In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Meat Samples
2.2. Isolation and Identification of Some Bacterial Species in the Meat Samples
2.3. Evaluation of the Antibacterial Activities of the Medicinal Plants against the Bacteria Isolated from Meat Samples by Well-Diffusion Technique
2.4. Assessment of the Impact of Essential Oil and Spices Powder on the Microbial Count in Tested Meat Samples
2.5. Statistical Analysis
3. Results
3.1. Isolation and Identification of Some Bacterial Species in the Meat Samples
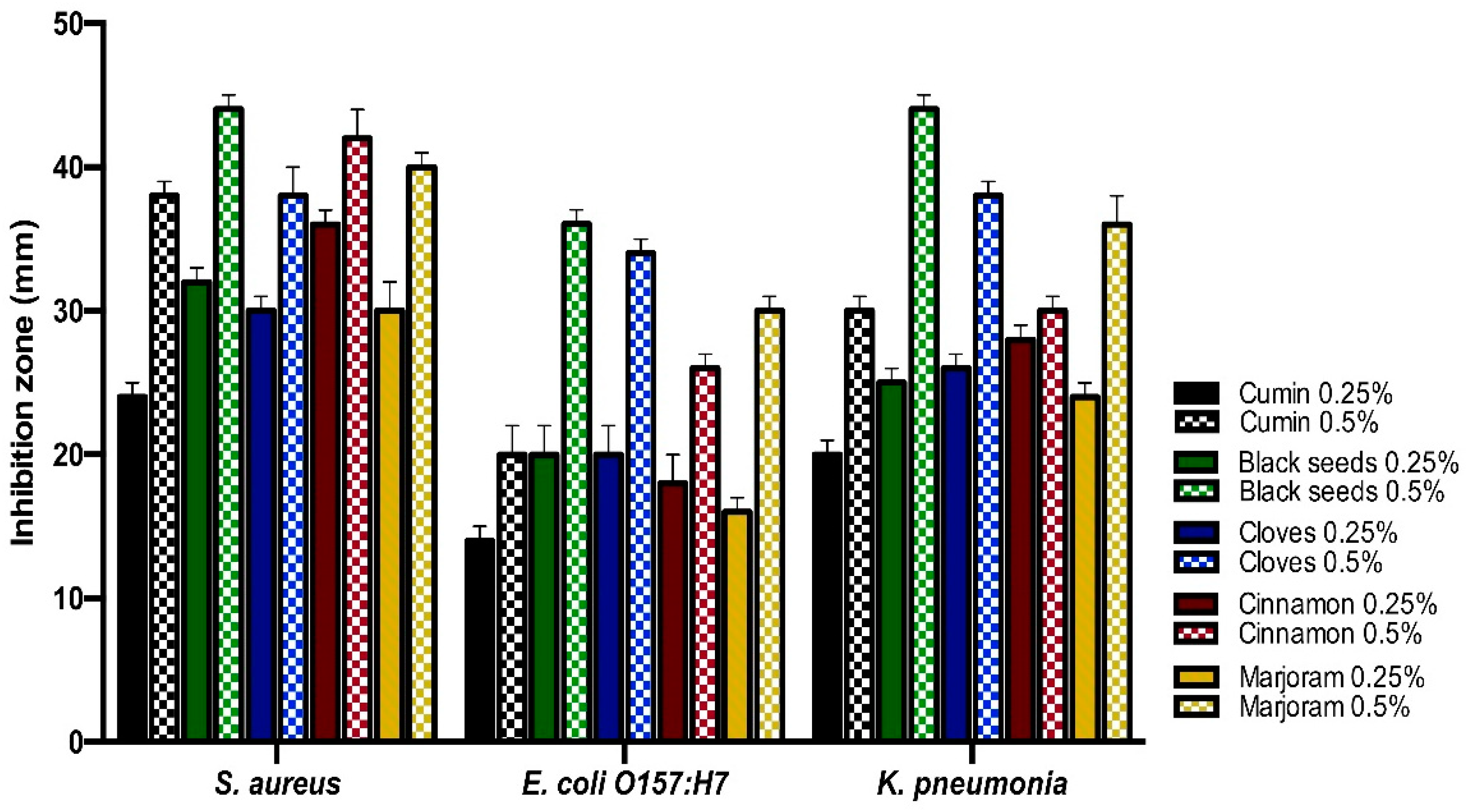
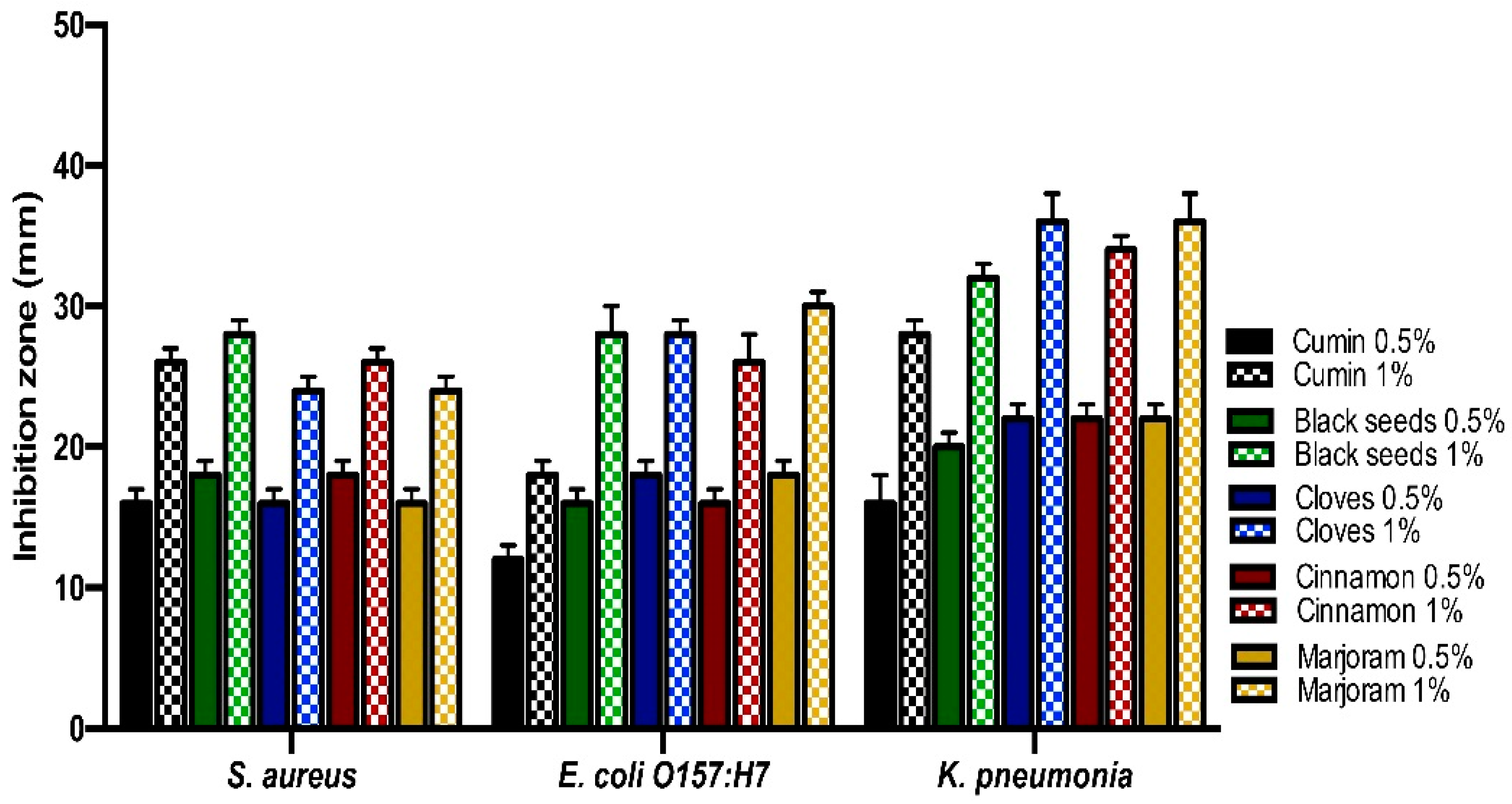
3.2. Antibacterial Activities of Essential Oils and Spices Powder of the Medicinal Plants against the Isolates by Well-Diffusion Assays Technique
3.3. Assessment of the Impact of Essential Oil and Spices Powder on the Microbial Count in Tested Meat Samples
3.3.1. Impact of Essential Oil and Spices Powder on the S. aureus Bacterial Count in Tested Meat Samples
3.3.2. Impact of Essential Oil and Spices Powder on the E. coli O157: H7 Bacterial Count in Tested Meat Samples
3.3.3. Impact of Essential Oil and Spices Powder on the K. pneumonia Bacterial Count in Tested Meat Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Antolak, H.; Czyzowska, A.; Kregiel, D. Antibacterial and antiadhesive activities of extracts from edible plants against soft drink spoilage by Asaia spp. J. Food Prot. 2017, 80, 25–34. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. Italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Chouhan, G.K.; Verma, J.P.; Jaiswal, D.K.; Mukherjee, A.; Singh, S.; de Araujo Pereira, A.P.; Liu, H.; Abd Allah, E.F.; Singh, B.K. Phytomicrobiome for promoting sustainable agriculture and food security: Opportunities, challenges, and solutions. Microbiol. Res. 2021, 248, 126763. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.; Tompkin, R. Meat and poultry products. Compend. Methods Microbiol. Exam. Foods 1992, 3, 821–835. [Google Scholar]
- Kotler, D.P.; Sordillo, E.M. A case of Staphylococcus aureus enterocolitis: A rare entity. Gastroenterol. Hepatol. 2010, 6, 117. [Google Scholar]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-resistant staphylococcus aureus (mrsa): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 2020, 13, 3255. [Google Scholar] [CrossRef] [PubMed]
- El-Mokhtar, M.A.; Hetta, H.F. Ambulance vehicles as a source of multidrug-resistant infections: A multicenter study in Assiut City, Egypt. Infect. Drug Resist. 2018, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H. Occurrence of methicillin-resistant staphylococcus aureus strains from cattle and chicken, and analyses of their meca, mecr1 and meci genes. Vet. Microbiol. 2006, 114, 155–159. [Google Scholar] [CrossRef]
