Crosslinked Hyaluronic Acid Gels for the Prevention of Intrauterine Adhesions after a Hysteroscopic Myomectomy in Women with Submucosal Myomas: A Prospective, Randomized, Controlled Trial
Abstract
1. Introduction
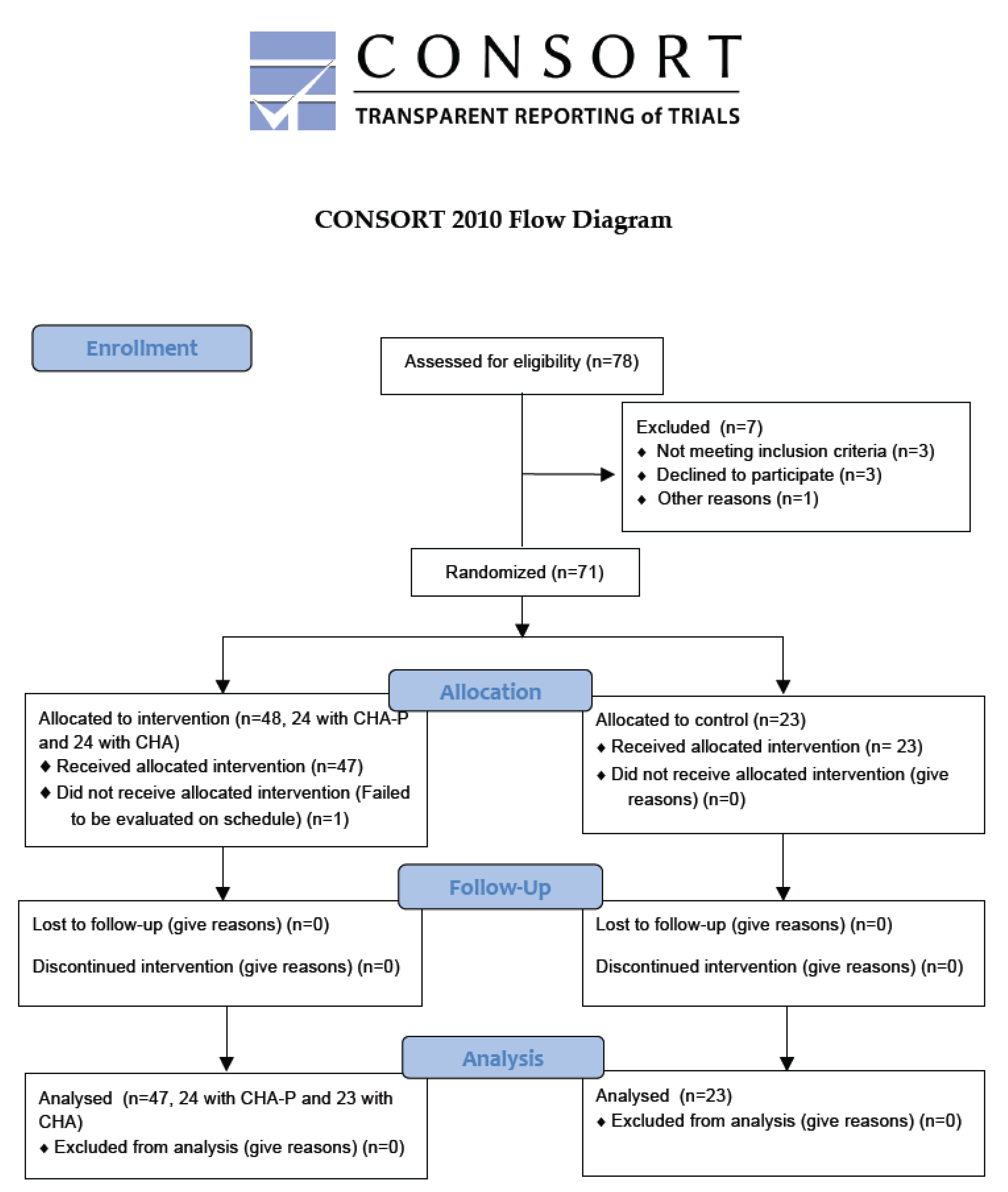
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. The Characteristics of the Study Subjects
3.2. The Outcomes
3.3. The Precipitating Factors for the Development of Modified American Fertility Society Stage II–III (Moderate and Severe) Intrauterine Adhesion
3.4. The Adverse Events
4. Discussion
4.1. Main Findings
4.2. Comparing with Other Randomized Controlled Trials (RCT) Using Hyaluronic Acid for the Primary Prevention of the Development of Intrauterine Adhesion after Hysteroscopic Myomectomy
4.3. Precipitating Factors Associated with an Increased Risk of the Development of Modified American Fertility Society Stage II–III (Moderate and Severe) Intrauterine Adhesion
4.4. The Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFS | American Fertility Society |
| CHA | Crosslinked hyaluronic acid |
| CHA-P | Crosslinked hyaluronic acid platform |
| CI | Confidence interval |
| D and C | Dilation and curettage |
| FIGO | International Federation of Gynecology and Obstetrics |
| IUA | Intrauterine adhesion or intrauterine adhesions |
| OR | Odds ratio |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
References
- Fritsch, H. Ein Fall von volligen Schwund der Gebaumutterhohle nach Auskratzung. Zentralbl. Gynaekol. 1894, 18, 1337–1342. [Google Scholar]
- Asherman, J.G. Amenorrhoea traumatica (atretica). J. Obstet. Gynaecol. Br. Emp. 1948, 55, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, D. The Effect of adjuvant treatment to prevent and treat intrauterine adhesions: A network meta-analysis of randomized controlled trials. J. Minim. Invasive. Gynecol. 2018, 25, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wong, Y.M.; Cheong, Y.; Xia, E.; Li, T.C. Asherman syndrome-one century later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef] [PubMed]
- March, C.M. Management of Asherman’s syndrome. Rerpod. BioMed. Online. 2011, 23, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Womens. Health. 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Deans, R.; Abbott, J. Review of intrauterine adhesions. J. Minim. Invasive. Gynecol. 2010, 17, 555–569. [Google Scholar] [CrossRef]
- Tu, C.H.; Yang, X.L.; Qin, X.Y.; Cai, L.P.; Zhang, P. Management of intrauterine adhesions: A novel intrauterine device. Med. Hypothesis 2013, 81, 394–396. [Google Scholar] [CrossRef]
