The Hypopiezotolerant Bacterium, Serratia liquefaciens, Failed to Grow in Mars Analog Soils under Simulated Martian Conditions at 7 hPa
Abstract
1. Introduction
2. Methods
2.1. Microbiological Procedures
2.2. Mars Analog Soils
2.3. Sonication to Enhance Recovery of Cells from Mars Analog Soils
2.4. Extraction of Bacterial Cells from Doped Soils
2.5. Growth of S. liquefaciens in a Minimal Liquid Growth Medium
2.6. Growth of S. liquefaciens in Mars Analog Soils
2.7. Growth of S. liquefaciens under Simulated Mars Low-PTA Conditions
2.8. Statistical Methods
3. Results
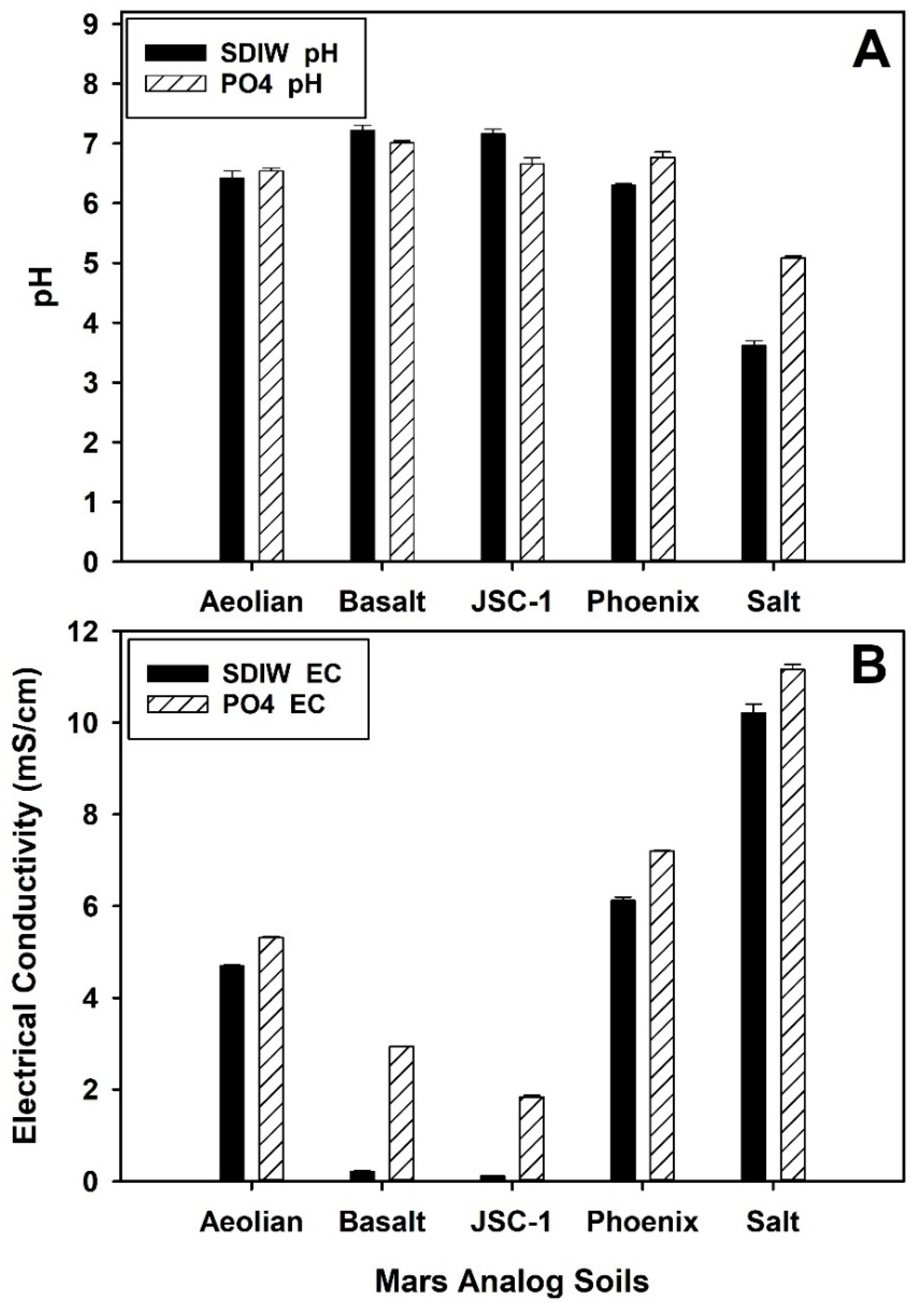
3.1. pH and EC of Mars Analog Soils
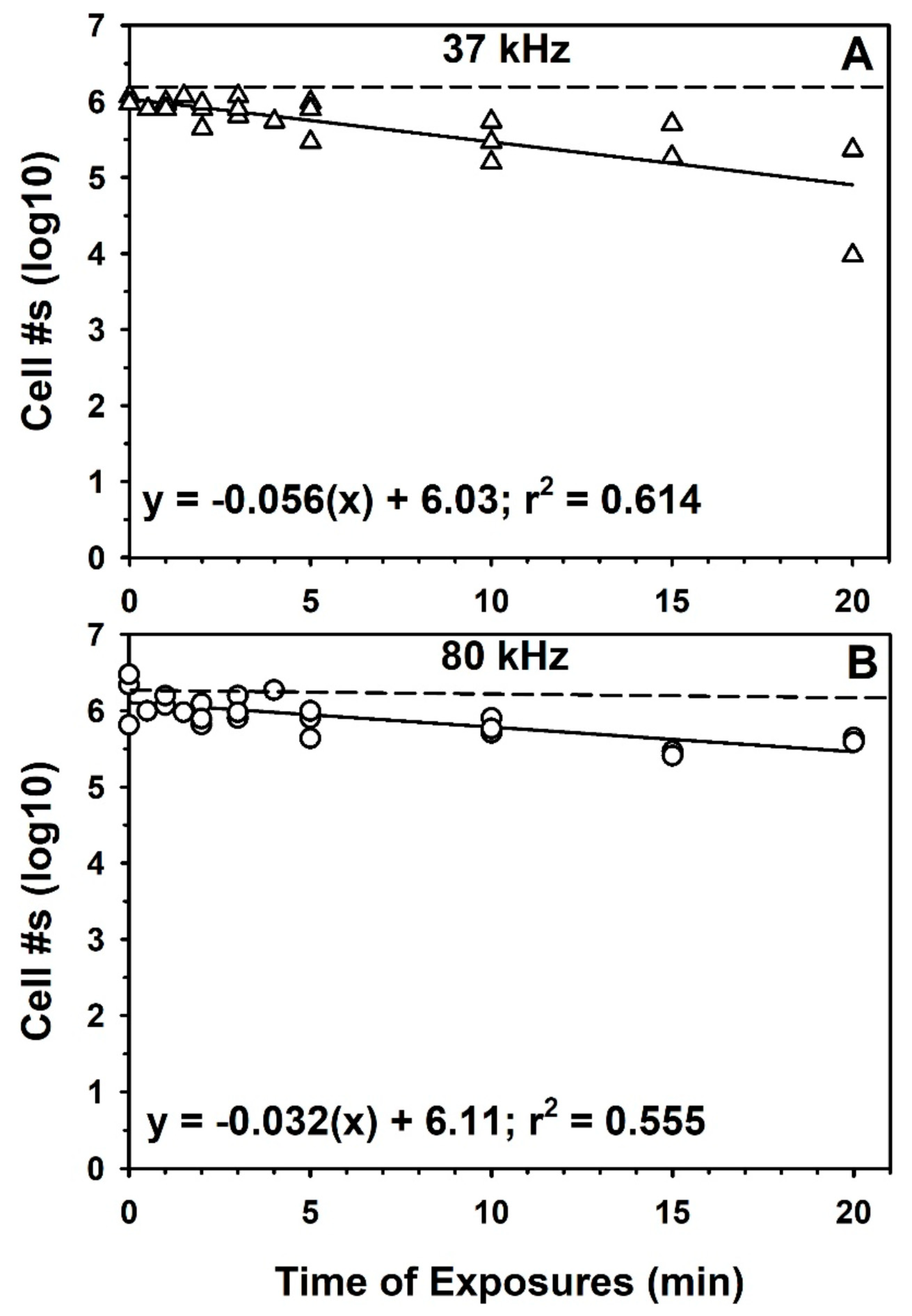
3.2. Sonication
3.3. Soil Extraction of S. liquefaciens Cells from JSC-1
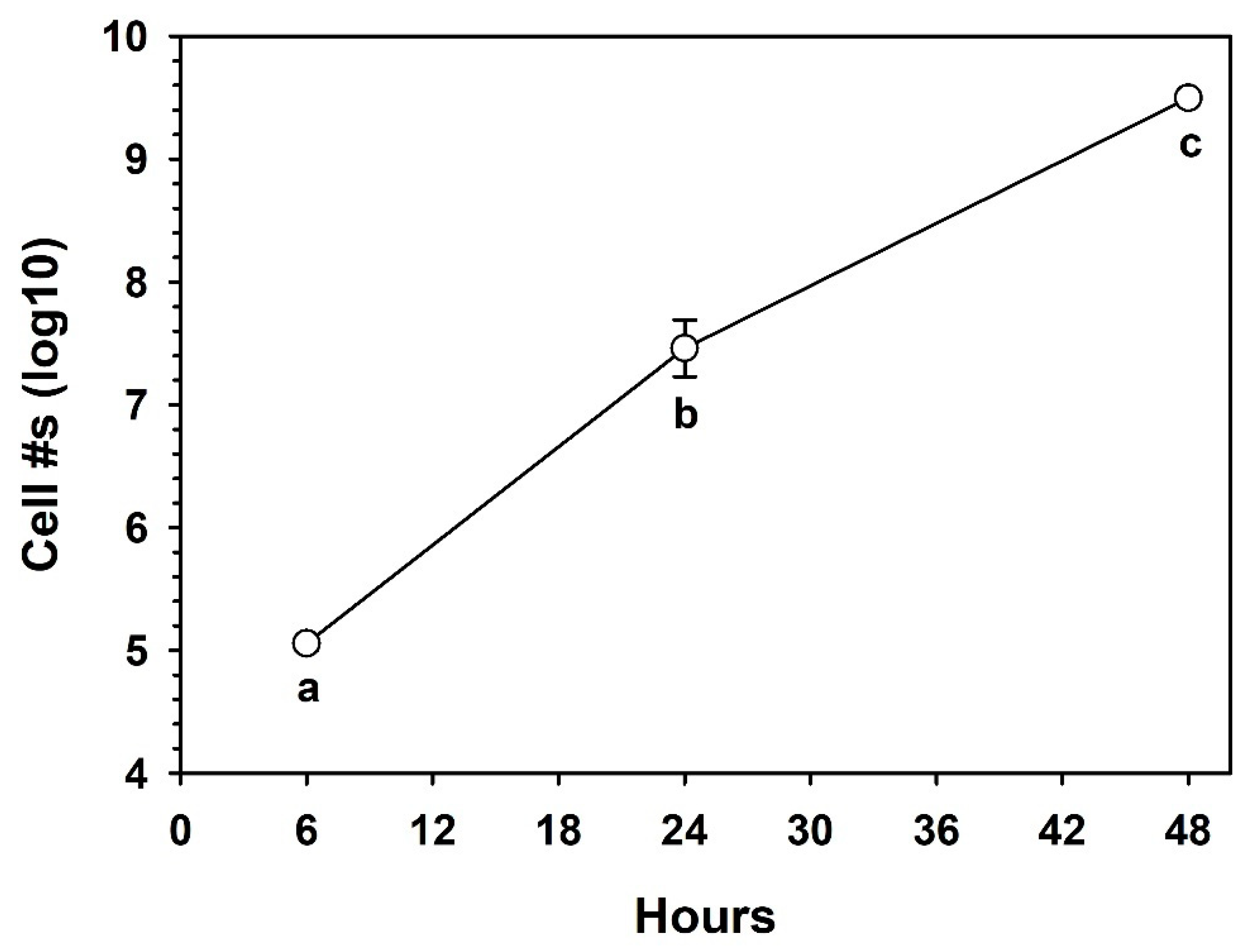
3.4. Growth in the Minimal Basal Medium (MBM)
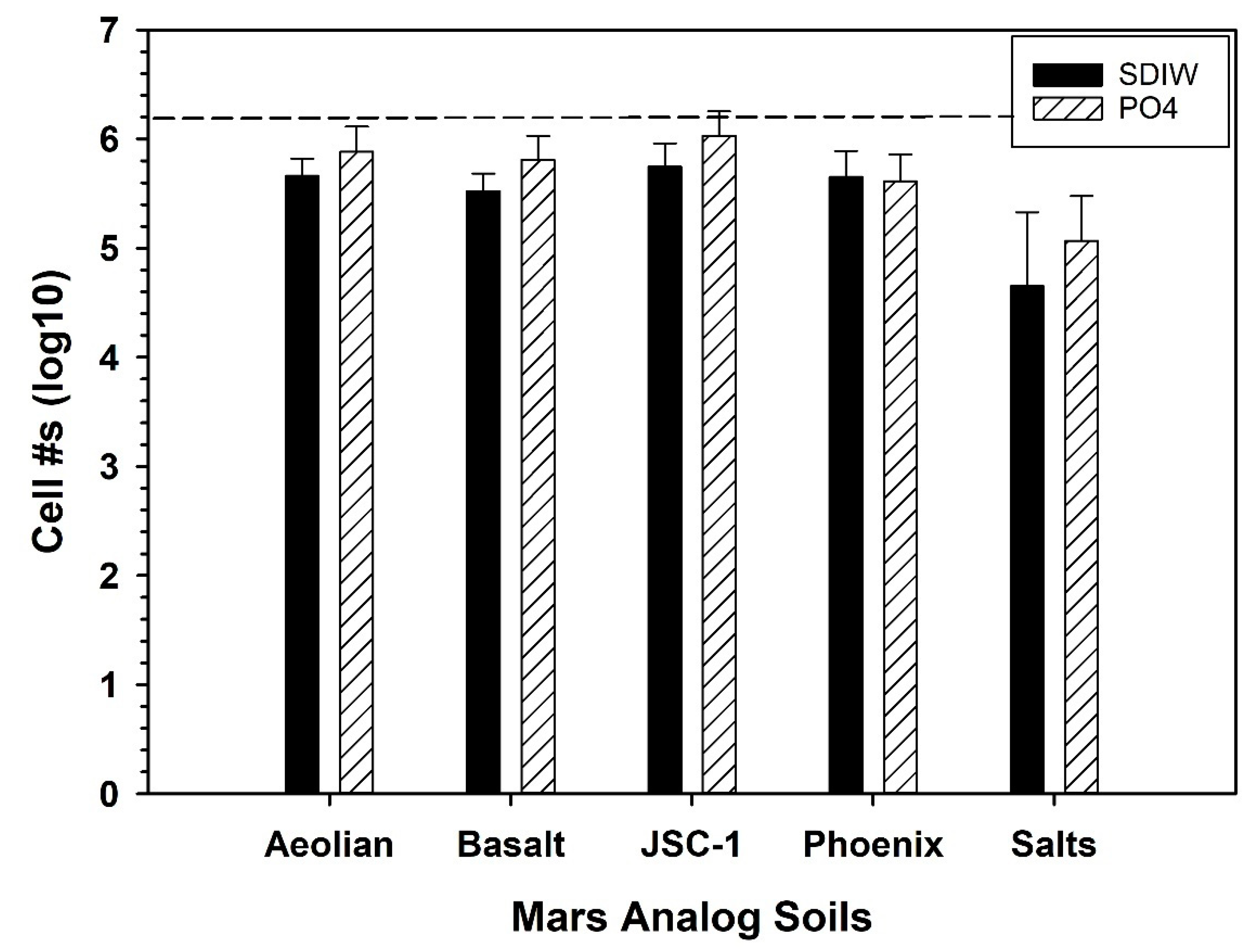
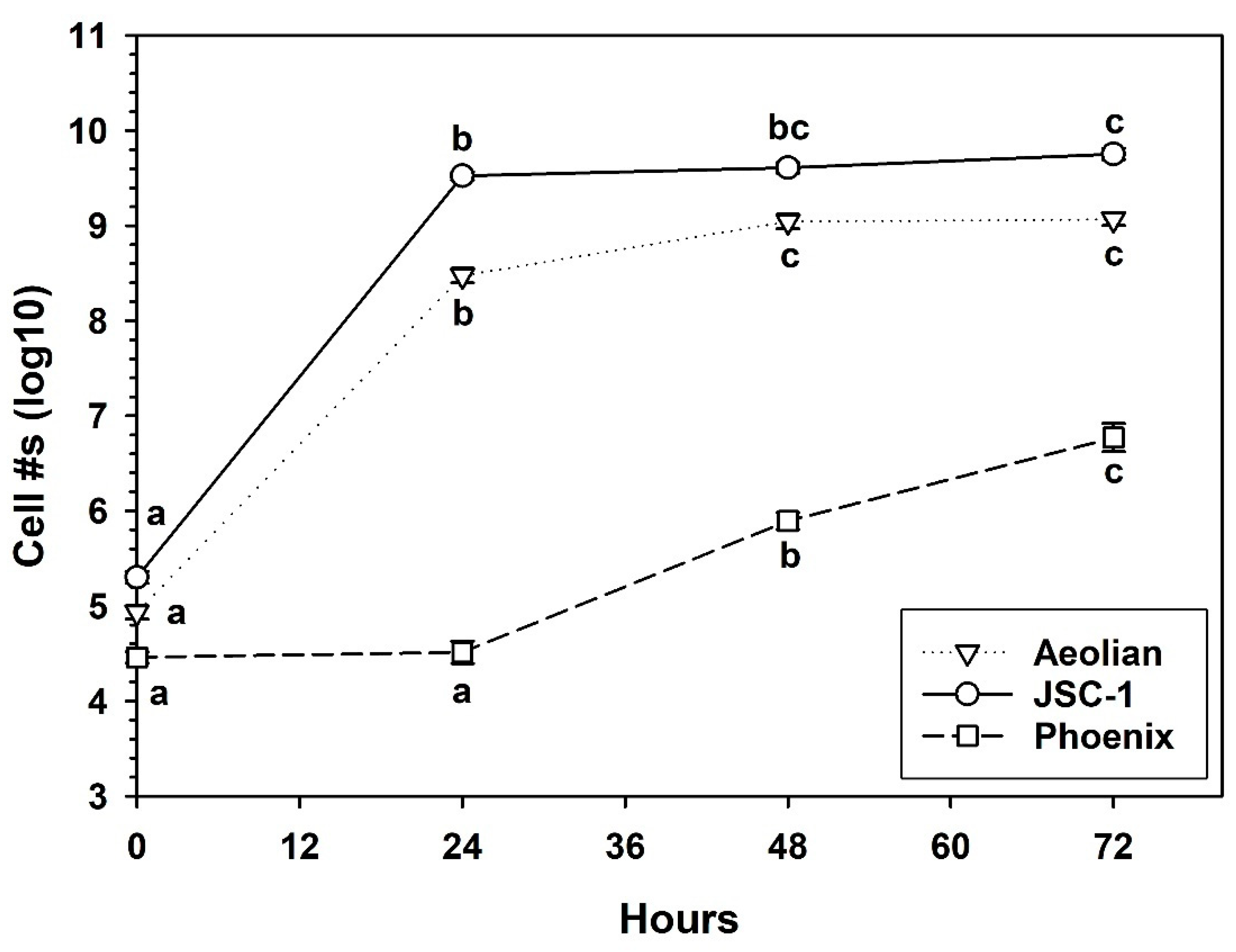
3.5. Growth of S. liquefaciens in Mars Analog Soils
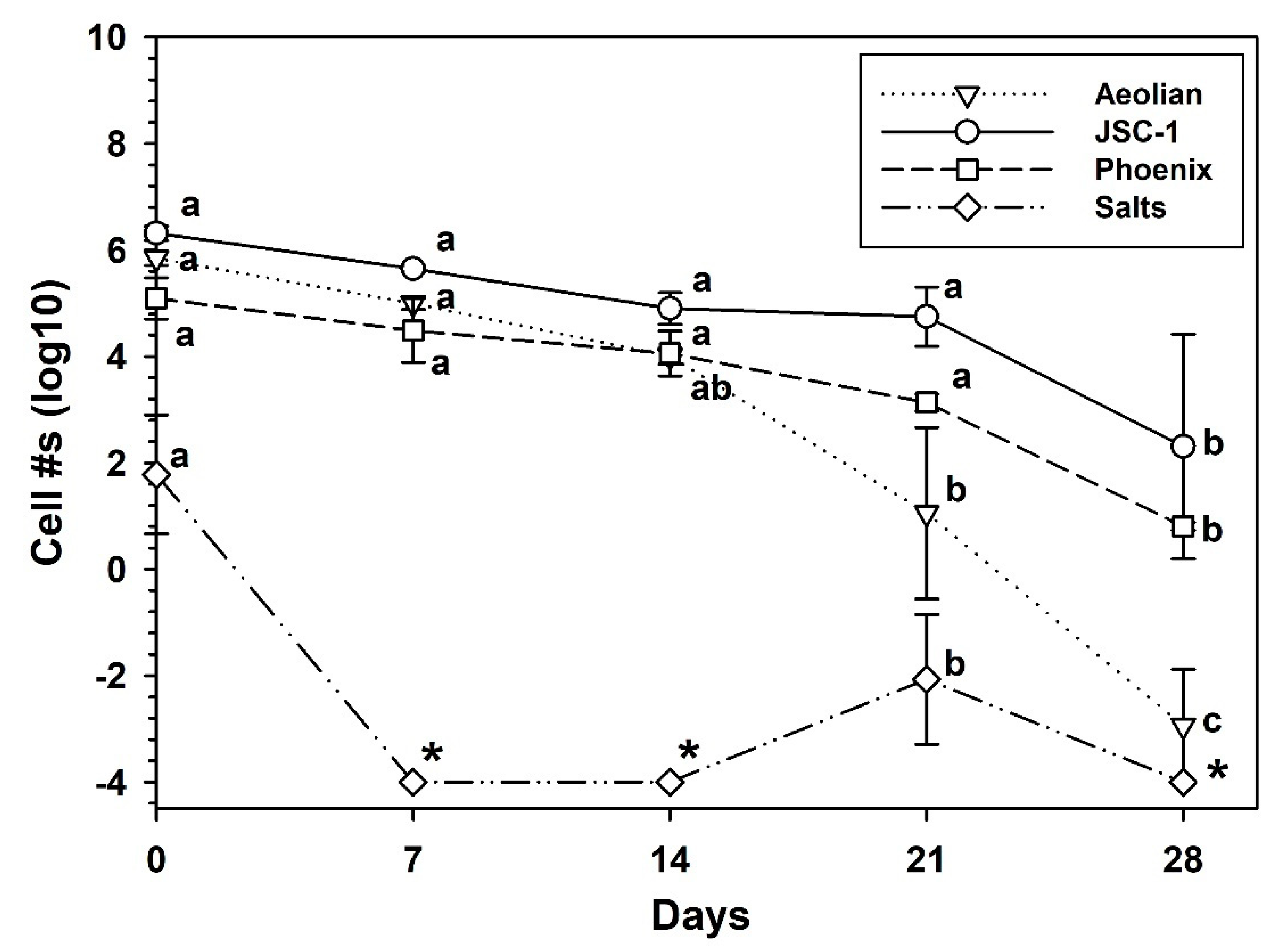
3.6. Growth of S. liquefaciens under Simulated Mars Low-PTA Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosure Statement
References
- Schuerger, A.C.; Golden, D.C.; Ming, D.W. Biotoxicity of Mars soils: 1. Dry deposition of analog soils on microbial colonies and survival under martian conditions. Planet. Space Sci. 2012, 72, 91–101. [Google Scholar] [CrossRef]
- Chan-Yam, K.; Goordial, J.; Greer, C.; Davila, A.; McKay, C.P.; Whyte, L.G. Microbial activity and habitability of an Antarctic dry valley water track. Astrobiology 2019, 19, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Schwendner, P.; Perras, A.; Rettberg, P.; Beblo-Vranesevic, K.; Bohmeier, M.; Rabbow, E.; Moissl-Eichinger, C.; Wink, L.; Marteinsson, V.; et al. Anaerobic microorganisms in astrobiological analogue environments: From field site to culture collection. Int. J. Astrobiol. 2017, 17, 314–328. [Google Scholar] [CrossRef]