- Moon, J.-S.; Lee, A.-R.; Kang, H.-M.; Lee, E.-S.; Kim, M.-N.; Paik, Y.; Park, Y.H.; Joo, Y.-S.; Koo, H. Phenotypic and genetic antibiogram of methicillin-resistant staphylococci isolated from bovine mastitis in Korea. J. Dairy Sci. 2007, 90, 1176–1185. [Google Scholar] [CrossRef]
- Kwon, N.H.; Park, K.T.; Jung, W.K.; Youn, H.Y.; Lee, Y.; Kim, S.H.; Bae, W.; Lim, J.Y.; Kim, J.Y.; Kim, J.M. Characteristics of methicillin resistant Staphylococcus aureus isolated from chicken meat and hospitalized dogs in korea and their epidemiological relatedness. Vet. Microbiol. 2006, 117, 304–312. [Google Scholar] [CrossRef]
- Jones, T.F.; Kellum, M.E.; Porter, S.S.; Bell, M.; Schaffner, W. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2002, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; Van Leeuwen, W.; Goessens, W.; Hollis, R.; Messer, S.; Herwaldt, L.; Bruining, H.; Heck, M.; Rost, J.; Van Leeuwen, N. Food-initiated outbreak of methicillin-resistant Staphylococcus aureus analyzed by pheno-and genotyping. J. Clin. Microbiol. 1995, 33, 1121–1128. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cachay, E.R.; Hunt, G.C. Klebsiella oxytoca: A rare cause of severe infectious colitis: First North American case report. Gastrointest. Endosc. 2004, 60, 142–145. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Kareem, S.M.; Al-Kadmy, I.M.; Kazaal, S.S.; Ali, A.N.M.; Aziz, S.N.; Makharita, R.R.; Algammal, A.M.; Al-Rejaie, S.; Behl, T.; Batiha, G.E.-S. Detection of gyra and parc mutations and prevalence of plasmid-mediated quinolone resistance genes in Klebsiella pneumoniae. Infect. Drug Resist. 2021, 14, 555. [Google Scholar] [CrossRef]
- Makharita, R.R.; El-Kholy, I.; Hetta, H.F.; Abdelaziz, M.H.; Hagagy, F.I.; Ahmed, A.A.; Algammal, A.M. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect. Drug Resist. 2020, 13, 3991. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [Green Version]
- Miliotis, M.D.; Bier, J.W. International Handbook of Foodborne Pathogens; CRC Press: Boca Raton, FL, USA, 2003; Volume 125. [Google Scholar]
- Algammal, A.M.; Hetta, H.F.; Batiha, G.E.; Hozzein, W.N.; El Kazzaz, W.M.; Hashem, H.R.; Tawfik, A.M.; El-Tarabili, R.M. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following fmd-outbreak in cattle. Sci. Rep. 2020, 10, 19779. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, A.; Gtari, M.; Boudabous, A.; Wagenlehner, F.M.E. Identification and susceptibility of Klebsiella and Enterobacter spp. isolated from meat products. Afr. J. Microbiol. Res. 2009, 3, 362–369. [Google Scholar]
- Yilma, Z.; Faye, B.; Loiseau, G. Occurrence and distribution of species of enterobacteriaceae in selected Ethiopian traditional dairy products: A contribution to epidemiology. Food Control 2007, 18, 1397–1404. [Google Scholar] [CrossRef]
- Gundogan, N.; Citak, S.; Yalcin, E. Virulence Properties of Extended Spectrum β-Lactamase–Producing Klebsiella Species in Meat Samples. J. Food Prot. 2011, 74, 559–564. [Google Scholar] [CrossRef]
