Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. UV-Visible Spectrophotometry
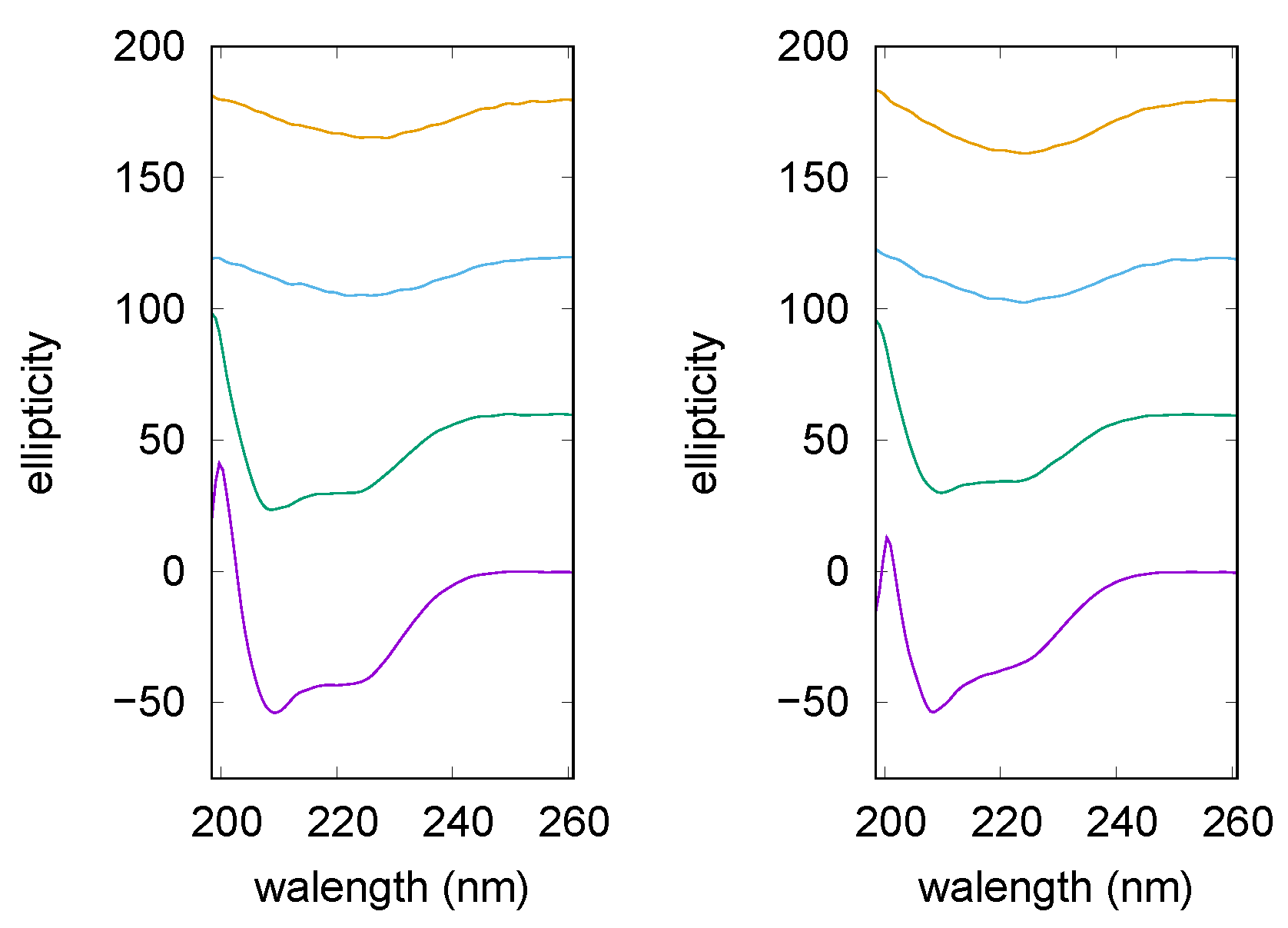
2.3. Circular Dichroism