- Horneck, G.; Baumstark-Khan, C. Astrobiology: The Quest for the Conditions of Life; Springer-Verlag: Köln, Germany, 2002; p. 411. [Google Scholar]
- Jakosky, B. The Search for Life on Other Planets; Cambridge University Press: Cambridge, UK, 1988; p. 326. [Google Scholar]
- Rummel, J.D.; Beaty, D.W.; Jones, M.A.; Bakermans, C.; Barlow, N.G.; Boston, P.J.; Chevrier, V.F.; Clark, B.C.; de Vera, J.-P.; Gough, R.V.; et al. A new analysis of Mars "Special Regions": Findings of the Second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 2014, 14, 887–968. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.A.; Lee, K.C.; Archer, S.D.J.; Cabrol, N.; Ng-Boyle, L.; Wettergreen, D.; Zacny, K.; Pointing, S.B.; The NASA life in the Atacama Project Team. Subsurface Microbial Habitats in an Extreme Desert Mars-Analog Environment. Front. Microbiol. 2019, 10, 69. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Mancinelli, R.L.; Kern, R.G.; Rothschild, L.J.; McKay, C.P. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: Implications for the forward contamination of Mars. Icarus 2003, 165, 253–276. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Ulrich, R.; Berry, B.J.; Nicholson, W.L. Growth of Serratia liquefaciens under 7 mbar, 0 °C, and CO2-enriched anoxic atmospheres. Astrobiology 2013, 13, 115–131. [Google Scholar] [CrossRef]
- Smith, S.A.; Benardini, J.N.; Anderl, D.; Ford, M.; Wear, E.; Schrader, M.; Schubert, W.; DeVeaux, L.; Paszczynski, A.; Childers, S.E. Identification and characterization of early mission phase microorganisms residing on the Mars Science Laboratory and assessment of their potential to survive Mars-like conditions. Astrobiology 2017, 17, 253–265. [Google Scholar] [CrossRef]
