Potential Biomarkers of Metastasizing Paragangliomas and Pheochromocytomas
Abstract
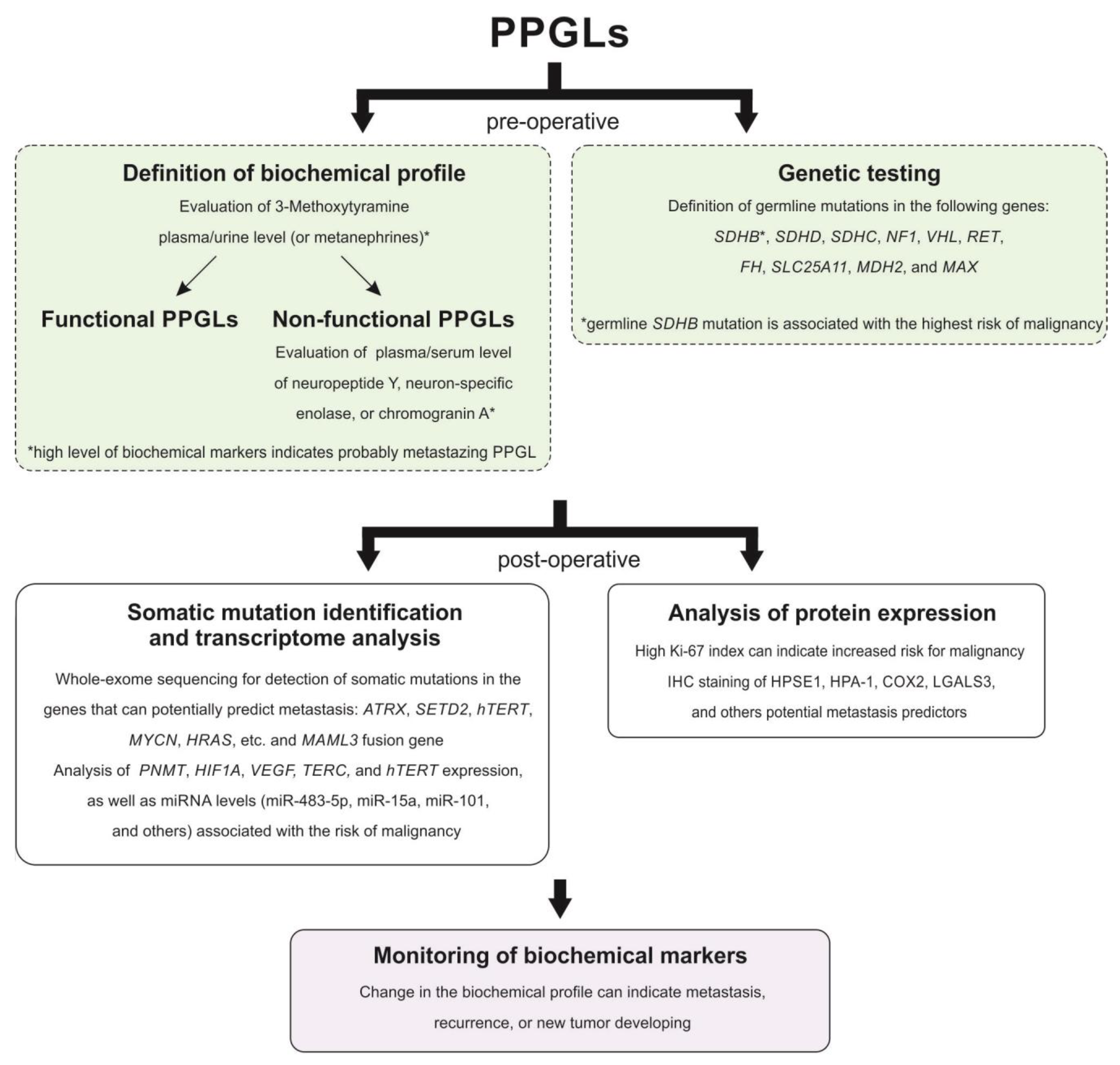
:1. Introduction
2. Definition, Localization, and Distribution
3. Metastatic Disease
3.1. Clinical Characteristics
3.2. Biochemical Markers
3.3. Genetic Markers
3.3.1. Mutations and DNA Methylation
3.3.2. Transcriptome Alterations
3.4. Histopathological Markers
3.4.1. Grading Systems
3.4.2. Immunoreactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J.; International Agency for Research on Cancer. WHO Classification of Head and Neck Tumours; WHO: Geneva, Switzerland, 2017.
- Lloyd, R.V. Endocrine Pathology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Barnes, L.; Universitäts-Spital, Z.; Department, P.; World Health Organization; International Agency for Research on cancer. Pathology and Genetics of Head and Neck Tumours; WHO: Geneva, Switzerland, 2017.
- Klein, R.D.; Jin, L.; Rumilla, K.; Young, W.F., Jr.; Lloyd, R.V. Germline SDHB mutations are common in patients with apparently sporadic sympathetic paragangliomas. Diagn. Mol. Pathol. 2008, 17, 94–100. [Google Scholar] [CrossRef]
- Pellitteri, P.K.; Rinaldo, A.; Myssiorek, D.; Gary Jackson, C.; Bradley, P.J.; Devaney, K.O.; Shaha, A.R.; Netterville, J.L.; Manni, J.J.; Ferlito, A. Paragangliomas of the head and neck. Oral Oncol. 2004, 40, 563–575. [Google Scholar] [CrossRef]
- van Duinen, N.; Corssmit, E.P.; de Jong, W.H.; Brookman, D.; Kema, I.P.; Romijn, J.A. Plasma levels of free metanephrines and 3-methoxytyramine indicate a higher number of biochemically active HNPGL than 24-h urinary excretion rates of catecholamines and metabolites. Eur. J. Endocrinol. 2013, 169, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, D.B.; Lippert, D.; Geer, C.P.; Edwards, H.D.; Russell, G.B.; Rees, C.J.; Browne, J.D. Clinical, histopathologic, and radiographic indicators of malignancy in head and neck paragangliomas. Otolaryngol. Head Neck Surg. 2010, 143, 531–537. [Google Scholar] [CrossRef]
- Mediouni, A.; Ammari, S.; Wassef, M.; Gimenez-Roqueplo, A.P.; Laredo, J.D.; Duet, M.; Tran Ba Huy, P.; Oker, N. Malignant head/neck paragangliomas. Comparative study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, R.V.; Or, K.G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Lee, J.H.; Barich, F.; Karnell, L.H.; Robinson, R.A.; Zhen, W.K.; Gantz, B.J.; Hoffman, H.T.; American College of Surgeons Commission on Cancer; American Cancer Society. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer 2002, 94, 730–737. [Google Scholar] [CrossRef]
- Rodriguez-Cuevas, S.; Lopez-Garza, J.; Labastida-Almendaro, S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck 1998, 20, 374–378. [Google Scholar] [CrossRef]
- Williams, M.D. Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics. Head Neck Pathol. 2017, 11, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliulo, G.; Zardo, F.; Varacalli, S.; D’Amico, R. Multiple paragangliomas of the head and neck. Otorrinolaringol. Ibero Am. 2003, 30, 31–38. [Google Scholar]
- Zanoletti, E.; Mazzoni, A. Vagal paraganglioma. Skull Base 2006, 16, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, O.; Young, W.F., Jr.; Iniguez-Ariza, N.M.; Kittah, N.E.; Gruber, L.; Bancos, C.; Tamhane, S.; Bancos, I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J. Clin. Endocrinol. Metab. 2017, 102, 3296–3305. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Harris, A.E.; Bell, W.R. Metastatic intravagal paraganglioma. Case report and review of the literature. Am. J. Med. 1985, 78, 1017–1024. [Google Scholar] [CrossRef]
- Martin, C.E.; Rosenfeld, L.; McSwain, B. Carotid body tumors: A 16-year follow-up of seven malignant cases. South Med. J. 1973, 66, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Manolidis, S.; Shohet, J.A.; Jackson, C.G.; Glasscock, M.E., 3rd. Malignant glomus tumors. Laryngoscope 1999, 109, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Moskovic, D.J.; Smolarz, J.R.; Stanley, D.; Jimenez, C.; Williams, M.D.; Hanna, E.Y.; Kupferman, M.E. Malignant head and neck paragangliomas: Is there an optimal treatment strategy? Head Neck Oncol. 2010, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Sethi, R.V.; Sethi, R.K.; Herr, M.W.; Deschler, D.G. Malignant head and neck paragangliomas: Treatment efficacy and prognostic indicators. Am. J. Otolaryngol. 2013, 34, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pamporaki, C.; Fliedner, S.M.J.; Timmers, H.J.L.M.; Nölting, S.; Beuschlein, F.; Prejbisz, A.; Remde, H.; Robledo, M.; Bornstein, S.R.; et al. Metastatic pheochromocytoma and paraganglioma: Signs and symptoms related to catecholamine secretion. Discov. Oncol. 2021, 12, 9. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Solorzano, C.C. Pheochromocytoma and Paraganglioma: Diagnosis, Genetics, and Treatment. Surg. Oncol. Clin. N. Am. 2016, 25, 119–138. [Google Scholar] [CrossRef]
- Abdel-Aziz, T.; Chung, T.-T.; Bomanji, J.; Gaze, M.; Kurzawinski, T. Patterns of recurrence, response to treatment and mortality in patients with malignant phaeochromocytomas and paragangliomas—A single centre experience. Endocr. Abstr. 2017. [Google Scholar] [CrossRef]
- Contrera, K.J.; Yong, V.; Reddy, C.A.; Liu, S.W.; Lorenz, R.R. Recurrence and Progression of Head and Neck Paragangliomas after Treatment. Otolaryngol. Head Neck Surg. 2020, 162, 504–511. [Google Scholar] [CrossRef]
- Khadilkar, K.; Sarathi, V.; Kasaliwal, R.; Pandit, R.; Goroshi, M.; Malhotra, G.; Dalvi, A.; Bakshi, G.; Bhansali, A.; Rajput, R.; et al. Predictors of malignancy in patients with pheochromocytomas/paragangliomas: Asian Indian experience. Endocr. Connect 2016, 5, 89–97. [Google Scholar] [CrossRef] [Green Version]
- de Wailly, P.; Oragano, L.; Rade, F.; Beaulieu, A.; Arnault, V.; Levillain, P.; Kraimps, J.L. Malignant pheochromocytoma: New malignancy criteria. Langenbecks Arch Surg. 2012, 397, 239–246. [Google Scholar] [CrossRef]
- Ohji, H.; Sasagawa, I.; Iciyanagi, O.; Suzuki, Y.; Nakada, T. Tumour angiogenesis and Ki-67 expression in phaeochromocytoma. BJU Int. 2001, 87, 381–385. [Google Scholar] [CrossRef] [PubMed]
- van der Harst, E.; Bruining, H.A.; Jaap Bonjer, H.; van der Ham, F.; Dinjens, W.N.; Lamberts, S.W.; de Herder, W.W.; Koper, J.W.; Stijnen, T.; Proye, C.; et al. Proliferative index in phaeochromocytomas: Does it predict the occurrence of metastases? J. Pathol. 2000, 191, 175–180. [Google Scholar] [CrossRef]