- Edberg, S.C.; Piscitelli, V.; Cartter, M. Phenotypic characteristics of coliform and noncoliform bacteria from a public water supply compared with regional and national clinical species. Appl. Environ. Microbiol. 1986, 52, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Aguiar, F.; Solarte, A.; Gómez-Gascón, L.; Galan-Relaño, A.; Luque, I.; Tarradas, C.; Rodriguez-Ortega, M.; Huerta, B. Antimicrobial susceptibility of cinnamon and red and common thyme essential oils and their main constituent compounds against Streptococcus suis. Lett. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Genet. 2019, 17, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Bamford, R.A.; Smith, A.; Metz, J.; Glover, G.; Titball, R.W.; Pagliara, S. Investigating the physiology of viable but non-culturable bacteria by microfluidics and time-lapse microscopy. BMC Biol. 2017, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef]
- Łapińska, U.; Glover, G.; Capilla-Lasheras, P.; Young, A.J.; Pagliara, S. Bacterial ageing in the absence of external stressors. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 2018044. [Google Scholar] [CrossRef]
- Manuse, S.; Shan, Y.; Canas-Duarte, S.J.; Bakshi, S.; Sun, W.-S.; Mori, H.; Paulsson, J.; Lewis, K. Bacterial persisters are a stochastically formed subpopulation of low-energy cells. PLoS Biol. 2021, 19, e3001194. [Google Scholar] [CrossRef]
- Smith, A.; Kaczmar, A.; Bamford, R.A.; Smith, C.; Frustaci, S.; Kovacs-Simon, A.; O’Neill, P.; Moore, K.; Paszkiewicz, K.; Titball, R.W.; et al. The Culture Environment Influences Both Gene Regulation and Phenotypic Heterogeneity in Escherichia coli. Front. Microbiol. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Goode, O.; Smith, A.; Łapińska, U.; Bamford, R.; Kahveci, Z.; Glover, G.; Attrill, E.; Carr, A.; Metz, J.; Pagliara, S. Heterologous Protein Expression Favors the Formation of Protein Aggregates in Persister and Viable but Nonculturable Bacteria. ACS Infect. Dis. 2021, 7, 1848–1858. [Google Scholar] [CrossRef]
- Goode, O.; Smith, A.; Zarkan, A.; Cama, J.; Invergo, B.M.; Belgami, D.; Caño-Muñiz, S.; Metz, J.; O’Neill, P.; Jeffries, A.; et al. Persister Escherichia coli Cells Have a Lower Intracellular pH than Susceptible Cells but Maintain Their pH in Response to Antibiotic Treatment. mBio 2021, 12, e0090921. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Ledeboer, N.A.; Doern, G.V. Haemophilus. Manual of Clinical Microbiology, 11th ed.; American Society of Microbiology: Washington, DC, USA, 2015; pp. 667–684. [Google Scholar]
- Farmer, J., III. Enterobacteriaceae: Introduction and Identification. In Manual of Clinical Microbiology, 8th ed.; ASM Press: Washington, DC, USA, 2003; Volume 1, pp. 636–653. [Google Scholar]