- Schwendner, P.; Schuerger, A.C. Exploring microbial activity in low-pressure environments. Curr. Issues Mol. Biol. 2020, 38, 163–196. [Google Scholar] [CrossRef]
- Fajardo-Cavazos, P.; Morrison, M.D.; Miller, K.M.; Schuerger, A.C.; Nicholson, W.L. Transcriptomic responses of Serratia liquefaciens cells grown under simulated Martian conditions of low temperature, low pressure, and CO2-enriched anoxic atmosphere. Sci. Rep. 2018, 8, 14938. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Krivushin, K.; Gilichinsky, D.; Schuerger, A.C. Growth of Carnobacterium spp. isolated from Siberian permafrost under simulated Mars conditions of pressure, temperature and atmosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kanervo, E.; Lehto, K.; Stahle, K.; Lehto, H.; Maenpaa, P. Characterization of growth and photosynthesis of Synechocystis sp. PCC6803 cultures under reduced atmospheric pressures and enhanced CO2 levels. Int. J. Astrobiol. 2005, 4, 97–100. [Google Scholar] [CrossRef]
- Sakon, J.J.; Burnap, R.L. An analysis of potential photosynthetic life on Mars. Int. J. Astrobiol. 2006, 5, 171–180. [Google Scholar] [CrossRef][Green Version]
- Schuerger, A.C.; Nicholson, W.L. Twenty species of hypobarophilic bacteria recovered from diverse soils exhibit growth under simulated martian conditions at 0.7 kPa. Astrobiology 2016, 16, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.M.; Robles-Martinez, J.A.; Nicholson, W.L. Exposure of Bacillus subtilis to low pressure (5 kilopascals) induces several global regulons, including those involved in the SigB-mediated general stress response. Appl. Environ. Microbiol. 2014, 80, 4788–4794. [Google Scholar] [CrossRef][Green Version]
- Schwendner, P.; Schuerger, A.C. Metabolic fingerprints of Serratia liquefaciens under simulated Martian conditions using Biolog GN2 microarrays. Sci. Rep. 2018, 8, 15721. [Google Scholar] [CrossRef]
