholar]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold atmospheric pressure plasma treatment of ready-to-eat-meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Shahidi, F.; Pegg, R.B. Nitrite-free meat curing systems: Update and review. Food Chem. 1992, 43, 185–191. [Google Scholar] [CrossRef]
- Eskandari, M.H.; Hosseunpur, S.; Mesbahi, R.; Shekarforoush, S. New composite nitrite-free and low-nitrite meat-curing systems using natural colorants. Food Sci. Nutr. 2013, 1, 392–401. [Google Scholar] [CrossRef]
- De González, M.T.; Osburn, W.N.; Hardin, M.D.; Longnecker, M.; Garg, H.K.; Bryan, N.S.; Keeton, J.T. A Survey of Nitrate and Nitrite Concentrations in Conventional and Organic-Labeled Raw Vegetables at Retail. J. Food Sci. 2015, 80, 942–949. [Google Scholar] [CrossRef]
- Chetty, A.A.; Prasad, S. Flow injection analysis of nitrate and nitrite in commercial baby foods. Food Chem. 2015, 197, 503–508. [Google Scholar] [CrossRef]
- Class Bivalvia (Pelecypoda). The Phylum Mollusca. Available online: http://www.manandmollusc.net/advanced_introduction/moll101pelecypoda.html (accessed on 12 October 2021).
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Wikswo, M.E.; Pringle, K.; Gould, L.H.; Parashar, U.D. Vital Signs: Foodborne Norovirus Outbreaks—United States, 2009–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 491–495. [Google Scholar]
- Koopmans, M. Foodborne Viruses and Seafood Safety in an Environmental Health Perspective. Epidemiology 2009, 20, S233. [Google Scholar] [CrossRef]
- Mizan, F.R.; Jahid, I.K.; Ha, S.D. Microbial biofilms in seafood: A food-hygiene challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.R.; Arias, C.; Dominguez, A.; Bartolomé, R.; Muntada, J.M. Foodborne outbreak of gastroenteritis due to Norovirus and Vibrio parahaemolyticus. Epidemol. Infect. 2008, 137, 626–629. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Alfano-Sobsey, M.; Davies, M.; Ledford, S.I. Norovirus Outbreak associated with undercooked oysters and secondary household transmission. Epidemiol. Infect. 2012, 140, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brucker, R.; Bui, T.; Kwan-Gett, T.; Stewart, L. Centers for Disease Control and Prevention, Notes from the Field: Norovirus Infections Associated with Frozen Raw Oysters, Washington. Morb. Mortal. Wkly. Rep. 2011, 307, 1480. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtmL/mm6106a3.htm (accessed on 29 September 2021).
- Chironna, M.; Germinario, C.; De Medici, D.; Fiore, A.; Di Pasquale, S.; Quartoa, M.; Barbuti, S. Detection of hepatitis A virus in mussels from different sources marketed in Puglia region (South Italy). Int. J. Food Microbiol. 2002, 75, 11–18. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Increase in Vibrio parahaemolyticus Illnesses Associated with Consumption of Shellfish from Several Atlantic Coast Harvest Areas, United States. 2013. Available online: https://www.cdc.gov/vibrio/investigations/vibriop-09-13/index.html (accessed on 12 October 2021).
- LeGuyader, F.S.; Miura, T.; Atmar, R.L. Prevalence and control of Norovirus and hepatitis A virus in shellfish. In Food Safety Assurance and Veterinary Public Health, Vol. 6: Foodborne Viruses and Prions and Their Significance for Public Health; Smulders, F.J.M., Nørrung, B., Budka, H., Eds.; ECVPH (European College of Veterinary Public Health/European Food Safety Authority), Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; Volume 6, pp. 137–168. [Google Scholar]
- Centers for Disease Control and Prevention. 2013. Available online: http://www.cdc.gov/drugresistance/ (accessed on 20 October 2016).
- Centers for Disease Control and Prevention. National Surveillance of Bacterial Foodborne Illnesses (Enteric Diseases). 2014. Available online: http://www.cdc.gov/nationalsurveillance/botulism-surveillance.html (accessed on 12 October 2021).