- Hooker, A.B.; Lemmers, M.; Thurkow, A.L.; Heymans, M.W.; Opmeer, B.C.; Brolmann, H.A.M.; Mol, B.W.; Huirne, J.A.F. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: Prevalence, risk factors and long-term reproductive outcome. Hum. Reprod. Update. 2014, 20, 262–278. [Google Scholar] [CrossRef]
- Chiu, C.S.; Hwu, Y.M.; Lee, R.K.; Lin, M.H. Intrauterine adhesion prevention with Malecot catheter after hysteroscopic myomectomy: A novel approach. Taiwan. J. Obstet. Gynecol. 2020, 59, 56–60. [Google Scholar] [CrossRef]
- Liao, W.L.; Ying, T.H.; Shen, H.P.; Wu, P.J. Combined treatment for big submucosal myoma with High Intensity Focused Ultrasound and hysteroscopic resection. Taiwan. J. Obstet. Gynecol. 2019, 58, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chen, M.J.; Chen, C.D.; Chen, S.U.; Ho, H.N.; Yang, Y.S. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil. Steril. 2013, 99, 2092–2096.e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chen, M.J.; Wu, M.Y.; Chao, K.H.; Ho, H.N.; Yang, Y.S. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil. Steril. 2008, 89, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Mettler, L.; Schollmeyer, T.; Tinelli, A.; Malvasi, A.; Alkatout, I. Complications of uterine fibroids and their management, surgical management of fibroids, laparoscopy and hysteroscopy versus hysterectomy, haemorrhage, adhesions, and complications. Obstet. Gynecol. Int. 2012, 2012, 791248. [Google Scholar] [CrossRef]
- Taskin, O.; Sadik, S.; Onoglu, A.; Gokdeniz, R.; Erturan, E.; Burak, F.; Wheeler, J.M. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J. Am. Assoc. Gynecol. Laparosc. 2000, 7, 351–354. [Google Scholar] [CrossRef]
- Friedman, J.A.; Wong, J.M.K.; Chaudhari, A.; Tsai, S.; Milad, M.P. Hysteroscopic myomectomy: A comparison of techniques and review of current evidence in the management of abnormal uterine bleeding. Curr. Opin. Obstet. Gynecol. 2018, 30, 243–251. [Google Scholar] [CrossRef]
- Healy, M.W.; Schexnayder, B.; Connell, M.T.; Terry, N.; DeCherney, A.H.; Csokmay, J.M.; Yauger, B.J.; Hill, M.J. Intrauterine adhesion prevention after hysteroscopy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2016, 215, 267–275.e7. [Google Scholar] [CrossRef]
- Haber, K.; Hawkins, E.; Levie, M.; Chudnoff, S. Hysteroscopic morcellation: Review of the manufacturer and user facility device experience (MAUDE) database. J. Minim. Invasive. Gynecol. 2015, 22, 110–114. [Google Scholar] [CrossRef]
- Ciebiera, M.; Łoziński, T.; Wojtyła, C.; Rawski, W.; Jakiel, G. Complications in modern hysteroscopic myomectomy. Ginekol. Pol. 2018, 89, 398–404. [Google Scholar] [CrossRef]
- Capmas, P.; Levaillant, J.M.; Fernandez, H. Surgical techniques and outcome in the management of submucous fibroids. Current. Opinion. Obst. Gyn. 2013, 25, 332–338. [Google Scholar] [CrossRef]