- Thompson, L.D. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: A clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, J.H.; Hong, A.R.; Seong, M.W.; Lee, K.E.; Kim, S.J.; Kim, S.W.; Shin, C.S.; Kim, S.Y. Disentangling of Malignancy from Benign Pheochromocytomas/Paragangliomas. PLoS ONE 2016, 11, e0168413. [Google Scholar] [CrossRef]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr.; Endocrine, S. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef] [PubMed]
- Plouin, P.F.; Amar, L.; Dekkers, O.M.; Fassnacht, M.; Gimenez-Roqueplo, A.P.; Lenders, J.W.; Lussey-Lepoutre, C.; Steichen, O.; Guideline Working, G. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 2016, 174, G1–G10. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.; Zhu, Y.; Wang, X.; Wu, Y.; Zhou, W.; Jin, X.; Zhang, R.; Sun, F.; Kasoma, Z.; Shen, Z. Predictive factors for malignant pheochromocytoma: Analysis of 136 patients. J. Urol. 2011, 185, 1583–1590. [Google Scholar] [CrossRef]
- Amar, L.; Peyrard, S.; Rossignol, P.; Zinzindohoue, F.; Gimenez-Roqueplo, A.P.; Plouin, P.F. Changes in urinary total metanephrine excretion in recurrent and malignant pheochromocytomas and secreting paragangliomas. Ann. N. Y. Acad. Sci. 2006, 1073, 383–391. [Google Scholar] [CrossRef]
- Gupta, P.; Khurana, M.L.; Khadgawat, R.; Bal, C.S.; Kumar, G.; Sharma, S.C.; Tandon, N. Plasma free metanephrine, normetanephrine, and 3-methoxytyramine for the diagnosis of pheochromocytoma/paraganglioma. Indian J. Endocrinol. Metab. 2015, 19, 633–638. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Lenders, J.W.; Siegert, G.; Bornstein, S.R.; Friberg, P.; Milosevic, D.; Mannelli, M.; Linehan, W.M.; Adams, K.; Timmers, H.J.; et al. Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur. J. Cancer 2012, 48, 1739–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, H.J.; Kozupa, A.; Eisenhofer, G.; Raygada, M.; Adams, K.T.; Solis, D.; Lenders, J.W.; Pacak, K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, S.H.; Chan, S.P.; Ananda, V.; Rajasingam, V. Dopamine-secreting phaeochromocytomas and paragangliomas: Clinical features and management. Singap. Med. J. 2010, 51, e89–e93. [Google Scholar]
- Marek, J.; Kopecka, J.; Musilova, J.; Horky, K.; Petrasek, J. New aspects and possibilities in the diagnosis of pheochromocytoma. Cas Lek Cesk 1989, 128, 907–914. [Google Scholar]
- John, H.; Ziegler, W.H.; Hauri, D.; Jaeger, P. Pheochromocytomas: Can malignant potential be predicted? Urology 1999, 53, 679–683. [Google Scholar] [CrossRef]
- Tippett, P.A.; McEwan, A.J.; Ackery, D.M. A re-evaluation of dopamine excretion in phaeochromocytoma. Clin. Endocrinol. 1986, 25, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Proye, C.; Vix, M.; Goropoulos, A.; Kerlo, P.; Lecomte-Houcke, M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J. Endocrinol. Investig. 1992, 15, 651–663. [Google Scholar] [CrossRef]
- Januszewicz, W.; Wocial, B.; Januszewicz, A.; Gryglas, P.; Prejbisz, A. Dopamine and dopa urinary excretion in patients with pheochromocytoma—Diagnostic implications. Blood Press 2001, 10, 212–216. [Google Scholar] [CrossRef]
- Tippett, P.A.; West, R.S.; McEwan, A.J.; Middleton, J.E.; Ackery, D.M. A comparison of dopamine and homovanillic acid excretion, as prognostic indicators in malignant phaeochromocytoma. Clin. Chim. Acta 1987, 166, 123–133. [Google Scholar] [CrossRef]
- Nolting, S.; Ullrich, M.; Pietzsch, J.; Ziegler, C.G.; Eisenhofer, G.; Grossman, A.; Pacak, K. Current Management of Pheochromocytoma/Paraganglioma: A Guide for the Practicing Clinician in the Era of Precision Medicine. Cancers 2019, 11, 1505. [Google Scholar] [CrossRef] [Green Version]
- Cavadas, C.; Silva, A.P.; Mosimann, F.; Cotrim, M.D.; Ribeiro, C.A.; Brunner, H.R.; Grouzmann, E. NPY regulates catecholamine secretion from human adrenal chromaffin cells. J. Clin. Endocrinol. Metab. 2001, 86, 5956–5963. [Google Scholar] [CrossRef]
- DeS, Senanayake, P.; Denker, J.; Bravo, E.L.; Graham, R.M. Production, characterization, and expression of neuropeptide Y by human pheochromocytoma. J. Clin. Investig. 1995, 96, 2503–2509. [Google Scholar] [CrossRef] [Green Version]
- Grouzmann, E.; Comoy, E.; Bohuon, C. Plasma neuropeptide Y concentrations in patients with neuroendocrine tumors. J. Clin. Endocrinol. Metab. 1989, 68, 808–813. [Google Scholar] [CrossRef]
- Grouzmann, E.; Gicquel, C.; Plouin, P.F.; Schlumberger, M.; Comoy, E.; Bohuon, C. Neuropeptide Y and neuron-specific enolase levels in benign and malignant pheochromocytomas. Cancer 1990, 66, 1833–1835. [Google Scholar] [CrossRef]
- Kuvshinoff, B.W.; Nussbaum, M.S.; Richards, A.I.; Bloustein, P.; McFadden, D.W. Neuropeptide Y secretion from a malignant extraadrenal retroperitoneal paraganglioma. Cancer 1992, 70, 2350–2353. [Google Scholar] [CrossRef]
- Helman, L.J.; Cohen, P.S.; Averbuch, S.D.; Cooper, M.J.; Keiser, H.R.; Israel, M.A. Neuropeptide Y expression distinguishes malignant from benign pheochromocytoma. J. Clin. Oncol. 1989, 7, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Thouennon, E.; Elkahloun, A.G.; Guillemot, J.; Gimenez-Roqueplo, A.P.; Bertherat, J.; Pierre, A.; Ghzili, H.; Grumolato, L.; Muresan, M.; Klein, M.; et al. Identification of potential gene markers and insights into the pathophysiology of pheochromocytoma malignancy. J. Clin. Endocrinol. Metab. 2007, 92, 4865–4872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korner, M.; Waser, B.; Reubi, J.C. High expression of neuropeptide y receptors in tumors of the human adrenal gland and extra-adrenal paraganglia. Clin. Cancer Res. 2004, 10, 8426–8433. [Google Scholar] [CrossRef] [Green Version]
- Oishi, S.; Sato, T. Elevated serum neuron-specific enolase in patients with malignant pheochromocytoma. Cancer 1988, 61, 1167–1170. [Google Scholar] [CrossRef]
- Tischler, A.S.; deKrijger, R.R. 15 YEARS OF PARAGANGLIOMA: Pathology of pheochromocytoma and paraganglioma. Endocr. Relat. Cancer 2015, 22, T123–T133. [Google Scholar] [CrossRef] [Green Version]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: More flaws than fame. Endocr. Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef] [PubMed]
- Bilek, R.; Vlcek, P.; Safarik, L.; Michalsky, D.; Novak, K.; Duskova, J.; Vaclavikova, E.; Widimsky, J., Jr.; Zelinka, T. Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma. Cancers 2019, 11, 586. [Google Scholar] [CrossRef] [Green Version]