- Cook, N.; Knight, A.; Richards, G.P. Persistence and elimination of human norovirus in food and on food contact surfaces: A critical review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef] [PubMed]
- Porsbo, L.J.; Jensen, T.; Nørrung, B. Occurrence and control of viruses in food handling environments and in ready-to-eat foods. In Food Safety Assurance and Veterinary Public Health, Vol. 6: Foodborne Viruses and Prions and Their Significance for Public Health; Smulders, F.J.M., Nørrung, B., Budka, H., Eds.; ECVPH (European College of Veterinary Public Health/European Food Safety Authority), Academic Publishers: Wageningen, The Netherlands, 2013; pp. 191–200. [Google Scholar]
- Zuber, S.C.; Butot, S.; Baert, L. Effects of treatments used in food processing on viruses. In Food Safety Assurance and Veterinary Public Health, Vol. 6: Foodborne Viruses and Prions and Their Significance for Public Health; Smulders, F.J.M., Nørrung, B., Budka, H., Eds.; ECVPH (European College of Veterinary Public Health/European Food Safety Authority), Academic Publishers: Wageningen, The Netherlands, 2013; pp. 113–136. [Google Scholar]
- Choi, M.S.; Jeon, E.B.; Kim, J.; Chou, E.H.; Lim, J.S.; Choi, J.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Virucidal Effects of Dielectric Barrier Discharge Plasma on Human Norovirus Infectivity in Fresh Oysters (Crassostrea gigas). Foods 2020, 9, 1731. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and Invertebrate Evolution. J. Biol. Chem. 2001, 19, 15563–15566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, D. The Many Colors of Blood. ChemMatters 2010, 28, 5–7. [Google Scholar]
- Verplaetse, J.; Tornout, P.; Defreyn, G.; Witters, R.; Lontie, R. The Reaction of Nitrogen Monoxide and of Nitrite with Deoxyhaemocyanin and Methaemocyanin of Helix pomatia. Eur. J. Biochem. 1979, 95, 327–331. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Chern, S.; Ye, X.; Liu, D.; Ding, T. Application of a Dielectric Barrier Discharge Atmospheric Cold Plasma (Dbd-Acp) for Escherichia Coli Inactivation in Apple Juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Makari, M.; Hojjati, M.; SHahbazi, S.; Askari, H.W.; Chunpeng, H. Elimination of Aspergillus flavus from Pistachio Nuts with Dielectric Barrier Discharge (DBD) Cold Plasma and its Impacts on Biochemical Indices. J. Food Qual. 2021, 2021, 9968711. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Verardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food. Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Murtaza, A.; Jun-Hu, C.; Da-Wen, S. Effects of dielectric barrier discharge cold plasma treatments on degradation of anilazine fungicide and quality of tomato (Lycopersicon esculentum Mill) juice. Int. J. Food Sci. 2021, 56, 69–75. [Google Scholar] [CrossRef]
- Albertos, I.; Martín-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Álvarez, C.; Rico, D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Umari, M.; Jabbar, S.; Nasiru, M.M.; Sultana, T.; Senan, A.; Awad, F.N.; Hong, Z. Exploring the potential of high-voltage electric field cold plasma (HVCP) using a dielectric barrier discharge (DBD) as a plasma source on the quality parameters of carrot juice. Antibiotics 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadnia, M.; Sadeghi, M.; Abbaszadeh, R.; Reza, H.; Marzdashti, G. Decontamination of whole strawberry via dielectric barrier discharge cold plasma and effects on quality attributes. J. Food Process. Preserv. 2021, 45, e15019. [Google Scholar] [CrossRef]
- Gao, Y.; Yeh, H.-Y.; Bowker, B.; Zhuang, H. Effects of different antioxidants on quality of meat patties treated with in-packages cold plasma. Innov. Food Sci. Emerg. Technol. 2021, 70, 102690. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Commun. 2002, L327, 1–27. [Google Scholar]
- Ni, Y.; Lynch, M.J.; Modic, M.; Whalley, R.D.; Walsh, J.L. A solar powered handheld plasma source for microbial decontamination applications. J. Phys. D Appl. Phys. 2016, 49, 355203. [Google Scholar] [CrossRef]
- Amtliche Sammlung von Untersuchungsverfahren nach §64 LFGB, Bestimmung des Rohproteingehaltes in Fleisch und Fleischerzeugnissen; Titrimetrisches Verfahren nach Kjeldahl; Referenzverfahren, 2014, L. 06.00-7; Beuth: Berlin. Available online: https://www.hygienicum.at/wp-content/uploads/2021/06/2021_Akkreditierungsumfang_Hygienicum_.pdf (accessed on 27 November 2021).