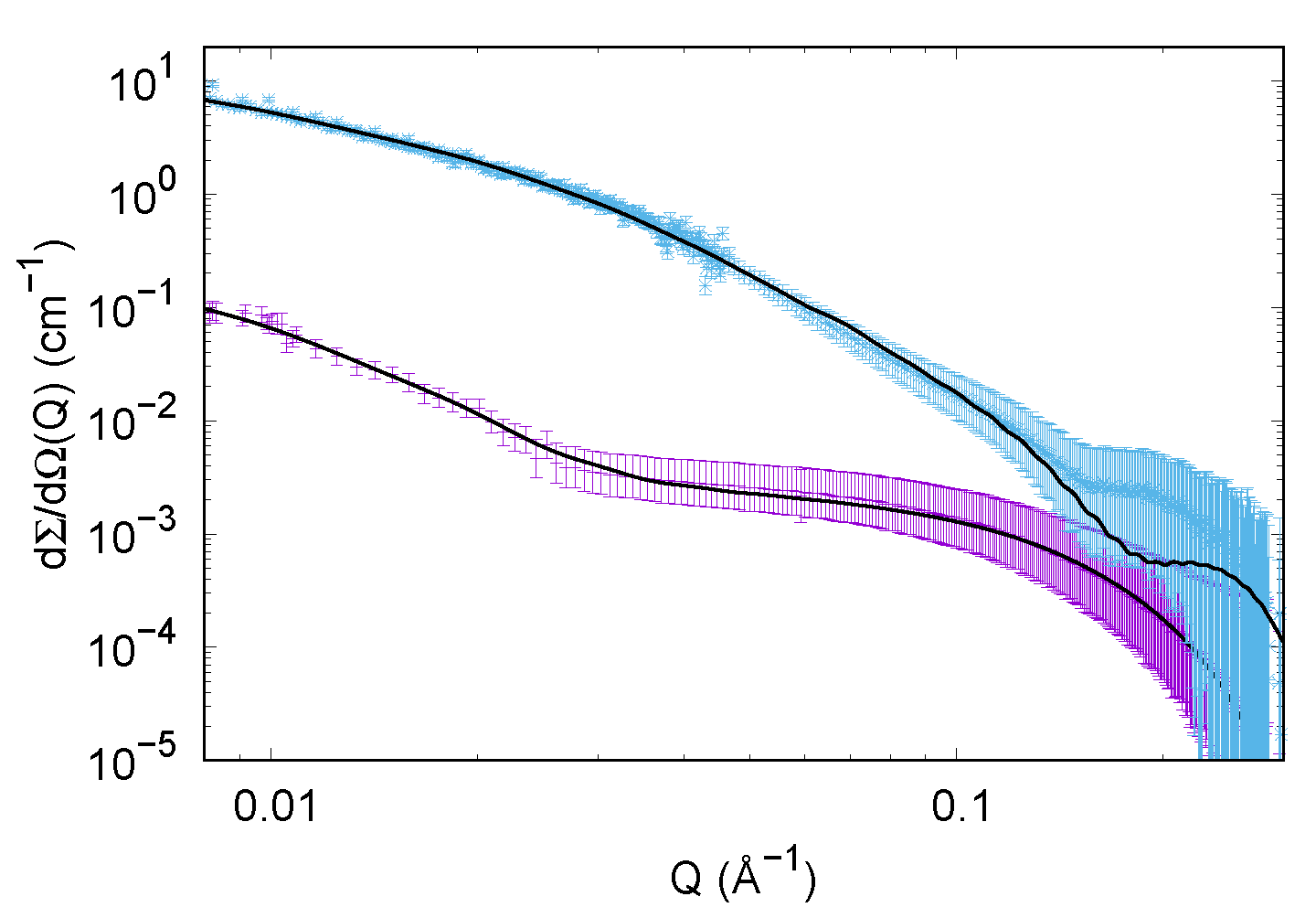
2.4. Small Angle X-ray Scattering
3. Results