- Gambadauro, P.; Gudmundsson, J.; Torrejón, R. Intrauterine adhesions following conservative treatment of uterine fibroids. Obst. Gyn. Int. 2012, 2012, 853269. [Google Scholar] [CrossRef] [PubMed]
- Litta, P.; Leggieri, C.; Conte, L.; Dalla Toffola, A.; Multinu, F.; Angioni, S. Monopolar versus bipolar device: Safety, feasibility, limits and perioperative complications in performing hysteroscopic myomectomy. Clin. Exp. Obstet. Gynecol. 2014, 41, 335–338. [Google Scholar] [PubMed]
- Sancho, J.M.; Delgado, V.S.C.; Valero, M.J.N.; Soteras, M.G.; Amate, V.P.; Carrascosa, A.A. Hysteroscopic myomectomy outcomes after 3-month treatment with either Ulipristal Acetate or GnRH analogues: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update. 2019, 25, 114–133. [Google Scholar] [CrossRef]
- Torres-De La Roche, L.A.; Campo, R.; Devassy, R.; Di Spiezio Sardo, A.; Hooker, A.; Koninckx, P.; Urman, B.; Wallwiener, M.; De Wilde, R.L. Adhesions and anti-adhesion systems highlights. Facts Views Vis. Obgyn. 2019, 11, 137–149. [Google Scholar]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Horng, H.C.; Chang, W.H.; Yeh, C.C.; Huang, B.S.; Chang, C.P.; Chen, Y.J.; Tsui, K.H.; Wang, P.H. Estrogen effects on wound healing. Int. J. Mol. Sci. 2017, 18, 2325. [Google Scholar] [CrossRef]
- Acunzo, G.; Guida, M.; Pellicano, M.; Tommaselli, G.A.; Di Spiezio Sardo, A.; Bifulco, G.; Cirillo, D.; Taylor, A.; Nappi, C. Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: A prospective, randomized, controlled study. Hum. Reprod. 2003, 18, 1918–1921. [Google Scholar] [CrossRef]
- Guida, M.; Acunzo, G.; Di Spiezio Sardo, A.; Bifulco, G.; Piccoli, R.; Pellicano, M.; Cerrota, G.; Cirillo, D.; Nappi, C. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: A prospective, randomized, controlled study. Hum. Reprod. 2004, 19, 1461–1464. [Google Scholar] [CrossRef]
- Bosteels, J.; Weyers, S.; D’Hooghe, T.M.; Torrance, H.; Broekmans, F.J.; Chua, S.J.; Mol, B.W.J. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane. Database. Syst. Rev. 2017, 11, CD011110. [Google Scholar] [CrossRef]
- Kim, T.; Ahn, K.H.; Choi, D.S.; Hwang, K.J.; Lee, B.I.; Jung, M.H.; Kim, J.W.; Kim, J.H.; Cha, S.H.; Lee, K.H.; et al. A randomized, multi-center, clinical trial to assess the efficacy and safety of alginate carboxymethylcellulose hyaluronic acid compared to carboxymethylcellulose hyaluronic acid to prevent postoperative intrauterine adhesion. J. Minim. Invasive. Gynecol. 2012, 19, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Di Spiezio Sardo, A.; Spinelli, M.; Bramante, S.; Scognamiglio, M.; Greco, E.; Guida, M.; Cela, V.; Nappi, C. Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J. Minim. Invasive. Gynecol. 2011, 18, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Can, S.; Kirpinar, G.; Dural, O.; Karamustafaoglu, B.B.; Tas, I.S.; Yasa, C.; Ugurlucan, F.G. Efficacy of a new crosslinked hyaluronan gel in the prevention of intrauterine adhesions. JSLS 2018, 22, e2018.00036. [Google Scholar]