- Schmidt, B.; Schwedt, G. HPLC method for determining nitrates in plant-based foods in comparison to photo- and potentiometric analysis. Dtsch. Lebensm.-Rundsch. 1984, 5, 137–140. [Google Scholar]
- Csadek, I.; Paulsen, P.; Bak, K.H.; Smulders, F.J.M. Application of atmospheric pressure cold plasma (ACP) on meat and meat products. Fleischwirtschaft 2021, 101, 96–104. [Google Scholar]
- Fuller, J.A.; Njenga, M.K.; Bigogo, G.; Aura, B.; Ope, M.O.; Nderitu, L.; Wakhule, L.; Erdman, D.D.; Breiman, R.F.; Feikin, D.R. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J. Med. Virol. 2013, 85, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; van der Ploeg, T.C.; Smeets, L.C.; de Groot, R.; Versteegh, F.G.A.; Hartwig, N.G. Pitfalls in interpretation of CT-values of RT-PCR in children with acute respiratory tract infections. J. Clin. Virol. 2017, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Park, S.Y.; Choe, W.; Ha, S.D. Inactivation of murine norovirus-1 and hepatitis A virus on fresh meats by amtopsheric pressure plasma jets. Food Res. Int. 2015, 76, 342–347. [Google Scholar] [CrossRef] [PubMed]
- .Guzel-Seydima, Z.P.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. Lebensm. Wiss. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Filipic, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetic, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Schlüter, O.C.; Cullen, P.J. Cold Plasma in Food and Agriculture. Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-801365-0. [Google Scholar]