- Cowan, S.T.; Steel, K.J. Cowan and Steel’s Manual for the Identification of Medical Bacteria; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- El-Hadedy, D.; El-Nour, S.A. Identification of Staphylococcus aureus and Escherichia coli isolated from egyptian food by conventional and molecular methods. J. Genet. Eng. Biotechnol. 2012, 10, 129–135. [Google Scholar] [CrossRef]
- Lane, D. 16s/23s rrna sequencing. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley and Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Yahia, R.; Hanora, A.; Fahmy, N.; Aly, K.A. Quorum sensing signal production by sponge-associated bacteria isolated from the Red Sea, Egypt. Afr. J. Biotechnol. 2017, 16, 1688–1698. [Google Scholar]
- Hetta, H.F.; Meshaal, A.K.; Algammal, A.M.; Yahia, R.; Makharita, R.R.; Marraiki, N.; Shah, M.A.; Hassan, H.-A.M.; Batiha, G.E.-S. In-vitro Antimicrobial Activity of Essential Oils and Spices Powder of some Medicinal Plants Against Bacillus Species Isolated from Raw and Processed Meat. Infect. Drug Resist. 2020, 13, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Rajasekar, A.; Wilkinson, S.; Sekar, R.; Bridge, J.; Medina-Roldán, E.; Moy, C.K. Biomineralisation performance of bacteria isolated from a landfill in China. Can. J. Microbiol. 2018, 64, 945–953. [Google Scholar] [CrossRef]
- Adiguzel, A.; Ozer, H.; KiliC, H.; CetiN, B. Screening of antimicrobial activity of essential oil and methanol extract of Satureja hortensis on foodborne bacteria and fungi. Czech J. Food 2007, 25, 81. [Google Scholar] [CrossRef] [Green Version]
- De, M.; De, A.K.; Banerjee, A.B. Screening of spices for antimicrobial activity. J. Spices Aromat. Crop. 1999, 8, 135–144. [Google Scholar]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Alam, S.; Ahmad, R.; Pranaw, K.; Mishra, P.; Khare, S.K. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour. Technol. 2018, 269, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Taniwaki, M.H.; Junqueira, V.C.A.; de Arruda Silveira, N.F.; Okazaki, M.M.; Gomes, R.A.R. Microbiological Examination Methods of Food and Water: A Laboratory Manual; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy. The Principles and Practice of Numerical Classification; W. H. Freeman and Co.: San Francisco, CA, USA, 1973. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Paudyal, N.; Anihouvi, V.; Hounhouigan, J.; Matsheka, M.I.; Sekwati-Monang, B.; Amoa-Awua, W.; Atter, A.; Ackah, N.B.; Mbugua, S.; Asagbra, A.; et al. Prevalence of foodborne pathogens in food from selected African countries—A meta-analysis. Int. J. Food Microbiol. 2017, 249, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Vorster, S.M.; Greebe, R.P.; Nortjé, G.L. Incidence of Staphylococcus aureus and Escherichia coli in Ground Beef, Broilers and Processed Meats in Pretoria, South Africa. J. Food Prot. 1994, 57, 305–310. [Google Scholar] [CrossRef]
- Ashater, A.M.; Farag, R.A.; Al-Ruthwani, E.K. Evaluation of bacterial load of frozen chicken thighs in Mosul markets. Iraqi J. Vet. Sci. 2012, 26, 63–69. [Google Scholar] [CrossRef]