- Berry, B.J.; Jenkins, D.G.; Schuerger, A.C. Effect of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl. Environ. Microbiol. 2010, 76, 2377–2386. [Google Scholar] [CrossRef]
- Novikova, N. Review of the knowledge of microbial contamination of the Russian manned spacecraft. Microbial Ecol. 2004, 47, 127–132. [Google Scholar] [CrossRef]
- Moissl, C.; Osman, S.; La Duc, M.T.; Dekas, A.; Brodie, E.; DeSantis, T.; Venkateswaran, K. Molecular bacterial community analysis of clean rooms where spacecraft are assembled. FEMS Microbiol. Ecol. 2007, 61, 509–521. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Vaishampayan, P.; Benardini, J.N., III; Rooney, A.P.; Spry, J.A. Deposition of extreme-tolerant bacterial strains isolated during different phases of Phoenix spacecraft assembly in a public culture collection. Astrobiology 2014, 14, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Schuerger, A.C.; Billi, D.; Friedmann, E.I.; Panitz, C. Effects of a simulated martian UV flux on the cyanobacterium, Chroococcidiopsis sp. 029. Astrobiology 2005, 5, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Rettberg, P.; Flemming, H.-C. Growth of the acidophilic iron-sulfur bacterium Acidithiobacillus ferrooxidans under Mars-like geochemical conditions. Planet. Space Sci. 2014, 98, 205–215. [Google Scholar] [CrossRef]
- Kral, T.A.; Altheide, T.S.; Lueders, A.E.; Schuerger, A.C. Low pressure and desiccation effects on methanogens: Implications for life on Mars. Planet. Space Sci. 2011, 59, 264–270. [Google Scholar] [CrossRef]
- Thomas, D.J.; Eubanks, L.M.; Rector, C.; Warrington, J.; Todd, P. Effects of atmospheric pressure on the survival of photosynthetic microorganisms during simulations of ecopoesis. Int. J. Astrobiol. 2008, 7, 243–249. [Google Scholar] [CrossRef][Green Version]