- Choi, M.-S.; Kim, J.Y.; Jeon, E.B.; Park, S.Y. Antibacterial activity of dielectric barrier discharge plasma against main food-borne bacteria in suspensions. Korean J. Fish. Aquat. Sci. 2019, 52, 617–624. [Google Scholar] [CrossRef]
- Pankaj, S.; Wan, Z.; Keener, K.M. Effects of Cold Plasma on Food Quality: A Review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gstraunthaler, G.; Lindl, T. Zell und Gewebekultur. Allgemeine Grundlagen und Spezielle Anwendungen; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Yu, J.-J.; Ji, H.; Chen, Y.; Zhang, Y.-f.; Zheng, X.-C.; Li, S.-H.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020, 158, 1194–1203. [Google Scholar] [CrossRef]
- Ji, H.; Tang, X.; Li, L.; Peng, S.; Gao, C.; Chen, Y. Improved physicochemical properties of peanut protein isolate glycated by atmospheric pressure cold plasma (ACP) treatment. Food Hydrocoll. 2020, 109, 106124. [Google Scholar] [CrossRef]
- Boulais, M.; Chenevert, K.J.; Demey, A.T.; Darrow, E.S.; Robison, M.R.; Roberts, J.P.; Volety, A. Oyster reproduction is compromised by acidification experienced seasonally in coastal regions. Sci. Rep. 2017, 7, 13276. [Google Scholar] [CrossRef] [Green Version]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
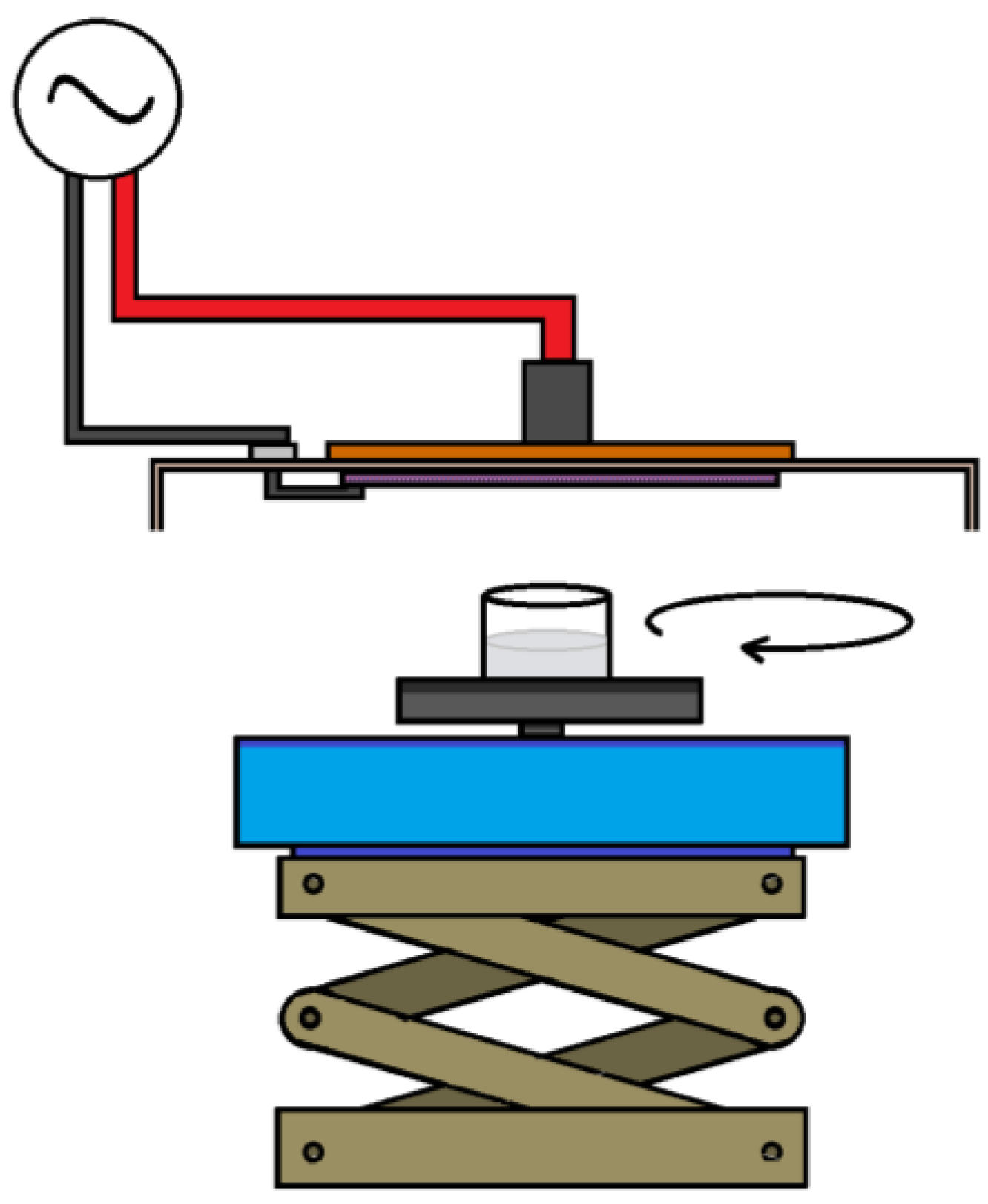
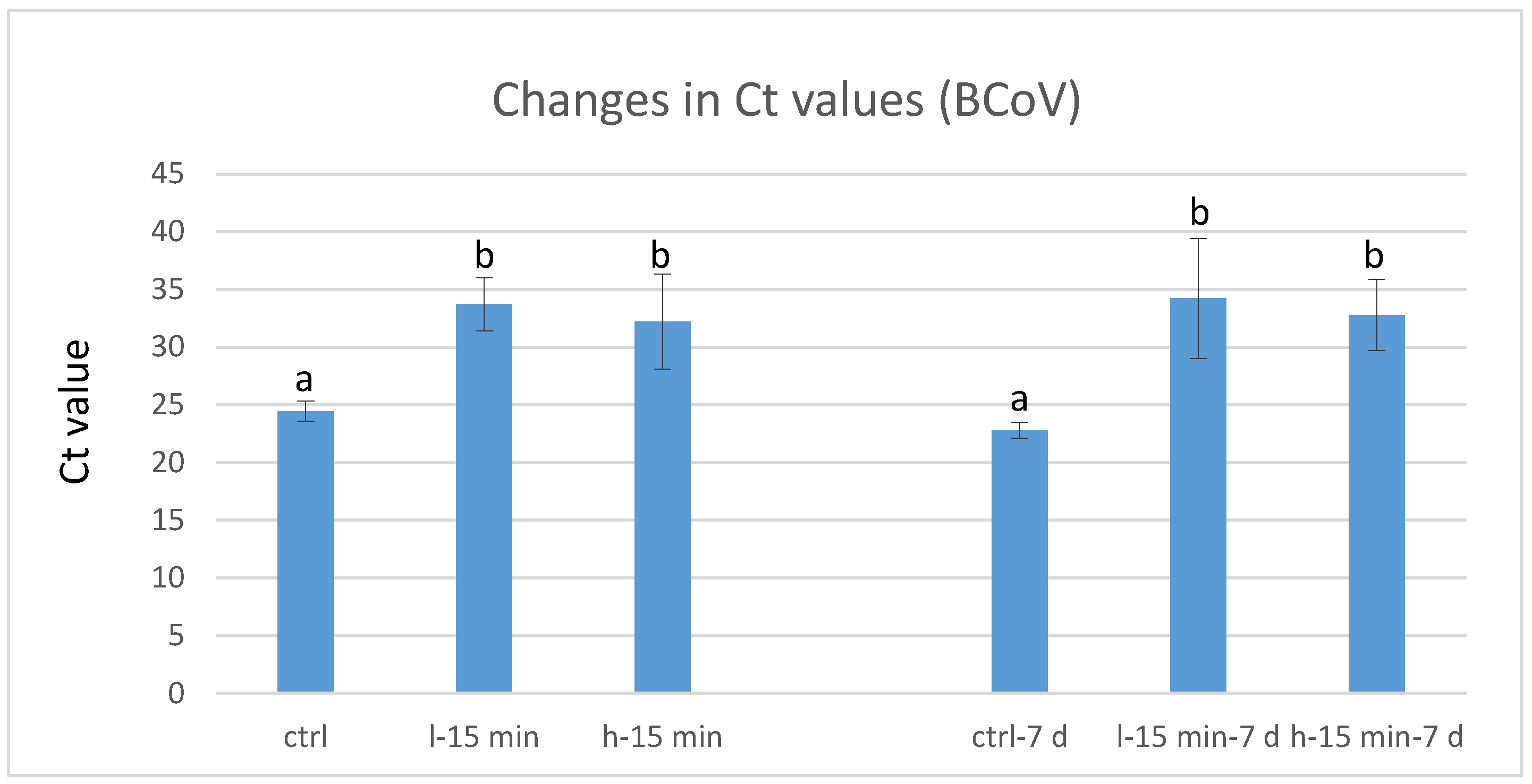

| Power | Power Input (Pin) [W] | Output Voltage (Vout) [kV] | Dissipated Power (Pout) [W] | Power Density [W/cm2] |
|---|---|---|---|---|
| Low | 20.70 | 8.16 | 17.87 | 0.48 |