- Grossrubatscher, E.; Dalino, P.; Vignati, F.; Gambacorta, M.; Pugliese, R.; Boniardi, M.; Rossetti, O.; Marocchi, A.; Bertuzzi, M.; Loli, P. The role of chromogranin A in the management of patients with phaeochromocytoma. Clin. Endocrinol. 2006, 65, 287–293. [Google Scholar] [CrossRef]
- Kimura, N.; Miura, W.; Noshiro, T.; Mizunashi, K.; Hanew, K.; Shimizu, K.; Watanabe, T.; Shibukawa, S.; Sohn, H.E.; Abe, K.; et al. Plasma chromogranin A in pheochromocytoma, primary hyperparathyroidism and pituitary adenoma in comparison with catecholamine, parathyroid hormone and pituitary hormones. Endocr. J. 1997, 44, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Szalat, A.; Fraenkel, M.; Doviner, V.; Salmon, A.; Gross, D.J. Malignant pheochromocytoma: Predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine 2011, 39, 160–166. [Google Scholar] [CrossRef]
- Parisien-La Salle, S.; Provencal, M.; Bourdeau, I. Chromogranin A in a Cohort of Pheochromocytomas and Paragangliomas: Usefulness at Diagnosis and as an Early Biomarker of Recurrence. Endocr. Pract. 2021, 27, 318–325. [Google Scholar] [CrossRef]
- Hescot, S.; Curras-Freixes, M.; Deutschbein, T.; van Berkel, A.; Vezzosi, D.; Amar, L.; de la Fouchardiere, C.; Valdes, N.; Riccardi, F.; Do Cao, C.; et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 2367–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrary, H.C.; Babajanian, E.; Calquin, M.; Carpenter, P.; Casazza, G.; Naumer, A.; Greenberg, S.; Kohlmann, W.; Cannon, R.; Monroe, M.M.; et al. Characterization of Malignant Head and Neck Paragangliomas at a Single Institution Across Multiple Decades. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef]
- Favier, J.; Amar, L.; Gimenez-Roqueplo, A.P. Paraganglioma and phaeochromocytoma: From genetics to personalized medicine. Nat. Rev. Endocrinol. 2015, 11, 101–111. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Vicha, A.; Musil, Z.; Pacak, K. Genetics of pheochromocytoma and paraganglioma syndromes: New advances and future treatment options. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 186–191. [Google Scholar] [CrossRef] [Green Version]
- van Hulsteijn, L.T.; Dekkers, O.M.; Hes, F.J.; Smit, J.W.; Corssmit, E.P. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: A systematic review and meta-analysis. J. Med. Genet. 2012, 49, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libe, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Hoffmann, M.M.; Neumann, H.P.; Erlic, Z. Somatic mutation analysis of the SDHB, SDHC, SDHD, and RET genes in the clinical assessment of sporadic and hereditary pheochromocytoma. Horm. Cancer 2012, 3, 187–192. [Google Scholar] [CrossRef]
- Papathomas, T.G.; Gaal, J.; Corssmit, E.P.; Oudijk, L.; Korpershoek, E.; Heimdal, K.; Bayley, J.P.; Morreau, H.; van Dooren, M.; Papaspyrou, K.; et al. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: A clinicopathological and molecular analysis. Eur. J. Endocrinol. 2014, 170, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Astuti, D.; Morris, M.; Krona, C.; Abel, F.; Gentle, D.; Martinsson, T.; Kogner, P.; Neumann, H.P.; Voutilainen, R.; Eng, C.; et al. Investigation of the role of SDHB inactivation in sporadic phaeochromocytoma and neuroblastoma. Br. J. Cancer 2004, 91, 1835–1841. [Google Scholar] [CrossRef] [Green Version]
- Letouze, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef] [Green Version]
- de Cubas, A.A.; Korpershoek, E.; Inglada-Perez, L.; Letouze, E.; Curras-Freixes, M.; Fernandez, A.F.; Comino-Mendez, I.; Schiavi, F.; Mancikova, V.; Eisenhofer, G.; et al. DNA Methylation Profiling in Pheochromocytoma and Paraganglioma Reveals Diagnostic and Prognostic Markers. Clin. Cancer Res. 2015, 21, 3020–3030. [Google Scholar] [CrossRef] [Green Version]
- Geli, J.; Kiss, N.; Karimi, M.; Lee, J.J.; Backdahl, M.; Ekstrom, T.J.; Larsson, C. Global and regional CpG methylation in pheochromocytomas and abdominal paragangliomas: Association to malignant behavior. Clin. Cancer Res. 2008, 14, 2551–2559. [Google Scholar] [CrossRef] [Green Version]
- Boedeker, C.C.; Neumann, H.P.; Maier, W.; Bausch, B.; Schipper, J.; Ridder, G.J. Malignant head and neck paragangliomas in SDHB mutation carriers. Otolaryngol. Head Neck Surg. 2007, 137, 126–129. [Google Scholar] [CrossRef]
- Neumann, H.P.; Pawlu, C.; Peczkowska, M.; Bausch, B.; McWhinney, S.R.; Muresan, M.; Buchta, M.; Franke, G.; Klisch, J.; Bley, T.A.; et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004, 292, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yao, W.; He, Q.; Yu, X.; Bian, B. Identification of a novel SDHB c.563 T > C mutation responsible for Paraganglioma syndrome and genetic analysis of the SDHB gene in China: A case report. BMC Med. Genet. 2020, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Donato, S.; Simoes, H.; Pinto, A.T.; Cavaco, B.M.; Leite, V. SDHx-related pheochromocytoma/paraganglioma—genetic, clinical, and treatment outcomes in a series of 30 patients from a single center. Endocrine 2019, 65, 408–415. [Google Scholar] [CrossRef]
- Hong, A.; Shanahan, M.; Schenberg, T.; Inder, W.; MacIsaac, R.; James, P.; Sachithanandan, N. Higher risk of phaeochromocytoma/paraganglioma (Phaeo-Pgl) in SDHD than SDHB carriers: An Australian cohort study. Intern. Med. J. 2019, 49, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Rijken, J.A.; Niemeijer, N.D.; Leemans, C.R.; Eijkelenkamp, K.; van der Horst-Schrivers, A.N.A.; van Berkel, A.; Timmers, H.; Kunst, H.P.M.; Bisschop, P.; van Dooren, M.F.; et al. Nationwide study of patients with head and neck paragangliomas carrying SDHB germline mutations. BJS Open 2018, 2, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemeijer, N.D.; Rijken, J.A.; Eijkelenkamp, K.; van der Horst-Schrivers, A.N.A.; Kerstens, M.N.; Tops, C.M.J.; van Berkel, A.; Timmers, H.; Kunst, H.P.M.; Leemans, C.R.; et al. The phenotype of SDHB germline mutation carriers: A nationwide study. Eur. J. Endocrinol. 2017, 177, 115–125. [Google Scholar] [CrossRef]
- Hensen, E.F.; Siemers, M.D.; Jansen, J.C.; Corssmit, E.P.; Romijn, J.A.; Tops, C.M.; van der Mey, A.G.; Devilee, P.; Cornelisse, C.J.; Bayley, J.P.; et al. Mutations in SDHD are the major determinants of the clinical characteristics of Dutch head and neck paraganglioma patients. Clin. Endocrinol. 2011, 75, 650–655. [Google Scholar] [CrossRef]