- Mickol, R.L.; Page, J.; Schuerger, A.C. Magnesium sulfate salt solutions and ices fail to protect Serratia liquefaciens from the biocidal effects of UV irradiation under martian conditions. Astrobiology 2017, 17, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.C.; Jager, K.M.; Morris, R.V.; Lindstrom, D.J.; Lindstrom, M.M.; Lockwood, J.P. JSC Mars-1: A Martian soil simulant. Proc. Conf. Am. Soc. Civ. Eng. 1998, 469–476. [Google Scholar]
- Schuerger, A.C.; Ming, D.W.; Golden, D.C. Biotoxicity of Mars soils: 2. Survival of Bacillus subtilis and Enterococcus faecalis in aqueous extracts derived from six Mars analog soils. Icarus 2017, 290, 215–223. [Google Scholar] [CrossRef]
- Weiblen, P.W.; Morey, G.B. A summary of the stratigraphy, petrology and structure of the Duluth Complex. Am. J. Sci. 1980, 280-A, 88–133. [Google Scholar]
- Spizizen, J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 1958, 44, 1072–1078. [Google Scholar] [CrossRef]
- Janssen, P.H.; Yates, P.S.; Grinton, B.E.; Taylor, P.M.; Sait, M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Protoebacteria, and Verrucomicrobia. Appl. Envrion. Microbiol. 2002, 68, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Courtois, S.; Frostegard, A.; Goransson, P.; Depret, G.; Jeannin, P.; Simonet, P. Quantification of bacterial subgroups in soil: Comparison of DNA extracted directly from soil and from cells previously released by density gradients centrifugation. Environ. Microbiol. 2001, 3, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Olsson-Francis, K.; Cockell, C.S. Experimental methods for studying microbial survival in extraterrestrial environments. J. Microbiol. Methods 2010, 80, 1–13. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Fajardo-Cavazos, P.; Fedenko, J.; Ortiz-Lugo, J.-L.; Rivas-Castillo, A.; Schuerger, A.C. Exploring the low-pressure growth limit: Laboratory evolution of Bacillus subtilis to enhanced growth at 5 kilopascals. Appl. Environ. Micro. 2010, 76, 7559–7565. [Google Scholar] [CrossRef] [PubMed]
