Evaluating the Death and Recovery of Lateral Line Hair Cells Following Repeated Neomycin Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Animal Husbandry
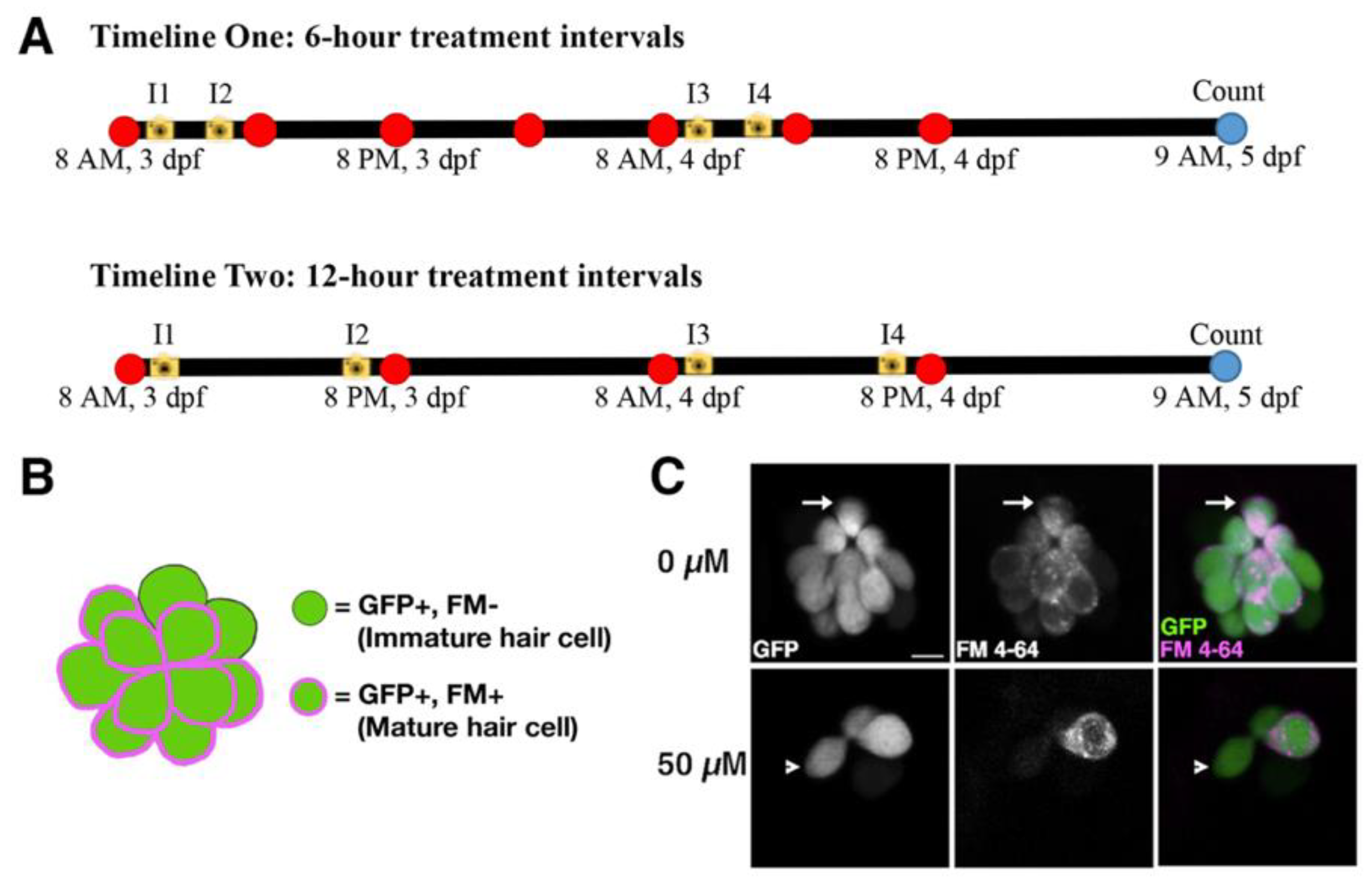
2.2. Neomycin Sulfate Treatments
2.3. Survivorship Counts
2.4. Hair Cell Counts and Strategy to Differentiate between Mature and Immature Hair Cells
2.5. Statistics
3. Results
3.1. Toxicity of Repeated Neomycin Treatments in 3–4 dpf Zebrafish
3.2. Hair Cell Proliferation and Maturation in Untreated Larvae
3.3. Effect of Neomycin Concentration and Treatment Interval on Hair Cell Death
3.4. Cellular and Functional Regeneration in 3 dpf Larval Zebrafish
3.5. Cellular and Functional Regeneration in 4 dpf Larval Zebrafish
3.6. Comparison of Hair Cell Death and Recovery between 3 and 4 dpf Zebrafish
4. Discussion
4.1. Toxicity Associated with Repeated Neomycin Treatments
4.2. Susceptibility of Lateral Line Hair Cells to Repeated Neomycin Treatments at 3–4 dpf
4.3. Hair Cell Maturation and Regeneration after Repeated Neomycin Treatments at 3–4 dpf
4.4. Mimicking the Chronic Loss of Lateral Line Function in Larval Zebrafish at 3–4 dpf
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogdans, J. Sensory ecology of the fish lateral-line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli. J. Fish Biol. 2019, 95, 53–72. [Google Scholar] [CrossRef]
- Montgomery, J.C.; Baker, C.F. Lateral line and fish behavior. In The Senses: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–142. [Google Scholar]
- Montgomery, J.C.; Baker, C.F.; Carton, A.G. The lateral line can mediate rheotaxis in fish. Nature 1997, 389, 960–963. [Google Scholar] [CrossRef]
- Baker, C.F.; Montgomery, J.C. The sensory basis of rheotaxis in the blind Mexican cave fish, Astyanax fasciatus. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1999, 184, 519–527. [Google Scholar] [CrossRef]
- Suli, A.; Watson, G.M.; Rubel, E.W.; Raible, D.W. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS ONE 2012, 7, e29727. [Google Scholar] [CrossRef] [Green Version]
- Coombs, S.; Finneran, J.J.; Conley, R.A. Hydrodynamic image formation by the peripheral lateral line system of the Lake Michigan mottled sculpin, Cottus bairdi. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1111–1114. [Google Scholar] [CrossRef] [Green Version]
- Stewart, W.J.; Cardenas, G.S.; McHenry, M.J. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 2013, 216, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Coffin, A.B.; Ramcharitar, J. Chemical ototoxicity of the fish inner ear and lateral line. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 877, pp. 419–437. [Google Scholar]
- Ton, C.; Parng, C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear. Res. 2005, 208, 79–88. [Google Scholar] [CrossRef]