- Papaspyrou, K.; Mewes, T.; Rossmann, H.; Fottner, C.; Schneider-Raetzke, B.; Bartsch, O.; Schreckenberger, M.; Lackner, K.J.; Amedee, R.G.; Mann, W.J. Head and neck paragangliomas: Report of 175 patients (1989–2010). Head Neck 2012, 34, 632–637. [Google Scholar] [CrossRef]
- Benn, D.E.; Gimenez-Roqueplo, A.P.; Reilly, J.R.; Bertherat, J.; Burgess, J.; Byth, K.; Croxson, M.; Dahia, P.L.; Elston, M.; Gimm, O.; et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J. Clin. Endocrinol. Metab. 2006, 91, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannelli, M.; Castellano, M.; Schiavi, F.; Filetti, S.; Giacche, M.; Mori, L.; Pignataro, V.; Bernini, G.; Giache, V.; Bacca, A.; et al. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J. Clin. Endocrinol. Metab. 2009, 94, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Backman, S.; Maharjan, R.; Falk-Delgado, A.; Crona, J.; Cupisti, K.; Stalberg, P.; Hellman, P.; Bjorklund, P. Global DNA Methylation Analysis Identifies Two Discrete clusters of Pheochromocytoma with Distinct Genomic and Genetic Alterations. Sci. Rep. 2017, 7, 44943. [Google Scholar] [CrossRef] [Green Version]
- Kiss, N.B.; Geli, J.; Lundberg, F.; Avci, C.; Velazquez-Fernandez, D.; Hashemi, J.; Weber, G.; Hoog, A.; Ekstrom, T.J.; Backdahl, M.; et al. Methylation of the p16INK4A promoter is associated with malignant behavior in abdominal extra-adrenal paragangliomas but not pheochromocytomas. Endocr. Relat. Cancer 2008, 15, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Iino, K.; Okawa, Y.; Kakizawa, K.; Matsunari, S.; Yamashita, M.; Taniguchi, T.; Maekawa, M.; Suda, T.; Oki, Y. DNA methylation analysis in malignant pheochromocytoma and paraganglioma. J. Clin. Transl. Endocrinol. 2017, 7, 12–20. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Fedorova, M.S.; Pavlov, V.S.; Kalinin, D.V.; Golovyuk, A.L.; Pudova, E.A.; Guvatova, Z.G.; Melnikova, N.V.; Dmitriev, A.A.; Razmakhaev, G.S.; et al. Mutation Frequency in Main Susceptibility Genes Among Patients with Head and Neck Paragangliomas. Front. Genet. 2020, 11, 614908. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Rohmer, V.; Amar, L.; Herman, P.; Leboulleux, S.; Darrouzet, V.; Niccoli, P.; Gaillard, D.; Chabrier, G.; Chabolle, F.; et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J. Clin. Endocrinol. Metab. 2009, 94, 2817–2827. [Google Scholar] [CrossRef]
- Lin, S.R.; Lee, Y.J.; Tsai, J.H. Mutations of the p53 gene in human functional adrenal neoplasms. J. Clin. Endocrinol. Metab. 1994, 78, 483–491. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Naruse, M.; Zeng, Z.; Nishikawa, T.; Kasajima, T.; Toma, H.; Yamamori, S.; Matsumoto, H.; Tanabe, A.; Naruse, K.; et al. The relatively high frequency of p53 gene mutations in multiple and malignant phaeochromocytomas. J. Endocrinol. 1998, 159, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, A.V.; Lukyanova, E.N.; Kalinin, D.V.; Zaretsky, A.R.; Pokrovsky, A.V.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Kharitonov, S.L.; Pavlov, V.S.; et al. Mutational load in carotid body tumor. BMC Med. Genom. 2019, 12, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, B.J.; Speel, E.J.; Korpershoek, E.; Claessen, S.M.; van Nederveen, F.H.; Giesen, V.; Dannenberg, H.; van der Harst, E.; Dinjens, W.N.; de Krijger, R.R. Frequent loss of 17p, but no p53 mutations or protein overexpression in benign and malignant pheochromocytomas. Mod. Pathol. 2008, 21, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Luchetti, A.; Walsh, D.; Rodger, F.; Clark, G.; Martin, T.; Irving, R.; Sanna, M.; Yao, M.; Robledo, M.; Neumann, H.P.; et al. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int. J. Endocrinol. 2015, 2015, 138573. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.S.; Kalinin, D.V.; Lukyanova, E.N.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Savvateeva, M.V.; Lipatova, A.V.; Guvatova, Z.G.; Kaprin, A.D.; et al. Multiple paragangliomas: A case report. BMC Med. Genom. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Gniado, E.; Carracher, C.P.; Sharma, S. Simultaneous Occurrence of Germline Mutations of SDHB and TP53 in a Patient with Metastatic Pheochromocytoma. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Lin, D.; Meng, L.; Xu, F.; Lian, J.; Xu, Y.; Xie, X.; Wang, X.; He, H.; Wang, C.; Zhu, Y. Enhanced wild-type p53 expression by small activating RNA dsP53-285 induces cell cycle arrest and apoptosis in pheochromocytoma cell line PC12. Oncol. Rep. 2017, 38, 3160–3166. [Google Scholar] [CrossRef]
- Castro-Vega, L.J.; Buffet, A.; De Cubas, A.A.; Cascon, A.; Menara, M.; Khalifa, E.; Amar, L.; Azriel, S.; Bourdeau, I.; Chabre, O.; et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum. Mol. Genet. 2014, 23, 2440–2446. [Google Scholar] [CrossRef] [Green Version]
- Buffet, A.; Morin, A.; Castro-Vega, L.J.; Habarou, F.; Lussey-Lepoutre, C.; Letouze, E.; Lefebvre, H.; Guilhem, I.; Haissaguerre, M.; Raingeard, I.; et al. Germline Mutations in the Mitochondrial 2-Oxoglutarate/Malate Carrier SLC25A11 Gene Confer a Predisposition to Metastatic Paragangliomas. Cancer Res. 2018, 78, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Cascon, A.; Comino-Mendez, I.; Curras-Freixes, M.; de Cubas, A.A.; Contreras, L.; Richter, S.; Peitzsch, M.; Mancikova, V.; Inglada-Perez, L.; Perez-Barrios, A.; et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrezk, R.; Suarez, A.; Tena, I.; Pacak, K. Update of Pheochromocytoma Syndromes: Genetics, Biochemical Evaluation, and Imaging. Front. Endocrinol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Castro-Vega, L.J.; Letouze, E.; Burnichon, N.; Buffet, A.; Disderot, P.H.; Khalifa, E.; Loriot, C.; Elarouci, N.; Morin, A.; Menara, M.; et al. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat. Commun. 2015, 6, 6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calsina, B.; Curras-Freixes, M.; Buffet, A.; Pons, T.; Contreras, L.; Leton, R.; Comino-Mendez, I.; Remacha, L.; Calatayud, M.; Obispo, B.; et al. Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genet. Med. 2018, 20, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Bausch, B.; Borozdin, W.; Neumann, H.P.; European-American Pheochromocytoma Study, G. Clinical and genetic characteristics of patients with neurofibromatosis type 1 and pheochromocytoma. N. Engl. J. Med. 2006, 354, 2729–2731. [Google Scholar] [CrossRef]