- Hindocha, A.; Beere, L.; Dias, S.; Watson, A.; Ahmad, G. Adhesion prevention agents for gynaecological surgery: An overview of Cochrane reviews. Cochrane. Database. Syst. Rev. 2015, 1, CD011254. [Google Scholar] [CrossRef]
- Lin, X.; Wei, M.; Li, T.C.; Huang, Q.; Huang, D.; Zhou, F.; Zhang, S. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: A cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Salma, U.; Xue, M.; Md Sayed, A.S.; Xu, D. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomed. Res. Int. 2014, 2014, 589296. [Google Scholar] [CrossRef]
- Azumaguchi, A.; Henmi, H.; Saito, T. Efficacy of silicone sheet as a personalized barrier for preventing adhesion reformation after hysteroscopic adhesiolysis of intrauterine adhesions. Reprod. Med. Biol. 2019, 18, 378–383. [Google Scholar] [CrossRef]
- Zhu, R.; Duan, H.; Gan, L.; Wang, S. Comparison of intrauterine suitable balloon and Foley balloon in the prevention of adhesion after hysteroscopic adhesiolysis. Biomed. Res. Int. 2018, 2018, 9494101. [Google Scholar] [CrossRef]
- Huang, H.; Xu, B.; Cheng, C.; Xu, D. A novel intrauterine stent for prevention of intrauterine adhesions. Ann. Transl. Med. 2020, 8, 61. [Google Scholar] [CrossRef]
- Thubert, T.; Dussaux, C.; Demoulin, G.; Rivain, A.L.; Trichot, C.; Deffieux, X. Influence of auto-cross-linked hyaluronic acid gel on pregnancy rate and hysteroscopic outcomes following surgical removal of intra-uterine adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wan, Y.; Zou, F.; Ye, M.; Deng, H.; Ma, J.; Wei, Y.; Tan, C.; Xue, M. Prevention of intrauterine adhesion with auto-crosslinked hyaluronic acid gel: A prospective, randomized, controlled clinical study. Zhonghua Fu Chan Ke Za Zhi 2015, 50, 32–36. (In Chinese) [Google Scholar] [PubMed]
- Ducarme, G.; Davitian, C.; Zarrouk, S.; Uzan, M.; Poncelet, C. Interest of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: A case-control study. J. Gynecol. Obstet. Biol. Reprod. 2006, 35, 691–695. [Google Scholar] [CrossRef]
- Krajcovicova, R.; Hudeck, R.; Ventruba, P.; Surgentova, K. The role of hyaluronan in Asherman’s syndrome therapy. J. Gynecol. Surg. 2015, 31, 250–254. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, B.; Xin, X.; He, F.; Cui, Y. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan. J. Obstet. Gynecol. 2019, 58, 731–736. [Google Scholar]