- Adegunloye, D. Microorganisms Associated with Poultry Faeces; FAO: Rome, Italy, 2006. [Google Scholar]
- Kitai, S.; Shimizu, A.; Kawano, J.; Sato, E.; Nakano, C.; Uji, T.; Kitagawa, H. Characterization of methicillin-resistant Staphylococcus aureus isolated from retail raw chicken meat in Japan. J. Vet. Med Sci. 2005, 67, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of Action of Spanish Oregano, Chinese Cinnamon, and Savory Essential Oils against Cell Membranes and Walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Schoeni, J.L. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl. Environ. Microbiol. 1987, 53, 2394–2396. [Google Scholar] [CrossRef] [Green Version]
- Stolka, M.; Yanus, J.F.; Pai, D.M. Hole transport in solid solutions of a diamine in polycarbonate. J. Phys. Chem. 1984, 88, 4707–4714. [Google Scholar] [CrossRef]
- Cagney, C.; Crowley, H.; Duffy, G.; Sheridan, J.; O’brien, S.; Carney, E.; Anderson, W.; McDowell, D.; Blair, I.; Bishop, R. Prevalence and numbers of Escherichia coli o157: H7 in minced beef and beef burgers from butcher shops and supermarkets in the republic of ireland. Food Microbiol. 2004, 21, 203–212. [Google Scholar] [CrossRef]
- Shafini, A.; Son, R.; Mahyudin, N.; Rukayadi, Y.; Tuan Zainazor, T. Prevalence of Salmonella spp. In chicken and beef from retail outlets in Malaysia. Int. Food Res. J. 2017, 24, 21–27. [Google Scholar]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea dc. Ex benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; Rose, T.S.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Van Vuuren, S.; Viljoen, A. Unravelling the Complex Antimicrobial Interactions of Essential Oils—The Case of Thymus vulgaris (Thyme). Molecules 2014, 19, 2896–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heer, A.; Guleria, S.; Razdan, V.K. Chemical composition, antioxidant and antimicrobial activities and characterization of bioactive compounds from essential oil of Cinnamomum tamala grown in north-western Himalaya. J. Plant Biochem. Biotechnol. 2017, 26, 191–198. [Google Scholar] [CrossRef]
- Radaelli, M.; da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; Da Costa, L.A.M.A.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, S.J.; Amin, H.H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Aziz, J.M.A.; Rahman, H. Structural Characterization, Antimicrobial Activity, and In Vitro Cytotoxicity Effect of Black Seed Oil. Evidence-Based Complement. Altern. Med. 2019, 2019, 6515671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugur, A.R.; Dagi, H.T.; Öztürk, B.; Tekin, G.; Findik, D. Assessment of In vitro antibacterial activity and cytotoxicity effect of Nigella sativa oil. Pharmacogn. Mag. 2016, 12, 471–S474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakathir, H.A.; Abbas, N.A. Detection of the antibacterial effect of Nigella sativa ground seedswith water. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 159–164. [Google Scholar] [CrossRef] [PubMed]