- Giovannoni, I.; Callea, F.; Boldrini, R.; Inserra, A.; Cozza, R.; Francalanci, P. Malignant pheochromocytoma in a 16-year-old patient with neurofibromatosis type 1. Pediatr. Dev. Pathol. 2014, 17, 126–129. [Google Scholar] [CrossRef]
- Otoukesh, S.; Cooper, C.J.; Lou, W.; Mojtahedzadeh, M.; Nasrazadani, A.; Wampler, M.; Nahleh, Z. Combination chemotherapy regimen in a patient with metastatic malignant pheochromocytoma and neurofibromatosis type 1. Am. J. Case Rep. 2014, 15, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa, G.; Blag, C.L.; Bota, M.; Zolog, A. A malignant pheochromocytoma in a child with von Hippel-Lindau mutation. Clujul. Med. 2017, 90, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Hasani-Ranjbar, S.; Amoli, M.M.; Ebrahim-Habibi, A.; Haghpanah, V.; Hejazi, M.; Soltani, A.; Larijani, B. Mutation screening of VHL gene in a family with malignant bilateral pheochromocytoma: From isolated familial pheochromocytoma to von Hippel-Lindau disease. Fam. Cancer 2009, 8, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Ong, R.K.S.; Flores, S.K.; Reddick, R.L.; Dahia, P.L.M.; Shawa, H. A Unique Case of Metastatic, Functional, Hereditary Paraganglioma Associated With an SDHC Germline Mutation. J. Clin. Endocrinol. Metab. 2018, 103, 2802–2806. [Google Scholar] [CrossRef]
- Bickmann, J.K.; Sollfrank, S.; Schad, A.; Musholt, T.J.; Springer, E.; Miederer, M.; Bartsch, O.; Papaspyrou, K.; Koutsimpelas, D.; Mann, W.J.; et al. Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: Lessons from three unrelated cases with an identical germline mutation (p.Arg133*). J. Clin. Endocrinol. Metab. 2014, 99, E489–E496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, T.; Jackson, M.; Roman-Gonzalez, A.; Shah, K.; Cote, G.J.; Jimenez, C. Metastatic sympathetic paraganglioma in a patient with loss of the SDHC gene. Fam. Cancer 2015, 14, 615–619. [Google Scholar] [CrossRef]
- Hinze, R.; Machens, A.; Schneider, U.; Holzhausen, H.J.; Dralle, H.; Rath, F.W. Simultaneously occurring liver metastases of pheochromocytoma and medullary thyroid carcinoma--a diagnostic pitfall with clinical implications for patients with multiple endocrine neoplasia type 2a. Pathol. Res. Pract. 2000, 196, 477–481. [Google Scholar] [CrossRef]
- Adachi, Y.; Mita, H.; Sasaki, Y.; Himori, R.; Onodera, K.; Nakamura, M.; Kikuchi, T.; Yamashita, K.; Yoshida, Y.; Ishii, Y.; et al. Malignant paraganglioma of the posterior mediastinum: A case report with genetic analysis. Mol. Clin. Oncol. 2019, 10, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Korpershoek, E.; Koffy, D.; Eussen, B.H.; Oudijk, L.; Papathomas, T.G.; van Nederveen, F.H.; Belt, E.J.; Franssen, G.J.; Restuccia, D.F.; Krol, N.M.; et al. Complex MAX Rearrangement in a Family With Malignant Pheochromocytoma, Renal Oncocytoma, and Erythrocytosis. J. Clin. Endocrinol. Metab. 2016, 101, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Cascon, A.; Robledo, M. MAX and MYC: A heritable breakup. Cancer Res. 2012, 72, 3119–3124. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, L.; Khare, S.; Wubbenhorst, B.; DeSloover, D.; D’Andrea, K.; Merrill, S.; Cho, N.W.; Greenberg, R.A.; Else, T.; Montone, K.; et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat. Commun. 2015, 6, 6140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiang, J.; Luo, Y.; Li, X.; Lu, Z.; Liu, Y.; Huang, J.; Hou, Y.; Pang, Y.; Sun, M.Y.F.; et al. Molecular evaluation of a sporadic paraganglioma with concurrent IDH1 and ATRX mutations. Endocrine 2018, 61, 216–223. [Google Scholar] [CrossRef]
- Job, S.; Draskovic, I.; Burnichon, N.; Buffet, A.; Cros, J.; Lepine, C.; Venisse, A.; Robidel, E.; Verkarre, V.; Meatchi, T.; et al. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2019, 25, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, T.; Konnick, E.Q.; Tretiakova, M.S. Malignant Intrarenal/Renal Pelvis Paraganglioma with Co-Occurring SDHB and ATRX Mutations. Endocr. Pathol. 2019, 30, 270–275. [Google Scholar] [CrossRef]
- Comino-Mendez, I.; Tejera, A.M.; Curras-Freixes, M.; Remacha, L.; Gonzalvo, P.; Tonda, R.; Leton, R.; Blasco, M.A.; Robledo, M.; Cascon, A. ATRX driver mutation in a composite malignant pheochromocytoma. Cancer Genet. 2016, 209, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Wilzen, A.; Rehammar, A.; Muth, A.; Nilsson, O.; Tesan Tomic, T.; Wangberg, B.; Kristiansson, E.; Abel, F. Malignant pheochromocytomas/paragangliomas harbor mutations in transport and cell adhesion genes. Int. J. Cancer 2016, 138, 2201–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, Y.J.; Choe, J.Y.; Park, H.J. Malignancy in Pheochromocytoma or Paraganglioma: Integrative Analysis of 176 Cases in TCGA. Endocr. Pathol. 2017, 28, 159–164. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lim, J.; Jeon, M.J.; Lee, Y.M.; Sung, T.Y.; Hong, E.G.; Lee, J.Y.; Jang, S.J.; Kim, W.G.; Song, D.E.; et al. Mutation Profile of Aggressive Pheochromocytoma and Paraganglioma with Comparison of TCGA Data. Cancers 2021, 13, 2389. [Google Scholar] [CrossRef]
- Brouwers, F.M.; Elkahloun, A.G.; Munson, P.J.; Eisenhofer, G.; Barb, J.; Linehan, W.M.; Lenders, J.W.; De Krijger, R.; Mannelli, M.; Udelsman, R.; et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann. N. Y. Acad. Sci. 2006, 1073, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Anouar, Y.; Yon, L.; Guillemot, J.; Thouennon, E.; Barbier, L.; Gimenez-Roqueplo, A.P.; Bertherat, J.; Lefebvre, H.; Klein, M.; Muresan, M.; et al. Development of novel tools for the diagnosis and prognosis of pheochromocytoma using peptide marker immunoassay and gene expression profiling approaches. Ann. N. Y. Acad. Sci. 2006, 1073, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, J.; Fendrich, V.; Holler, J.; Buchholz, M.; Heinmoller, E.; Langer, P.; Ramaswamy, A.; Samans, B.; Walz, M.K.; Rothmund, M.; et al. Microarray analysis reveals differential expression of benign and malignant pheochromocytoma. Endocr. Relat. Cancer 2010, 17, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Suh, I.; Shibru, D.; Eisenhofer, G.; Pacak, K.; Duh, Q.Y.; Clark, O.H.; Kebebew, E. Candidate genes associated with malignant pheochromocytomas by genome-wide expression profiling. Ann. Surg. 2009, 250, 983–990. [Google Scholar] [CrossRef]