- Zheng, F.; Xin, X.; He, F.; Cui, Y. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 1–4. [Google Scholar]
- Fuchs, N.; Smorgick, N.; Ben Ami, I.; Vaknin, Z.; Tovbin, Y.; Halperin, R.; Pansky, M. Intercoat (Oxiplex/AP gel) for preventing intrauterine adhesions after operative hysteroscopy for suspected retained products of conception: Double-blind, prospective, randomized pilot study. J. Minim. Invasive. Gynecol. 2014, 21, 126–130. [Google Scholar] [CrossRef]
- Hooker, A.B.; de Leeuw, R.; van de Ven, P.M.; Bakkum, E.A.; Thurkow, A.L.; Vogel, N.E.A.; van Vliet, H.A.A.M.; Bongers, M.Y.; Emanuel, M.H.; Verdonkschot, A.E.M.; et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: Short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil. Steril. 2017, 107, 1223–1231.e3. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Zhou, Y.; Fan, X.; Huang, J.; Wu, J.; Yu, R.; Lou, J.; Yang, M.; Yao, Z.; et al. New crosslinked hyaluronan gel for the prevention of intrauterine adhesions after dilation and curettage in patients with delayed miscarriage: A prospective, multicenter, randomized, controlled trial. J. Minim. Invasive. Gynecol. 2019, 26, 94–99. [Google Scholar] [CrossRef]
- Tsapanos, V.S.; Stathopoulou, L.P.; Papathanassopoulou, V.S.; Tzingounis, V.A. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J. Biomed. Mater. Res. 2002, 63, 10–14. [Google Scholar] [CrossRef]
- Zheng, F.; Xin, X.; He, F.; Liu, J.; Cui, Y. Meta-analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterineoperations. Exp. Ther. Med. 2020, 19, 2672–2678. [Google Scholar] [PubMed]
- Shokeir, T.A.; Fawzy, M.; Tatongy, M. The nature of intrauterine adhesions following reproductive hysteroscopic surgery as determined by early and late follow-up hysteroscopy: Clinical implications. Arch. Gynecol. Obstet. 2008, 277, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Even, M.; Fay, S.; Naoura, I.; Revaux, A.; Carbonnel, M.; Pirtea, P.; de Ziegler, D.; Ayoubi, J.M. Early second-look hysteroscopy: Prevention and treatment of intrauterine post-surgical adhesions. Front. Surg. 2019, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Laughlin-Tommaso, S.K.; Hesley, G.K.; Hopkins, M.R.; Brandt, K.R.; Zhu, Y.; Stewart, E.A. Clinical limitations of the International Federation of Gynecology and Obstetrics (FIGO) classification of uterine fibroids. Int. J. Gynaecol. Obstet. 2017, 139, 143–148. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, P.A.; Muzzupapa, G.; Bovicelli, A.; Marconi, S.; Bitti, S.R.; Sansovini, M.; Bovicelli, L. Hyaluronan derivative gel in intrauterine adhesion (IUA) prevention after operative hysteroscopy. Ellipse 2003, 19, 15–18. [Google Scholar]