| Food Samples (Number) | Frequency of S. aureus (Number, %) | Frequency of E. coli O157:H7 (Number, %) | Frequency of K. pneumonia (Number, %) |
|---|---|---|---|
| Fresh veal meat (6) | 2 (33.3%) | 3 (50%) | 1 (16.7%) |
| Fresh chicken meat (6) | 3 (50%) | 3 (50%) | 2 (33.3%) |
| Fresh mutton meat (3) | 00 (0.0%) | 2 (66.7%) | 0 (0.0%) |
| Beef luncheon (12) | 7 (58.4%) | 6 (50%) | 4 (33.3%) |
| Chicken luncheon (12) | 7 (58.4%) | 6 (50%) | 5 (41.7%) |
| Ground beef (12) | 8 (66.7%) | 4 (33.4%) | 1 (8.4%) |
| Basterma (6) | 3 (50%) | 3 (50%) | 1 (16.7%) |
| Beef burger (3) | 3 (100%) | 3 (100%) | 1 (33.3%) |
| Total (60) | 33 (55%) | 30 (50%) | 15 (25%) |
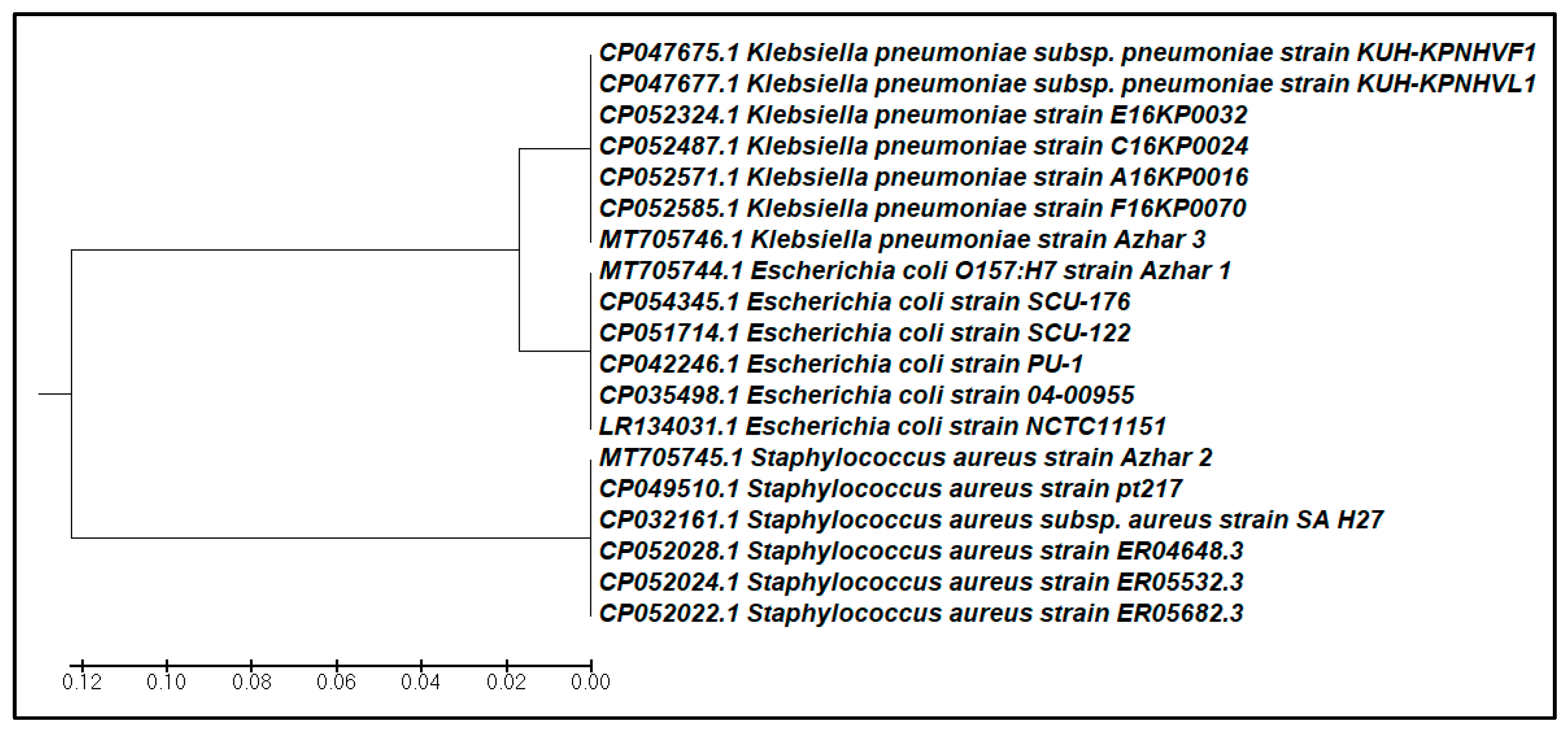
| Isolates | Name of Closely Associated Strain | Identity % | Gene Bank Accession Number of the Closely Associated Strain |
|---|---|---|---|
| Azhar1 | Staphylococcus aureus subsp. Aureus str. Newbould 305 | 100% | EJE55184.1 |
| Azhar2 | Klebsiella pneumoniae strain F16KP0070 | 100% | CP052585.1 |
| Azhar3 | Escherichia coli strain SCU-176 | 99.76% | CP054345.1 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.339 | 4.333 | 4.368 | 4.415 | 4.399 | 4.464 | 4.386 ± 0.04987 | - |
| Cumin oil 0.25% | 4.269 | 4.049 | 3.909 | 3.613 | 0.00 | 0.00 | 2.640 ± 2.056 | 0.0048 |
| Cumin oil 0.50% | 4.218 | 3.992 | 3.827 | 3.545 | 0.00 | 0.00 | 2.597 ± 2.024 | 0.0028 |
| Black seeds oil 0.25% | 4.236 | 4.359 | 4.013 | 3.478 | 0.00 | 0.00 | 2.681 ± 2.099 | 0.0100 |
| Black seeds oil 0.50% | 4.046 | 3.852 | 3.177 | 0.00 | 0.00 | 0.00 | 1.846 ± 2.042 | 0.0002 |
| Cloves oil 0.25% | 4.179 | 3.978 | 3.881 | 3.725 | 3.323 | 0.00 | 3.181 ± 1.585 | 0.0062 |
| Cloves oil 0.50% | 4.159 | 3.959 | 3.741 | 3.398 | 0.00 | 0.00 | 2.543 ± 1.986 | 0.0016 |
| Cinnamon oil 0.25% | 4.189 | 4.083 | 3.819 | 3.279 | 0.00 | 0.00 | 2.562 ± 2.009 | 0.0023 |
| Cinnamon oil 0.50% | 4.284 | 4.268 | 3.654 | 0.00 | 0.00 | 0.00 | 2.034 ± 2.240 | 0.0022 |
| Marjoram oil 0.25% | 4.207 | 4.121 | 3.919 | 3.624 | 3.042 | 0.00 | 3.152 ± 1.601 | 0.0078 |
| Marjoram oil 0.50% | 4.251 | 3.914 | 3.663 | 3.398 | 0.00 | 0.00 | 2.538 ± 1.986 | 0.0018 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.358 | 4.325 | 4.419 | 4.454 | 4.469 | 4.493 | 4.420 ± 0.06596 | - |
| Cumin 0.50% | 4.284 | 4.291 | 3.786 | 3.492 | 3.042 | 0.00 | 3.149 ± 1.615 | 0.0086 |
| Cumin 1.0% | 4.233 | 4.046 | 3.887 | 3.613 | 0.00 | 0.00 | 2.630 ± 2.047 | 0.0033 |
| Black seeds 0.50% | 4.188 | 4.018 | 3.839 | 0.00 | 0.00 | 0.00 | 2.008 ± 2.202 | 0.0010 |
| Black seeds 1.0% | 4.179 | 3.992 | 3.741 | 0.00 | 0.00 | 0.00 | 1.985 ± 2.179 | 0.0007 |
| Cloves 0.50% | 4.182 | 4.049 | 3.833 | 3.613 | 0.00 | 0.00 | 2.613 ± 2.033 | 0.0023 |
| Cloves 1.0% | 4.124 | 3.978 | 3.691 | 3.079 | 0.00 | 0.00 | 2.479 ± 1.953 | 0.0008 |