| High | 29.90 | 9.44 | 25.38 | 0.67 |
| Minutes | Temperature Increase in °C | pH Increase | ||
|---|---|---|---|---|
| Low Power | High Power | Low Power | High Power | |
| 0 to 5 | 8.6 ± 1.8 | 8.5 ± 3.1 a | 0.36 ± 0.27 | 0.24 ± 0.29 a |
| 0 to 10 | 8.8 ± 1.8 | 11.4 ± 2.1 b | 0.34 ± 0.31 | 0.46 ± 0.35 b |
| 0 to 15 | 8.7 ± 2.3 | 11.9 ± 2.1 b | 0.29 ± 0.52 | 0.57 ± 0.36 b |
| Minutes | Temperature Increase in °C | pH Decrease | ||
|---|---|---|---|---|
| Low Power | High Power | Low Power | High Power | |
| 0 to 5 | 2.8 ± 1.2 a | 4.2 ± 1.1 a | 0.92 ± 0.08 a | 0.59 ± 0.28 a |
| 0 to 10 | 4.4 ± 0.3 b | 3.9 ± 0.9 a | 1.28 ± 0.02 a | 4.67 ± 0.81 b |
| 0 to 15 | 6.2 ± 0.2 b | 7.0 ± 2.3 b | 5.19 ± 0.55 b | 5.82 ± 1.87 b |
| Minutes | Temperature Increase in °C | pH Decrease | ||
|---|---|---|---|---|
| Low Power | High Power | Low Power | High Power | |
| 0 to 5 | 6.5 ± 2.2 a | 9.2 ± 3.1 a | 0.09 ± 0.03 a | 0.16 ± 0.02 a |
| 0 to 10 | 8.1 ± 2.8 a,b | 11.0 ± 2.8 a,b | 0.11 ± 0.06 a | 0.17 ± 0.03 a |
| 0 to 15 | 9.2 ± 2.3 b | 13.6 ± 2.9 b | 0.24 ± 0.02 b | 0.21 ± 0.02 b |
| mg/kg Fresh Matter | N (Kjeldahl Method) | N from Nitrate | N from Nitrite |
|---|---|---|---|
| Controls | 13,520 ± 679 a | 705 ± 37 a | 297 ± 23 a |
| Low power | |||
| 5 min | 13,670 ± 1273 a | 724 ± 24 a | 304 ± 36 a |
| 10 min | 14,015 ± 1768 b | 872 ± 16 b | 297 ± 23 a |
| 15 min | 14,130 ± 424 b | 1019 ± 9 c | 308 ± 14 a |
| High power | |||
| 5 min | 14,225 ± 212 c | 764 ± 25 d | 284 ± 20 a |
| 10 min | 14,210 ± 424 c | 1020 ± 19 e | 309 ± 39 a |