| Scores | Description |
|---|---|
| 1 | Filmy adhesions with less than 1/3 enclosure |
| 2 | Filmy adhesions with 1/3 to 2/3 enclosure |
| 4 | Filmy adhesions with more than 2/3 enclosure |
| 4 | Dense adhesions with less than 1/3 enclosure |
| 8 | Dense adhesions with 1/3 to 2/3 enclosure |
| 16 | Dense adhesions with more than 2/3 enclosure |
| Characteristics | Treatment (n = 47) | No Treatment (n = 23) | p Value | |
|---|---|---|---|---|
| Characteristics of Patients | ||||
| Age (years) | 45.4 ± 3.6 | 45.2 ± 4.4 | 0.825 | |
| Body mass index (Kg/m2) | 23.8 ± 3.6 | 22.7 ± 2.7 | 0.202 | |
| *Previous Uterine Surgery | 0.190 | |||
| No | 37 (78.7%) | 21 (91.3%) | ||
| Yes | 10 (21.3%) | 2 (8.7%) | ||
| Abortion History | 0.265 | |||
| No | 33 (70.2%) | 19 (82.6%) | ||
| Yes | 14 (29.8%) | 4 (17.4%) | ||
| Gravidity | 1.15 ± 0.98 | 0.78 ± 0.85 | 0.130 | |
| Gravidity, times | 0.625 | |||
| 0 | 13 (27.7%) | 10 (43.5%) | ||
| 1 | 19 (40.4%) | 9 (39.1%) | ||
| 2 | 11 (23.4%) | 3 (13.0%) | ||
| 3 | 3 (6.4%) | 1 (4.3%) | ||
| 4 | 1 (2.1%) | 0 | ||
| Parity | 0.79 ± 0.72 | 0.61 ± 0.66 | 0.320 | |
| Parity, times | 0.579 | |||
| 0 | 18 (38.3%) | 11 (47.8%) | ||
| 1 | 21 (44.7%) | 10 (43.5%) | ||
| 2 | 8 (17.0%) | 2 (8.7%) | ||
| Characteristics of Myomas | 0.900 | |||
| FIGO type | Type 1 | 32 (68.1%) | 16 (69.6%) | |
| Type 2 | 15 (31.9%) | 7 (30.4%) | ||
| Number | 1.3 ± 0.5 | 1.3 ± 0.5 | 0.957 | |
| Maximal diameter (cm) | 2.4 ± 0.5 | 2.2 ± 0.8 | 0.250 | |
| Maximal volume (cm3) | 5.4 ± 3.2 | 4.1 ± 4.0 | 0.154 | |
| Treatment (n = 47) | No Treatment (n = 23) | p Value | |
|---|---|---|---|
| Intrauterine Adhesion | 0.012 | ||
| No | 41 (87.2%) | 14 (60.9%) | |
| Yes | 6 (12.8%) | 9 (39.1%) | |
| Modified AFS Stage | 0.002 | ||
| 0 | 41 (87.2%) | 14 (60.9%) | |
| I (mild) | 5 (10.6%) | 1 (4.3%) | |
| II (moderate) | 1 (2.1%) | 4 (17.4%) | |
| III (severe) | 0 | 4 (17.4%) |
| CHA-P Gel (n = 24) | CHA Gel (n = 23) | No (n = 23) | p-Value | |
|---|---|---|---|---|
| Intrauterine Adhesion | 0.031 | |||
| No | 22 (91.7%) a | 19 (82.6%) a | 14 (60.9%) | |
| Yes | 2 (8.3%) a | 4 (17.4%) a | 9 (39.1%) | |
| Modified AFS Stage | 0.014 | |||
| 0 | 22 (91.7%) b | 19 (82.6%) b | 14 (60.9%) | |
| I (mild) | 2 (8.3%) b | 3 (13.0%) b | 1 (4.3%) | |
| II (moderate) | 0 b | 1 (4.3%) b | 4 (17.4%) | |
| III (severe) | 0 b | 0b | 4 (17.4%) |
| Characteristics | n | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Characteristics of Patients | |||
| Age (years) | |||
| <43.9 | 27 | 1 (Reference) | |
| ≥43.9 | 43 | 3.03 (1.90–4.83) | <0.0001 |
| Body Mass Index (Kg/m2) | |||
| <23.22 | 32 | 1 (Reference) | |