| Cinnamon 0.50% | 4.188 | 4.124 | 3.858 | 3.673 | 3.492 | 0.00 | 3.223 ± 1.601 | 0.0074 |
| Cinnamon 1.0% | 4.144 | 4.104 | 3.756 | 0.00 | 0.00 | 0.00 | 2.001 ± 2.196 | 0.0008 |
| Marjoram 0.50% | 4.218 | 4.124 | 3.978 | 3.699 | 2.699 | 0.00 | 3.120 ± 1.625 | 0.0083 |
| Marjoram 1.0% | 4.144 | 4.076 | 3.864 | 3.613 | 3.463 | 0.00 | 3.193 ± 1.586 | 0.0047 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.325 | 4.308 | 4.345 | 4.373 | 4.405 | 4.461 | 4.370 ± 0.0565 | - |
| Cumin oil 0.25% | 4.277 | 4.177 | 4.049 | 3.949 | 3.492 | 0.00 | 3.324 ± 1.651 | 0.0111 |
| Cumin oil 0.5% | 4.265 | 4.159 | 4.083 | 3.839 | 3.343 | 0.00 | 3.282 ± 1.641 | 0.0078 |
| Black seeds oil 0.25% | 4.239 | 4.065 | 3.959 | 3.858 | 3.114 | 0.00 | 3.206 ± 1.618 | 0.0029 |
| Black seeds oil 0.5% | 4.209 | 3.949 | 3.733 | 3.079 | 0.00 | 0.00 | 2.495 ± 1.969 | 0.0003 |
| Cloves oil 0.25% | 4.241 | 4.072 | 3.929 | 3.623 | 0.00 | 0.00 | 2.644 ± 2.058 | 0.0013 |
| Cloves oil 0.5% | 4.162 | 4.118 | 3.978 | 3.506 | 0.00 | 0.00 | 2.627 ± 2.048 | 0.0015 |
| Cinnamon oil 0.25% | 4.228 | 4.156 | 4.46 | 3.959 | 3.624 | 0.00 | 3.405 ± 1.691 | 0.0272 |
| Cinnamon oil 0.5% | 4.046 | 4.083 | 3.869 | 2.955 | 2.612 | 0.00 | 2.928 ± 1.558 | 0.0004 |
| Marjoram oil 0.25% | 4.223 | 4.089 | 4.005 | 3.945 | 3.869 | 3.279 | 3.902 ± 0.3285 | 0.0132 |
| Marjoram oil 0.5% | 4.258 | 4.061 | 3.987 | 3.89 | 3.771 | 3.415 | 3.897 ± 0.2876 | 0.0104 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.349 | 4.359 | 4.377 | 4.417 | 4.405 | 4.455 | 4.394 ± 0.03977 | - |
| Cumin 0.5% | 4.282 | 4.228 | 4.083 | 3.964 | 0.00 | 0.00 | 2.760 ± 2.140 | 0.0068 |
| Cumin 1.0% | 4.277 | 4.185 | 3.323 | 2.584 | 1.688 | 0.00 | 2.676 ± 1.637 | 0.0014 |
| Black seeds 0.5% | 4.258 | 4.111 | 4.057 | 3.876 | 0.00 | 0.00 | 2.717 ± 2.108 | 0.0031 |
| Black seeds 1.0% | 4.236 | 3.983 | 3.799 | 3.323 | 0.00 | 0.00 | 2.557 ± 2.003 | 0.0004 |
| Cloves 0.5% | 4.261 | 4.087 | 3.959 | 3.699 | 0.00 | 0.00 | 2.668 ± 2.074 | 0.0016 |
| Cloves 1.0% | 4.191 | 4.124 | 4.005 | 3.557 | 0.00 | 0.00 | 2.646 ± 2.062 | 0.0017 |
| Cinnamon 0.5% | 4.083 | 4.101 | 3.909 | 3.343 | 3.079 | 0.00 | 3.086 ± 1.568 | 0.0011 |
| Cinnamon 1.0% | 4.239 | 4.162 | 4.083 | 4.026 | 0.00 | 0.00 | 2.752 ± 2.133 | 0.0056 |
| Marjoram 0.5% | 4.233 | 4.111 | 4.046 | 3.987 | 3.819 | 3.323 | 3.920 ± 0.3229 | 0.0140 |
| Marjoram 1.0% | 4.275 | 4.079 | 4.038 | 3.909 | 3.725 | 0.00 | 3.338 ± 1.645 | 0.0055 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.297 | 4.347 | 4.364 | 4.435 | 4.452 | 4.479 | 4.396 ± 0.07039 | - |
| Cumin oil 0.25% | 4.239 | 3.992 | 3.807 | 3.463 | 0.00 | 0.00 | 2.584 ± 2.017 | 0.0036 |
| Cumin oil 0.5% | 4.188 | 3.939 | 3.613 | 0.00 | 0.00 | 0.00 | 1.957 ± 2.151 | 0.0010 |
| Black seeds oil 0.25% | 4.221 | 4.076 | 3.929 | 3.579 | 0.00 | 0.00 | 2.634 ± 2.052 | 0.0050 |
| Black seeds oil 0.5% | 4.076 | 3.819 | 0.00 | 0.00 | 0.00 | 0.00 | 1.316 ± 2.040 | 0.0002 |