| 15 min | 14,765 ± 248 d | 1095 ± 36 e | 332 ± 21 b |
| Sample | Expressed as KNO3 | Expressed as NaNO2 | Expressed as N from KNO3 | Expressed as N from NaNO2 |
|---|---|---|---|---|
| Controls | 441.0 ± 44.2 | 1293.0 ± 20.0 | 61.0 ± 6.1 | 262.0 ± 4.1 |
| KNO3 addition (calculated) | 1000.0 | - | 138.6 | - |
| Total NO3 expected | 1441.0 | - | 199.6 | - |
| 15 min after KNO3 addition | 973.0 ± 3.6 | 37.0 ± 17.7 | 135.0 ± 0.5 | 7.0 ± 3.6 |
| 24 h after KNO3 addition | 1097.0 ± 28.0 | Not detected | 152.0 ± 3.9 | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csadek, I.; Paulsen, P.; Weidinger, P.; Bak, K.H.; Bauer, S.; Pilz, B.; Nowotny, N.; Smulders, F.J.M. Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment. Life 2021, 11, 1333. https://doi.org/10.3390/life11121333
Csadek I, Paulsen P, Weidinger P, Bak KH, Bauer S, Pilz B, Nowotny N, Smulders FJM. Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment. Life. 2021; 11(12):1333. https://doi.org/10.3390/life11121333
Chicago/Turabian StyleCsadek, Isabella, Peter Paulsen, Pia Weidinger, Kathrine H. Bak, Susanne Bauer, Brigitte Pilz, Norbert Nowotny, and Frans J. M. Smulders. 2021. "Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment" Life 11, no. 12: 1333. https://doi.org/10.3390/life11121333
APA StyleCsadek, I., Paulsen, P., Weidinger, P., Bak, K. H., Bauer, S., Pilz, B., Nowotny, N., & Smulders, F. J. M. (2021). Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment. Life, 11(12), 1333. https://doi.org/10.3390/life11121333