| ≥23.22 | 38 | 4.11 (2.38–7.13) | <0.0001 |
| *Previous Uterine Surgery | |||
| No | 58 | 1 (Reference) | |
| Yes | 12 | 2.63 (1.81–3.80) | <0.0001 |
| Abortion History | |||
| No | 52 | 1 (Reference) | |
| Yes | 18 | 2.99 (1.97–4.53) | <0.0001 |
| Gravidity | |||
| <2 | 51 | 1 (Reference) | |
| ≥2 | 19 | 2.99 (1.97–4.55) | <0.0001 |
| Parity | |||
| <2 | 60 | 1 (Reference) | |
| ≥2 | 10 | 2.72 (1.87–3.96) | <0.0001 |
| Characteristics of Myoma | |||
| FIGO Type | |||
| 1 | 48 | 1 (Reference) | |
| 2 | 22 | 3.75 (2.28–6.17) | <0.0001 |
| Number | |||
| 1 | 49 | 1 (Reference) | |
| >1 | 21 | 3.32 (2.11–5.23) | <0.0001 |
| Maximal diameter | |||
| ≤2.7 cm | 51 | 1 (Reference) | |
| >2.7 cm | 19 | 3.70 (2.27–6.02) | <0.0001 |
| Maximal volume | |||
| ≤6.8 cm3 | 47 | 1 (Reference) | |
| >6.8 cm3 | 23 | 3.54 (2.20–5.72) | <0.0001 |
| Application of Postoperative Anti-Adhesive Gels | |||
| No | 23 | 1 (Reference) | |
| Yes | 47 | 0.25 (0.15–0.42) | <0.0001 |
| Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|
| FIGO type | ||
| 1 | 1 (Reference) | |
| 2 | 108.36 (8.95–1312.54) | <0.0001 |
| Application of Postoperative Anti-Adhesive Gels | ||
| No | 1 (Reference) | |
| Yes | 0.04 (0.00–0.33) | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Chang, W.-H.; Cheng, M.; Huang, H.-Y.; Horng, H.-C.; Chen, Y.-J.; Lee, W.-L.; Wang, P.-H. Crosslinked Hyaluronic Acid Gels for the Prevention of Intrauterine Adhesions after a Hysteroscopic Myomectomy in Women with Submucosal Myomas: A Prospective, Randomized, Controlled Trial. Life 2020, 10, 67. https://doi.org/10.3390/life10050067
Huang C-Y, Chang W-H, Cheng M, Huang H-Y, Horng H-C, Chen Y-J, Lee W-L, Wang P-H. Crosslinked Hyaluronic Acid Gels for the Prevention of Intrauterine Adhesions after a Hysteroscopic Myomectomy in Women with Submucosal Myomas: A Prospective, Randomized, Controlled Trial. Life. 2020; 10(5):67. https://doi.org/10.3390/life10050067
Chicago/Turabian StyleHuang, Chen-Yu, Wen-Hsun Chang, Min Cheng, Hsin-Yi Huang, Huann-Cheng Horng, Yi-Jen Chen, Wen-Ling Lee, and Peng-Hui Wang. 2020. "Crosslinked Hyaluronic Acid Gels for the Prevention of Intrauterine Adhesions after a Hysteroscopic Myomectomy in Women with Submucosal Myomas: A Prospective, Randomized, Controlled Trial" Life 10, no. 5: 67. https://doi.org/10.3390/life10050067
APA StyleHuang, C.-Y., Chang, W.-H., Cheng, M., Huang, H.-Y., Horng, H.-C., Chen, Y.-J., Lee, W.-L., & Wang, P.-H. (2020). Crosslinked Hyaluronic Acid Gels for the Prevention of Intrauterine Adhesions after a Hysteroscopic Myomectomy in Women with Submucosal Myomas: A Prospective, Randomized, Controlled Trial. Life, 10(5), 67. https://doi.org/10.3390/life10050067