| Cloves oil 0.25% | 4.185 | 4.087 | 3.813 | 3.613 | 0.00 | 0.00 | 2.616 ± 2.037 | 0.0047 |
| Cloves oil 0.5% | 4.177 | 3.644 | 0.00 | 0.00 | 0.00 | 0.00 | 1.304 ± 2.026 | 0.0002 |
| Cinnamon oil 0.25% | 4.228 | 4.153 | 4.079 | 3.749 | 3.398 | 0.00 | 3.268 ± 1.630 | 0.0212 |
| Cinnamon oil 0.5% | 4.149 | 3.929 | 3.624 | 0.00 | 0.00 | 0.00 | 1.950 ± 2.143 | 0.0008 |
| Marjoram oil 0.25% | 4.291 | 4.244 | 3.978 | 3.708 | 3.519 | 0.00 | 3.290 ± 1.639 | 0.0281 |
| Marjoram oil 0.5% | 4.144 | 3.869 | 3.557 | 3.177 | 0.00 | 0.00 | 2.458 ± 1.931 | 0.0011 |
| Treatments | Storage Periods (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | Mean ± S.D | p Value | |
| Control | 4.299 | 4.343 | 4.358 | 4.409 | 4.437 | 4.455 | 4.384 ± 0.06009 | - |
| Cumin 0.5% | 4.246 | 4.009 | 3.852 | 3.519 | 0.00 | 0.00 | 2.604 ± 2.031 | 0.0027 |
| Cumin 1.0% | 4.207 | 3.969 | 3.741 | 3.415 | 0.00 | 0.00 | 2.555 ± 1.997 | 0.0017 |
| Black seeds 0.5% | 4.231 | 4.345 | 3.959 | 3.644 | 0.00 | 0.00 | 2.697 ± 2.103 | 0.0088 |
| Black seeds 1.0% | 4.087 | 3.852 | 3.302 | 0.00 | 0.00 | 0.00 | 1.874 ± 2.068 | 0.0002 |
| Cloves 0.5% | 4.207 | 4.114 | 3.864 | 3.741 | 0.00 | 0.00 | 2.654 ± 2.063 | 0.0044 |
| Cloves 1.0% | 4.202 | 3.749 | 3.569 | 0.00 | 0.00 | 0.00 | 1.920 ± 2.113 | 0.0003 |
| Cinnamon 0.5% | 4.248 | 4.194 | 4.118 | 3.852 | 3.592 | 0.00 | 3.334 ± 1.652 | 0.0233 |
| Cinnamon 1.0% | 4.177 | 3.964 | 3.733 | 3.177 | 0.00 | 0.00 | 2.509 ± 1.971 | 0.0011 |
| Marjoram 0.5% | 4.297 | 4.262 | 4.038 | 3.819 | 3.624 | 0.00 | 3.340 ± 1.656 | 0.0284 |
| Marjoram 1.0% | 4.156 | 3.909 | 3.654 | 3.398 | 3.042 | 0.00 | 3.027 ± 1.533 | 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshaal, A.K.; Hetta, H.F.; Yahia, R.; Abualnaja, K.M.; Mansour, A.T.; Al-Kadmy, I.M.S.; Alghamdi, S.; Dablool, A.S.; Emran, T.B.; Sedky, H.; et al. In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life 2021, 11, 1178. https://doi.org/10.3390/life11111178
Meshaal AK, Hetta HF, Yahia R, Abualnaja KM, Mansour AT, Al-Kadmy IMS, Alghamdi S, Dablool AS, Emran TB, Sedky H, et al. In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life. 2021; 11(11):1178. https://doi.org/10.3390/life11111178
Chicago/Turabian StyleMeshaal, Ahmed Kh., Helal F. Hetta, Ramadan Yahia, Khamael M. Abualnaja, Abdallah Tageldein Mansour, Israa M. S. Al-Kadmy, Saad Alghamdi, Anas S. Dablool, Talha Bin Emran, Haitham Sedky, and et al. 2021. "In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat" Life 11, no. 11: 1178. https://doi.org/10.3390/life11111178
APA StyleMeshaal, A. K., Hetta, H. F., Yahia, R., Abualnaja, K. M., Mansour, A. T., Al-Kadmy, I. M. S., Alghamdi, S., Dablool, A. S., Emran, T. B., Sedky, H., Batiha, G. E.-S., & El-Kazzaz, W. (2021). In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life, 11(11), 1178. https://doi.org/10.3390/life11111178