- Linbo, T.L.; Stehr, C.M.; Incardona, J.P.; Scholz, N.L. Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ. Toxicol. Chem. 2006, 25, 597–603. [Google Scholar] [CrossRef]
- Ou, H.C.; Raible, D.W.; Rubel, E.W. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear. Res. 2007, 233, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A.; Holder, N. Cell turnover in neuromasts of zebrafish larvae. Hear. Res. 2000, 143, 171–181. [Google Scholar] [CrossRef]
- Harris, J.A.; Cheng, A.G.; Cunningham, L.L.; MacDonald, G.; Raible, D.W.; Rubel, E.W. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). JARO—J. Assoc. Res. Otolaryngol. 2003, 4, 219–234. [Google Scholar] [CrossRef]
- Olivari, F.A.; Hernández, P.P.; Allende, M.L. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008, 1244, 1–12. [Google Scholar] [CrossRef]
- Owens, K.N.; Cunningham, D.E.; Macdonald, G.; Rubel, E.W.; Raible, D.W.; Pujol, R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J. Comp. Neurol. 2007, 502, 522–543. [Google Scholar] [CrossRef]
- Esterberg, R.; Linbo, T.; Pickett, S.B.; Wu, P.; Ou, H.C.; Rubel, E.W.; Raible, D.W. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Investig. 2016, 126, 3556–3566. [Google Scholar] [CrossRef] [Green Version]
- Sha, S.-H.; Schacht, J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear. Res. 1999, 128, 112–118. [Google Scholar] [CrossRef]
- Hirose, K.; Hockenbery, D.M.; Rubel, E.W. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear. Res. 1997, 104, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neuro-Otol. 2000, 5, 3–22. [Google Scholar] [CrossRef]
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and cisplatin-induced ototoxicity: Mechanisms and otoprotective strategies. Cold Spring Harb. Perspect. Med. 2018, 9, a033548. [Google Scholar] [CrossRef]
- Murakami, S.L.; Cunningham, L.L.; Werner, L.A.; Bauer, E.; Pujol, R.; Raible, D.W.; Rubel, E.W. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio). Hear. Res. 2003, 186, 47–56. [Google Scholar] [CrossRef]
- Santos, F.; MacDonald, G.; Rubel, E.W.; Raible, D.W. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio). Hear. Res. 2006, 213, 25–33. [Google Scholar] [CrossRef]
- Ghysen, A.; Dambly-Chaudière, C. The lateral line microcosmos. Genes Dev. 2007, 21, 2118–2130. [Google Scholar] [CrossRef] [Green Version]
- Ledent, V. Postembryonic development of the posterior lateral line in zebrafish. Development 2002, 129, 597–604. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Kimmel, C.B.; Schabtach, E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J. Comp. Neurol. 1985, 233, 377–389. [Google Scholar] [CrossRef]
- Raible, D.W.; Kruse, G.J. Organization of the lateral line system in embryonic zebrafish. J. Comp. Neurol. 2000, 421, 189–198. [Google Scholar] [CrossRef]