- Suh, Y.J.; Park, J.H.; Bilegsaikhan, S.E.; Lee, D.J. Transcriptome Analysis Reveals Significant Differences in Gene Expression of Malignant Pheochromocytoma or Paraganglioma. Int. J. Endocrinol. 2019, 2019, 7014240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Oh, E.; Lee, B.; Kim, Y.J.; Oh, D.Y.; Jung, K.; Choi, J.S.; Kim, J.; Kim, S.J.; Yang, J.W.; et al. Phenylethanolamine N-methyltransferase downregulation is associated with malignant pheochromocytoma/paraganglioma. Oncotarget 2016, 7, 24141–24153. [Google Scholar] [CrossRef] [Green Version]
- Span, P.N.; Rao, J.U.; Oude Ophuis, S.B.; Lenders, J.W.; Sweep, F.C.; Wesseling, P.; Kusters, B.; van Nederveen, F.H.; de Krijger, R.R.; Hermus, A.R.; et al. Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant pheochromocytoma/paraganglioma. Endocr. Relat. Cancer 2011, 18, 323–331. [Google Scholar] [CrossRef]
- Lan, H.; Lu, H.; Wang, X.; Jin, H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. BioMed Res. Int. 2015, 2015, 125094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Rochow, G.Y.; Jackson, N.E.; Conaglen, J.V.; Whittle, D.E.; Kunnimalaiyaan, M.; Chen, H.; Westin, G.; Sandgren, J.; Stalberg, P.; Khanafshar, E.; et al. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr. Relat. Cancer 2010, 17, 835–846. [Google Scholar] [CrossRef]
- Patterson, E.; Webb, R.; Weisbrod, A.; Bian, B.; He, M.; Zhang, L.; Holloway, A.K.; Krishna, R.; Nilubol, N.; Pacak, K.; et al. The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endocr. Relat. Cancer 2012, 19, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Zong, L.; Meng, L.; Shi, R. Role of miR-101 in pheochromocytoma patients with SDHD mutation. Int. J. Clin. Exp. Pathol. 2015, 8, 1545–1554. [Google Scholar]
- Tsang, V.H.; Dwight, T.; Benn, D.E.; Meyer-Rochow, G.Y.; Gill, A.J.; Sywak, M.; Sidhu, S.; Veivers, D.; Sue, C.M.; Robinson, B.G.; et al. Overexpression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr. Relat. Cancer 2014, 21, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Le, Q.T.; Giaccia, A.J. MiR-210--micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, S.M.; Ayabe, R.I.; Malekzadeh, P.; Good, M.L.; Wach, M.M.; Gonzales, M.K.; Tirosh, A.; Nilubol, N.; Pacak, K.; Kebebew, E.; et al. MicroRNA-210 May Be a Preoperative Biomarker of Malignant Pheochromocytomas and Paragangliomas. J. Surg. Res. 2019, 243, 1–7. [Google Scholar] [CrossRef]
- Calsina, B.; Castro-Vega, L.J.; Torres-Perez, R.; Inglada-Perez, L.; Curras-Freixes, M.; Roldan-Romero, J.M.; Mancikova, V.; Leton, R.; Remacha, L.; Santos, M.; et al. Integrative multi-omics analysis identifies a prognostic miRNA signature and a targetable miR-21-3p/TSC2/mTOR axis in metastatic pheochromocytoma/paraganglioma. Theranostics 2019, 9, 4946–4958. [Google Scholar] [CrossRef]
- Cong, Y.S.; Wright, W.E.; Shay, J.W. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trybek, T.; Kowalik, A.; Gozdz, S.; Kowalska, A. Telomeres and telomerase in oncogenesis. Oncol. Lett. 2020, 20, 1015–1027. [Google Scholar] [CrossRef]
- Kubota, Y.; Nakada, T.; Sasagawa, I.; Yanai, H.; Itoh, K. Elevated levels of telomerase activity in malignant pheochromocytoma. Cancer 1998, 82, 176–179. [Google Scholar] [CrossRef]
- Boltze, C.; Mundschenk, J.; Unger, N.; Schneider-Stock, R.; Peters, B.; Mawrin, C.; Hoang-Vu, C.; Roessner, A.; Lehnert, H. Expression profile of the telomeric complex discriminates between benign and malignant pheochromocytoma. J. Clin. Endocrinol. Metab. 2003, 88, 4280–4286. [Google Scholar] [CrossRef] [Green Version]
- Isobe, K.; Yashiro, T.; Omura, S.; Kaneko, M.; Kaneko, S.; Kamma, H.; Tatsuno, I.; Takekoshi, K.; Kawakami, Y.; Nakai, T. Expression of the human telomerase reverse transcriptase in pheochromocytoma and neuroblastoma tissues. Endocr. J. 2004, 51, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, H.; Ogawa, O.; Mishina, M.; Oka, H.; Okumura, K.; Yamabe, H.; Terachi, T.; Yoshida, O. Telomerase activity in adrenal cortical tumors and pheochromocytomas with reference to clinicopathologic features. Urol. Res. 1998, 26, 29–32. [Google Scholar] [CrossRef]
- Hirano, Y.; Nobata, S.; Takahashi, H.; Kageyama, S.; Sudoko, H.; Ushiyama, T.; Suzuki, K.; Fujita, K. Histologically benign but telomerase positive adrenal pheochromocytoma. Int. J. Urol. 2002, 9, 697–699. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Else, T.; Bamberger, A.M.; Frilling, A.; Beil, F.U.; Allolio, B.; Schulte, H.M. Telomerase activity in benign and malignant adrenal tumors. Exp. Clin. Endocrinol. Diabetes 1999, 107, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, J.; Qin, Y.; Ma, Y.; Liang, X.; Xian, J.; Lu, D.; Wei, M.; Yang, J.Y.; Yang, M.Q.; et al. Differential expression of human telomerase catalytic subunit mRNA by in situ hybridization in pheochromocytomas. Endocr. Pathol. 2006, 17, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Brown, T.C.; Juhlin, C.C.; Andreasson, A.; Wang, N.; Backdahl, M.; Healy, J.M.; Prasad, M.L.; Korah, R.; Carling, T.; et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr. Relat. Cancer 2014, 21, 427–434. [Google Scholar] [CrossRef]
- Svahn, F.; Juhlin, C.C.; Paulsson, J.O.; Fotouhi, O.; Zedenius, J.; Larsson, C.; Stenman, A. Telomerase reverse transcriptase promoter hypermethylation is associated with metastatic disease in abdominal paraganglioma. Clin. Endocrinol. 2018, 88, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Dwight, T.; Flynn, A.; Amarasinghe, K.; Benn, D.E.; Lupat, R.; Li, J.; Cameron, D.L.; Hogg, A.; Balachander, S.; Candiloro, I.L.M.; et al. TERT structural rearrangements in metastatic pheochromocytomas. Endocr. Relat. Cancer 2018, 25, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Papathomas, T.G.; Oudijk, L.; Zwarthoff, E.C.; Post, E.; Duijkers, F.A.; van Noesel, M.M.; Hofland, L.J.; Pollard, P.J.; Maher, E.R.; Restuccia, D.F.; et al. Telomerase reverse transcriptase promoter mutations in tumors originating from the adrenal gland and extra-adrenal paraganglia. Endocr. Relat. Cancer 2014, 21, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Fliedner, S.M.; Lehnert, H.; Pacak, K. Metastatic paraganglioma. Semin. Oncol. 2010, 37, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Kumari, K.; Jain, D.; Kumar, R.; Mohan, A.; Kumar, R. Metastatic carotid body paraganglioma of lungs and lymph nodes: Unsuspected diagnosis on EBUS-TBNA. Diagn. Cytopathol. 2017, 45, 327–332. [Google Scholar] [CrossRef]