- Haberle, R.M.; McKay, C.P.; Schaeffer, J.; Cabrol, N.A.; Grin, E.A.; Zent, A.P.; Quinn, R. On the possibility of liquid water on present-day Mars. J. Geophys. Res. 2001, 106, 23317–23326. [Google Scholar] [CrossRef]
- Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Schulze-Makuch, D. Enhanced microbial survival in subzero brines. Astrobiology 2018, 18, 1171–1180. [Google Scholar] [CrossRef]
- Gordon, A.S.; Millero, F.J. Electrolyte effects on attachment of an estuarine bacterium. Appl. Environ. Microbiol. 1984, 47, 496–499. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Richards, J.T.; Newcombe, D.A.; Venkateswaran, K.J. Rapid inactivation of seven Bacillus spp. under simulated Mars UV irradiation. Icarus 2006, 181, 52–62. [Google Scholar] [CrossRef]
- Stoker, C.R.; Zent, A.; Catling, D.C.; Douglas, S.; Marshall, J.R.; Archer Jr., D.; Clark, B.; Kounaves, S.P.; Lemmon, M.T.; Quinn, R.; et al. Habitability of the Phoenix landing site. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Grau, F.H. Role of pH, lactate, and anaerobiosis in controlling the growth of some fermentative Gram-negative bacteria on beef. Appl. Environ. Microbiol. 1981, 42, 1043–1050. [Google Scholar] [CrossRef]
- Starliper, C.E.; Watten, B.J. Bactericidal efficacy of elevated pH on fish pathogenic and environmental bacteria. J. Adv. Res. 2013, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.K.; Shelegedin, V.N.; Vdovina, M.A.; Pavlov, A.A. Growth of microorganisms in Martian-like shallow subsurface conditions: Laboratory modeling. Int. J. Astrobiol. 2010, 9, 51–58. [Google Scholar] [CrossRef]