- Mackenzie, S.M.; Raible, D.W. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS ONE 2012, 7, e47257. [Google Scholar] [CrossRef]
- Alharazneh, A.; Luk, L.; Huth, M.; Monfared, A.; Steyger, P.S.; Cheng, A.G.; Ricci, A.J. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS ONE 2011, 6, e22347. [Google Scholar] [CrossRef] [Green Version]
- Owens, K.N.; Coffin, A.B.; Hong, L.S.; Bennett, K.O.; Rubel, E.W.; Raible, D.W. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear. Res. 2009, 253, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Seiler, C.; Nicolson, T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J. Neurobiol. 1999, 41, 424–434. [Google Scholar] [CrossRef]
- Hernández, P.P.; Olivari, F.A.; Sarrazin, A.F.; Sandoval, P.C.; Allende, M.L. Regeneration in zebrafish lateral line neuromasts: Expression of the neural progenitor cell marker sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev. Neurobiol. 2007, 67, 637–654. [Google Scholar] [CrossRef]
- McHenry, M.J.; Feitl, K.E.; Strother, J.A.; van Trump, W.J. Larval zebrafish rapidly sense the water flow of a predator’s strike. Biol. Lett. 2009, 5, 477–479. [Google Scholar] [CrossRef] [Green Version]
- Erickson, T.; Pacentine, I.V.; Venuto, A.; Clemens, R.; Nicolson, T. The lhfpl5 ohnologs lhfpl5a and lhfpl5b are required for mechanotransduction in distinct populations of sensory hair cells in zebrafish. Front. Mol. Neurosci. 2020, 12, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon: Eugene, OR, USA, 2000. [Google Scholar]
- Kindt, K.S.; Finch, G.; Nicolson, T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev. Cell 2012, 23, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Meyers, J.R.; MacDonald, R.B.; Duggan, A.; Lenzi, D.; Standaert, D.G.; Corwin, J.T.; Corey, D.P. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003, 23, 4054–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio PBC: Boston, MA, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Han, J.; Liu, K.; Wang, R.; Zhang, Y.; Zhou, B. Exposure to cadmium causes inhibition of otolith development and behavioral impairment in zebrafish larvae. Aquat. Toxicol. 2019, 214, 105236. [Google Scholar] [CrossRef]
- Hansen, J.A.; Rose, J.D.; Jenkins, R.A.; Gerow, K.G.; Bergman, H.L. Chinook salmon (Oncorhynchus Tshawytscha) and rainbow trout (Oncorhynchus Mykiss) exposed to copper: Neurophysiological and histological effects on the olfactory system. Environ. Toxicol. Chem. 1999, 18, 1979–1991. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, R.; Zhang, T.; Zhao, G.; Huang, Y.; Wang, H.; Liu, J.-X. Copper impairs zebrafish swimbladder development by down-regulating Wnt signaling. Aquat. Toxicol. 2017, 192, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Carreau, N.D.; Pyle, G.G. Effect of copper exposure during embryonic development on chemosensory function of juvenile fathead minnows (Pimephales promelas). Ecotoxicol. Environ. Saf. 2005, 61, 1–6. [Google Scholar] [CrossRef]