- Javidiparsijani, S.; Brickman, A.; Lin, D.M.; Rohra, P.; Ghai, R.; Bitterman, P.; Reddi, V.; Al-Khudari, S.; Gattuso, P. Is Regional Lymph Node Metastasis of Head and Neck Paraganglioma a Sign of Aggressive Clinical Behavior: A Clinical/Pathologic Review. Ear. Nose Throat J. 2021, 100, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Strong, V.E.; Kennedy, T.; Al-Ahmadie, H.; Tang, L.; Coleman, J.; Fong, Y.; Brennan, M.; Ghossein, R.A. Prognostic indicators of malignancy in adrenal pheochromocytomas: Clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery 2008, 143, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mehrotra, P.K.; Jain, M.; Gupta, S.K.; Mishra, A.; Chand, G.; Agarwal, G.; Verma, A.K.; Mishra, S.K.; Singh, U. Size of the tumor and pheochromocytoma of the adrenal gland scaled score (PASS): Can they predict malignancy? World J. Surg. 2010, 34, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tischler, A.S.; Lloyd, R.V.; DeLellis, R.A.; de Krijger, R.; van Nederveen, F.; Nose, V. Observer variation in the application of the Pheochromocytoma of the Adrenal Gland Scaled Score. Am. J. Surg. Pathol. 2009, 33, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Bialas, M.; Okon, K.; Dyduch, G.; Ciesielska-Milian, K.; Buziak, M.; Hubalewska-Dydejczyk, A.; Sobrinho-Simoes, M. Neuroendocrine markers and sustentacular cell count in benign and malignant pheochromocytomas—A comparative study. Pol. J. Pathol. 2013, 64, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.M.; Khandeparkar, S.G.; Deshmukh, S.D.; Karekar, R.R.; Gaopande, V.L.; Joshi, A.R.; Kesari, M.V.; Shelke, R.R. Risk Stratification in Paragangliomas with PASS (Pheochromocytoma of the Adrenal Gland Scaled Score) and Immunohistochemical Markers. J. Clin. Diagn. Res. 2016, 10, EC01–EC04. [Google Scholar] [CrossRef]
- Konosu-Fukaya, S.; Omata, K.; Tezuka, Y.; Ono, Y.; Aoyama, Y.; Satoh, F.; Fujishima, F.; Sasano, H.; Nakamura, Y. Catecholamine-Synthesizing Enzymes in Pheochromocytoma and Extraadrenal Paraganglioma. Endocr. Pathol. 2018, 29, 302–309. [Google Scholar] [CrossRef]
- Stenman, A.; Svahn, F.; Hojjat-Farsangi, M.; Zedenius, J.; Soderkvist, P.; Gimm, O.; Larsson, C.; Juhlin, C.C. Molecular Profiling of Pheochromocytoma and Abdominal Paraganglioma Stratified by the PASS Algorithm Reveals Chromogranin B as Associated With Histologic Prediction of Malignant Behavior. Am. J. Surg. Pathol. 2019, 43, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, J.M.; Ahn, S.H.; Kim, H.; Kim, B.J.; Sung, T.Y.; Kim, Y.H.; Hong, S.J.; Song, D.E.; Lee, S.H. Validation of pathological grading systems for predicting metastatic potential in pheochromocytoma and paraganglioma. PLoS ONE 2017, 12, e0187398. [Google Scholar] [CrossRef] [Green Version]
- Stenman, A.; Zedenius, J.; Juhlin, C.C. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas-A Meta-Analysis and Systematic Review of the Literature. Cancers 2019, 11, 225. [Google Scholar] [CrossRef] [Green Version]
- Cheung, V.K.Y.; Gill, A.J.; Chou, A. Old, New, and Emerging Immunohistochemical Markers in Pheochromocytoma and Paraganglioma. Endocr. Pathol. 2018, 29, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018, 72, 106–116. [Google Scholar] [CrossRef]
- van Nederveen, F.H.; Gaal, J.; Favier, J.; Korpershoek, E.; Oldenburg, R.A.; de Bruyn, E.M.; Sleddens, H.F.; Derkx, P.; Riviere, J.; Dannenberg, H.; et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: A retrospective and prospective analysis. Lancet Oncol. 2009, 10, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Snezhkina, A.V.; Kalinin, D.V.; Pavlov, V.S.; Lukyanova, E.N.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Savvateeva, M.V.; Stepanov, O.A.; Poloznikov, A.A.; et al. Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas. Int. J. Mol. Sci. 2020, 21, 6950. [Google Scholar] [CrossRef]
- Richter, S.; Gieldon, L.; Pang, Y.; Peitzsch, M.; Huynh, T.; Leton, R.; Viana, B.; Ercolino, T.; Mangelis, A.; Rapizzi, E.; et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet. Med. 2019, 21, 705–717. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Chang, J.; Kim, S.-H.; Min, H.S.; Kim, H.-Y.; Jung, S.-E.; Park, K.-W.; Lee, S.-C. Risk Factors for Malignancy of Pheochromocytoma and Abdominal Paraganglioma in Children: Clinicopathologic Perspectives. J. Korean Assoc. Pediatr. Surg. 2013, 19, 108–121. [Google Scholar] [CrossRef]
- Clarke, M.R.; Weyant, R.J.; Watson, C.G.; Carty, S.E. Prognostic markers in pheochromocytoma. Hum. Pathol. 1998, 29, 522–526. [Google Scholar] [CrossRef]
- Kovacs, K.; Bell, D.; Gardiner, G.W.; Honey, R.J.; Goguen, J.; Rotondo, F. Malignant paraganglioma of the urinary bladder: Immunohistochemical study of prognostic indicators. Endocr. Pathol. 2005, 16, 363–369. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, X.; Wang, A.; Xie, Q.; Xu, X.; Sun, J. PD-L1 expression and association with malignant behavior in pheochromocytomas/paragangliomas. Hum. Pathol. 2019, 86, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Black, J.R.; Trousil, S.; Dina, R.E.; Trivedi, P.; Mauri, F.A.; Sharma, R. Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: Relationship with the hypoxic response, immune evasion and malignant behavior. Oncoimmunology 2017, 6, e1358332. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, K.E.; Wen, D.R.; Cancilla, P.A.; Cochran, A.J. Paragangliomas: Assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. Hum. Pathol. 1989, 20, 29–39. [Google Scholar] [CrossRef]
- Nastos, C.; Yiallourou, A.; Kotsis, T.; Mizamtsidi, M.; Delaportas, D.; Kondi-Pafiti, A.; Polymeneas, G. Immunohistochemical expression patterns of S100, synaptophysin, chromogranin A and neuron specific enolase in predicting malignant behaviour in paragangliomas. J. BUON 2018, 23, 1540–1545. [Google Scholar]
- Robertson, D.I.; Cooney, T.P. Malignant carotid body paraganglioma: Light and electron microscopic study of the tumor and its metastases. Cancer 1980, 46, 2623–2633. [Google Scholar] [CrossRef]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Quiros, R.M.; Kim, A.W.; Maxhimer, J.; Gattuso, P.; Xu, X.; Prinz, R.A. Differential heparanase-1 expression in malignant and benign pheochromocytomas. J. Surg. Res. 2002, 108, 44–50. [Google Scholar] [CrossRef]