- Smith, D.J.; Schuerger, A.C.; Davidson, M.M.; Onstott, T.C.; Pacala, S.W.; Bakermans, C. Survivability of Psychrobacter cryohalolentis K5 under simulated martian surface conditions. Astrobiology 2009, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Moat, A.G.; Foster, J.W.; Spector, M.P. Microbial Physiology, 4th ed.; Wiley-Liss, Inc.: New York, NY, USA, 2002; p. 696. [Google Scholar]
- Gooding, J.L. Soil Mineralogy and Chemistry on Mars: Possible clues from slats and clays in SNC meteorites. Icarus 1992, 99, 28–41. [Google Scholar] [CrossRef]
- Treiman, A.H.; Gleason, J.D.; Bogard, D.D. The SNC meteorites are from Mars. Planet. Space Sci. 2000, 48, 1213–1230. [Google Scholar] [CrossRef]
- Ming, D.W.; Mittlefehldt, D.W.; Morris, R.V.; Golden, D.C.; Gellert, R.; Yen, A.; Clark, B.C.; Squyres, S.W.; Farrand, W.H.; Ruff, S.W.; et al. Geochemical and mineralogical indicators for aqueous processes in the Columbia Hills of Gusev Crater, Mars. J. Geophys. Res. Planets 2006, 111, E02S12. [Google Scholar] [CrossRef]
- Morris, R.V.; Golden, D.C.; Bell, J.F., III; Shelfer, T.D.; Scheinost, A.C.; Hinman, N.W.; Furniss, G.; Mertzman, S.A.; Bishop, J.L.; Ming, D.W. Mineralogy, composition, and alteration of Mars Pathfinder rocks and soils: Evidence from multispectral, elemental, and magnetic data on terrestrial analogue, SNC meteorite, and Pathfinder samples. J. Geophys. Res. 2000, 105, 1757–1817. [Google Scholar] [CrossRef]
- Stern, J.C.; Sutter, B.; Freissinet, C.; Navarro-Gonzalez, R.; McKay, C.P.; Archer, P.D.; Buch, A.; Brunner, A.E.; Coll, P.; Eignenbrode, J.L.; et al. Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale Crater, Mars. Proc. Natl. Acad. Sci. USA 2015, 112, 4245–4250. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M. Weathering profiles in phosphorus-rich rocks at Gusev Crater, Mars, suggest dissolution of phosphate minerals into potentially habitable near-neutral waters. Astrobiology 2015, 15, 1060–1075. [Google Scholar] [CrossRef]