- Han, E.; Ho Oh, K.; Park, S.; Chan Rah, Y.; Park, H.C.; Koun, S.; Choi, J. Analysis of behavioral changes in zebrafish (Danio rerio) larvae caused by aminoglycoside-induced damage to the lateral line and muscles. NeuroToxicology 2020, 78, 134–142. [Google Scholar] [CrossRef]
- Cruz, I.A.; Kappedal, R.; Mackenzie, S.M.; Hailey, D.W.; Hoffman, T.L.; Schilling, T.F.; Raible, D.W. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev. Biol. 2015, 402, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Hardy, K.; Amariutei, A.E.; de Faveri, F.; Hendry, A.; Marcotti, W.; Ceriani, F. Functional development and regeneration of hair cells in the zebrafish lateral line. J. Physiol. 2021, 599, 3913–3936. [Google Scholar] [CrossRef]
- Carrillo, A.; McHenry, M.J. Zebrafish learn to forage in the dark. J. Exp. Biol. 2016, 219, 582–589. [Google Scholar] [CrossRef] [Green Version]






| Beta | Estimate | Standard Error | p Value | Significance Level |
|---|---|---|---|---|
| Intercept (0 µM, 6 h) | 4.1322996 | 0.4913248 | 4.08 × 10−17 | **** |
| 50 µM Neomycin | −0.9457874 | 0.5789734 | 0.102 | ns |
| 100 µM Neomycin | −2.6188895 | 0.5660887 | 3.72 × 10−6 | **** |
| 200 µM Neomycin | −3.2026104 | 0.5625068 | 1.24 × 10−8 | **** |
| 300 µM Neomycin | −5.2428102 | 0.6140916 | 1.37 × 10−17 | **** |
| 400 µM Neomycin | −5.8515287 | 0.6317583 | 2.00 × 10−20 | **** |
| 12-Hour Treatment Intervals | 2.5645373 | 0.3409579 | 5.41 × 10−14 | **** |
| Beta | Estimate | Standard Error | p Value | Significance Level |
|---|---|---|---|---|
| Intercept (0 µM, I1) | 0.1024 | 0.1330 | 0.441 | ns |
| 4 dpf | 1.1435 | 0.2106 | 5.68 × 10−8 | **** |
| Number of Treatments | −0.6593 | 0.0655 | <2 × 10−16 | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuto, A.; Erickson, T. Evaluating the Death and Recovery of Lateral Line Hair Cells Following Repeated Neomycin Treatments. Life 2021, 11, 1180. https://doi.org/10.3390/life11111180
Venuto A, Erickson T. Evaluating the Death and Recovery of Lateral Line Hair Cells Following Repeated Neomycin Treatments. Life. 2021; 11(11):1180. https://doi.org/10.3390/life11111180
Chicago/Turabian StyleVenuto, Alexandra, and Timothy Erickson. 2021. "Evaluating the Death and Recovery of Lateral Line Hair Cells Following Repeated Neomycin Treatments" Life 11, no. 11: 1180. https://doi.org/10.3390/life11111180
APA StyleVenuto, A., & Erickson, T. (2021). Evaluating the Death and Recovery of Lateral Line Hair Cells Following Repeated Neomycin Treatments. Life, 11(11), 1180. https://doi.org/10.3390/life11111180