- Zhu, Y.; He, H.C.; Yuan, F.; Zhang, J.; Rui, W.B.; Zhao, J.P.; Shen, Z.J.; Ning, G. Heparanase-1 and Cyclooxygenase-2: Prognostic indicators of malignancy in pheochromocytomas. Endocrine 2010, 38, 93–99. [Google Scholar] [CrossRef]
- Salmenkivi, K.; Haglund, C.; Ristimaki, A.; Arola, J.; Heikkila, P. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J. Clin. Endocrinol. Metab. 2001, 86, 5615–5619. [Google Scholar] [CrossRef]
- Saffar, H.; Sanii, S.; Heshmat, R.; Haghpanah, V.; Larijani, B.; Rajabiani, A.; Azimi, S.; Tavangar, S.M. Expression of galectin-3, nm-23, and cyclooxygenase-2 could potentially discriminate between benign and malignant pheochromocytoma. Am. J. Clin. Pathol. 2011, 135, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Gimm, O.; Krause, U.; Brauckhoff, M.; Hoang-Vu, C.; Dralle, H. Distinct expression of galectin-3 in pheochromocytomas. Ann. N. Y. Acad. Sci. 2006, 1073, 571–577. [Google Scholar] [CrossRef]
- Deng, L.; Chen, T.; Xu, H.; Li, Y.; Deng, M.; Mo, D.; Tian, H.; Ren, Y. The Expression of Snail, Galectin-3, and IGF1R in the Differential Diagnosis of Benign and Malignant Pheochromocytoma and Paraganglioma. BioMed Res. Int. 2020, 2020, 4150735. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; AlSadek, D.M. Galectin-3 as a Potential Target to Prevent Cancer Metastasis. Clin. Med. Insights Oncol. 2015, 9, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savin, S.; Cvejic, D.; Isic, T.; Paunovic, I.; Tatic, S.; Havelka, M. Thyroid peroxidase and galectin-3 immunostaining in differentiated thyroid carcinoma with clinicopathologic correlation. Hum. Pathol. 2008, 39, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, R.I.; Lack, E.E.; Steinberg, S.M.; Keiser, H.R. Decreased expression of neuropeptides in malignant paragangliomas: An immunohistochemical study. Hum. Pathol. 1988, 19, 41–50. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Ahlman, H.; Jansson, S.; Nilsson, O. N-cadherin expression in adrenal tumors: Upregulation in malignant pheochromocytoma and downregulation in adrenocortical carcinoma. Endocr. Pathol. 2002, 13, 99–110. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, Y.; Rui, W.; Zhu, Y.; Zhang, C.; Zhao, J.; Wei, Q.; Wu, Y.; Shen, Z.; Ning, G. Expression and diagnostic relevance of heat shock protein 90 and signal transducer and activator of transcription 3 in malignant pheochromocytoma. J. Clin. Pathol. 2013, 66, 286–290. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z. Expression of PDK1 in malignant pheochromocytoma as a new promising potential therapeutic target. Clin. Transl. Oncol. 2019, 21, 1312–1318. [Google Scholar] [CrossRef]
| Parameter | HNPGLs | PHEO | Other Extra-Adrenal PGLs | ||
|---|---|---|---|---|---|
| CPGL | MEPGL | VPGL | |||
| Mean age at diagnosis | 40–50 * [7,8] | 55 [4] | 41–47 [4] | 40–50 [9] | 40–50 [9] |
| Female/male ratio | 2:1–8:1 ** [10,11] | 3:1–9:1 [12] | 2:1–8:1 [12] | 1:1 [9] | 1:1 [9] |
| Multifocal cases, % | 10–25 [1] | 10–50 [13] | 10 *** [14] | 8 [15] | 33 [15] |
| Metastatic cases, % | 4–6 [12] | 2 [12] | 16–19 [10,12] | 10 [9] | 2.5–50 [9] |
| Mutated Gene | Incidence of Patients with Metastatic Disease (nmetastasizing/nmutated) * | Reference |
|---|---|---|
| SDHB | 7/13 (53.8%) 3/10 (30%) 5/6 (83%) 5/18 (28%) 3/9 (33%) 1/8 (12.5%) 3/54 (5.6%) 0/8 (0%) 1/5 (20%) | Boedeker et al. [77] Neumann et al. [78] McCrary et al. [63] Chen et al. [79] Donato et al. [80] Hong et al. [81] Rijken et al. [82,83] Hensen et al. [84] Papaspyrou et al. [85] |
| SDHD | 0/6 (0%) 2/25 (8%) 0/10 (0%) 0/45 (0%) 5/22 (22.7%) 1/28 (3.6%) | McCrary et al. [63] Benn et al. [86] Neumann et al. [78] Boedeker et al. [77] Papaspyrou et al. [85] Mannelli et al. [87] |
| Potential Marker | Characteristics Associated with Malignancy |
|---|---|
| Histopathological markers | |
| Grading system for adrenal pheochromocytoma and paraganglioma (GAPP) | Well-differentiated and moderately differentiated tumors |
| Tumor size and weight | On average, larger than 10 cm and more than 500 g |
| Adrenal gland scaled score (PASS) | ≥4 |
| Ki-67 proliferation index | >2% |
| Sustentacular cells | Cell density depletion or absent |
| Galectin-3 (LGALS3) | Increased expression detected using IHC staining |
| Succinate dehydrogenase complex subunit B (SDHB) | Negative or weak diffuse IHC staining |
| Heparanase-1 (HPSE1) | Positive IHC staining |
| Cyclooxygenase-2 (COX2) | |
| Genetic markers | |
| Succinate dehydrogenase complex subunit B (SDHB) | Germline mutation |
| Succinate dehydrogenase complex subunit D (SDHD) | |
| Fumarate hydratase (FH) | |
| Solute carrier family 25 member 11 (SLC25A11) | |
| Malate dehydrogenase 2 (MDH2) | |
| ATRX chromatin remodeler (ATRX) | Somatic mutation |
| Histone-lysine N-methyltransferase SETD2 (SETD2) | |
| Telomerase reverse transcriptase (hTERT) | |
| Mastermind-like transcriptional coactivator 3 (MAML3) | Fusion gene |
| CpG island methylator phenotype (CIMP) | High CIMP |
| MicroRNA miR-15a | Downregulation |
| Phenylethanolamine N-methyltransferase (PNMT) | |
| MicroRNA miR-483-5p | Overexpression |
| MicroRNA miR-101 | |
| MicroRNA miR-210 | |
| MicroRNA miR-21-3p | |
| MicroRNA miR-183-5p | |
| Telomerase reverse transcriptase (hTERT) | |
| Biochemical markers | |
| Normetanephrine and 3-methoxytyramine | Increased plasma or urine level |
| Neuron-specific enolase (NSE) | Increased serum level |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snezhkina, A.; Pavlov, V.; Dmitriev, A.; Melnikova, N.; Kudryavtseva, A. Potential Biomarkers of Metastasizing Paragangliomas and Pheochromocytomas. Life 2021, 11, 1179. https://doi.org/10.3390/life11111179
Snezhkina A, Pavlov V, Dmitriev A, Melnikova N, Kudryavtseva A. Potential Biomarkers of Metastasizing Paragangliomas and Pheochromocytomas. Life. 2021; 11(11):1179. https://doi.org/10.3390/life11111179
Chicago/Turabian StyleSnezhkina, Anastasiya, Vladislav Pavlov, Alexey Dmitriev, Nataliya Melnikova, and Anna Kudryavtseva. 2021. "Potential Biomarkers of Metastasizing Paragangliomas and Pheochromocytomas" Life 11, no. 11: 1179. https://doi.org/10.3390/life11111179
APA StyleSnezhkina, A., Pavlov, V., Dmitriev, A., Melnikova, N., & Kudryavtseva, A. (2021). Potential Biomarkers of Metastasizing Paragangliomas and Pheochromocytomas. Life, 11(11), 1179. https://doi.org/10.3390/life11111179