- Freissinet, C.; Glavin, D.P.; Mahaffy, P.R.; Miller, K.E.; Eigenbrode, J.L.; Summons, R.E.; Brunner, A.E.; Buch, A.; Szopa, C.; Archer, P.D., Jr.; et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J. Geophys. Res. Planets 2015, 120, 495–514. [Google Scholar] [CrossRef]
- Eigenbrode, J.L.; Summons, R.E.; Steele, A.; Freissinet, C.; Millan, M.; Navarro-González, R.; Sutter, B.; McAdam, A.C.; Franz, H.B.; Glavin, D.P.; et al. Organic matter preserved in 3-billion-year-old mudstones at Gale Crater, Mars. Science 2018, 360, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Trainer, M.G.; Wong, M.H.; McConnochie, T.H.; Franz, H.B.; Atreya, S.K.; Conrad, P.G.; Lefevre, F.; Mahaffy, P.R.; Malespin, C.A.; Manning, H.L.K.; et al. Seasonal variations in atmopsheric composition as measured in Gale Crater, Mars. J. Geophys. Res. Planets 2019, 124, 3000–3024. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuerger, A.C.; Mickol, R.L.; Schwendner, P. The Hypopiezotolerant Bacterium, Serratia liquefaciens, Failed to Grow in Mars Analog Soils under Simulated Martian Conditions at 7 hPa. Life 2020, 10, 77. https://doi.org/10.3390/life10060077
Schuerger AC, Mickol RL, Schwendner P. The Hypopiezotolerant Bacterium, Serratia liquefaciens, Failed to Grow in Mars Analog Soils under Simulated Martian Conditions at 7 hPa. Life. 2020; 10(6):77. https://doi.org/10.3390/life10060077
Chicago/Turabian StyleSchuerger, Andrew C., Rebecca L. Mickol, and Petra Schwendner. 2020. "The Hypopiezotolerant Bacterium, Serratia liquefaciens, Failed to Grow in Mars Analog Soils under Simulated Martian Conditions at 7 hPa" Life 10, no. 6: 77. https://doi.org/10.3390/life10060077
APA StyleSchuerger, A. C., Mickol, R. L., & Schwendner, P. (2020). The Hypopiezotolerant Bacterium, Serratia liquefaciens, Failed to Grow in Mars Analog Soils under Simulated Martian Conditions at 7 hPa. Life, 10(6), 77. https://doi.org/10.3390/life10060077