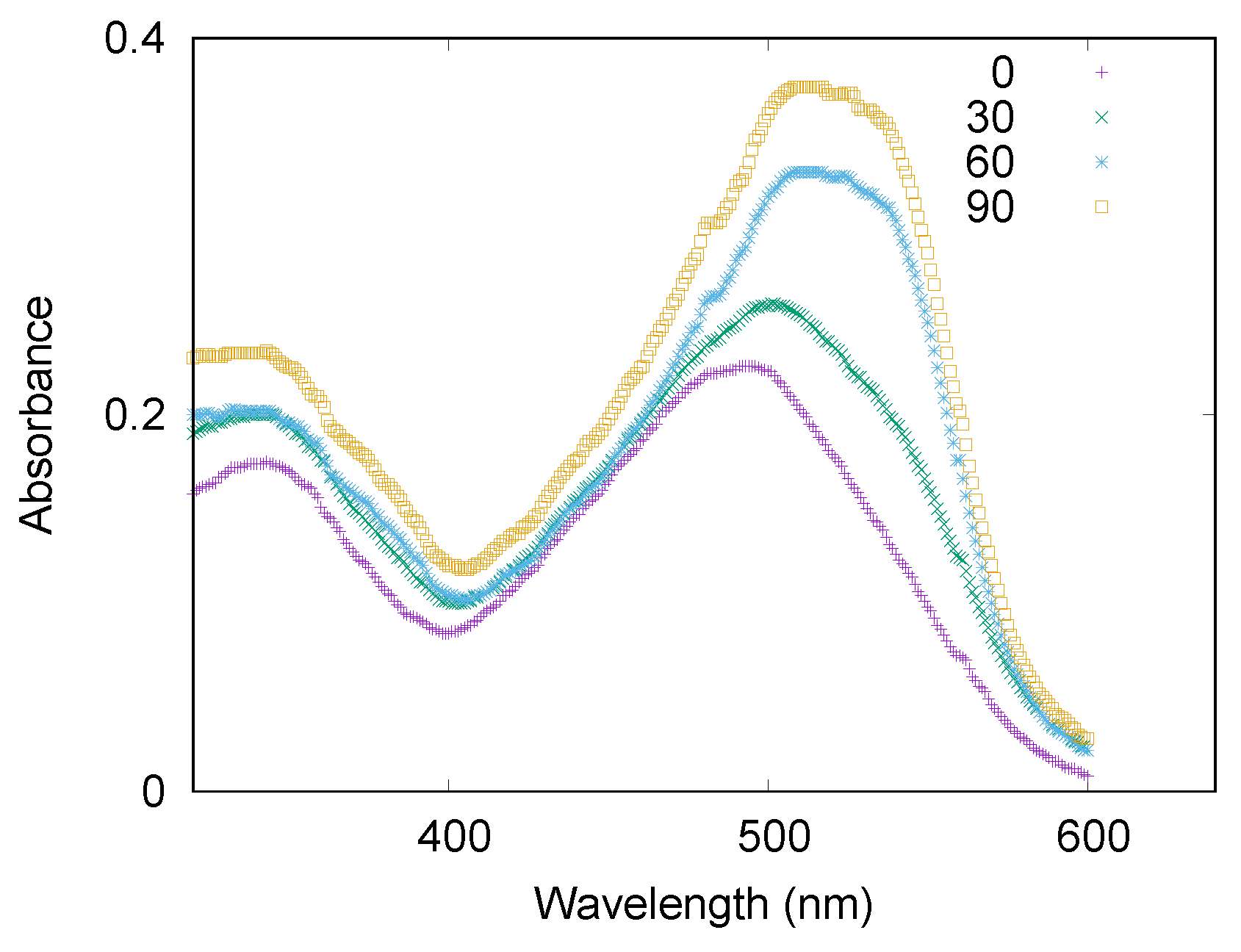
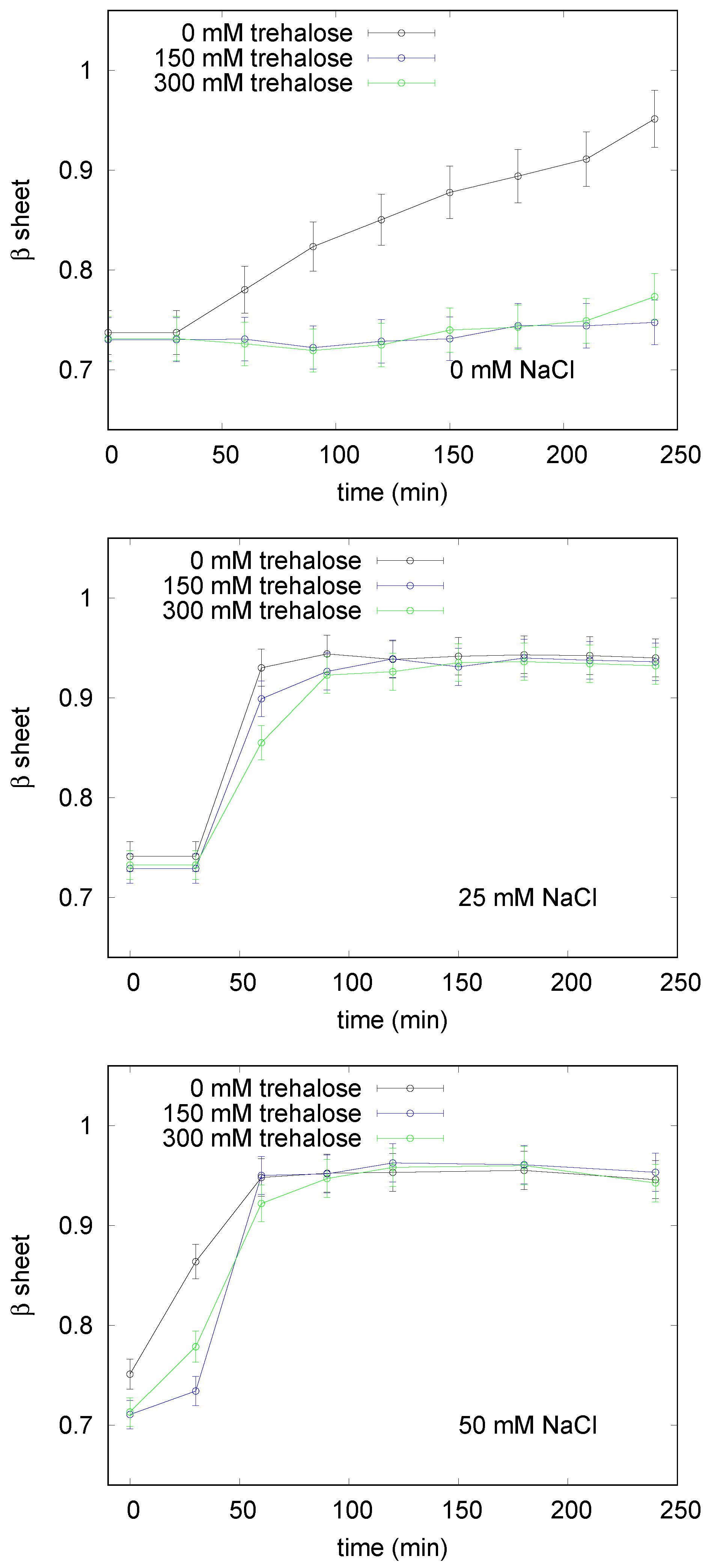
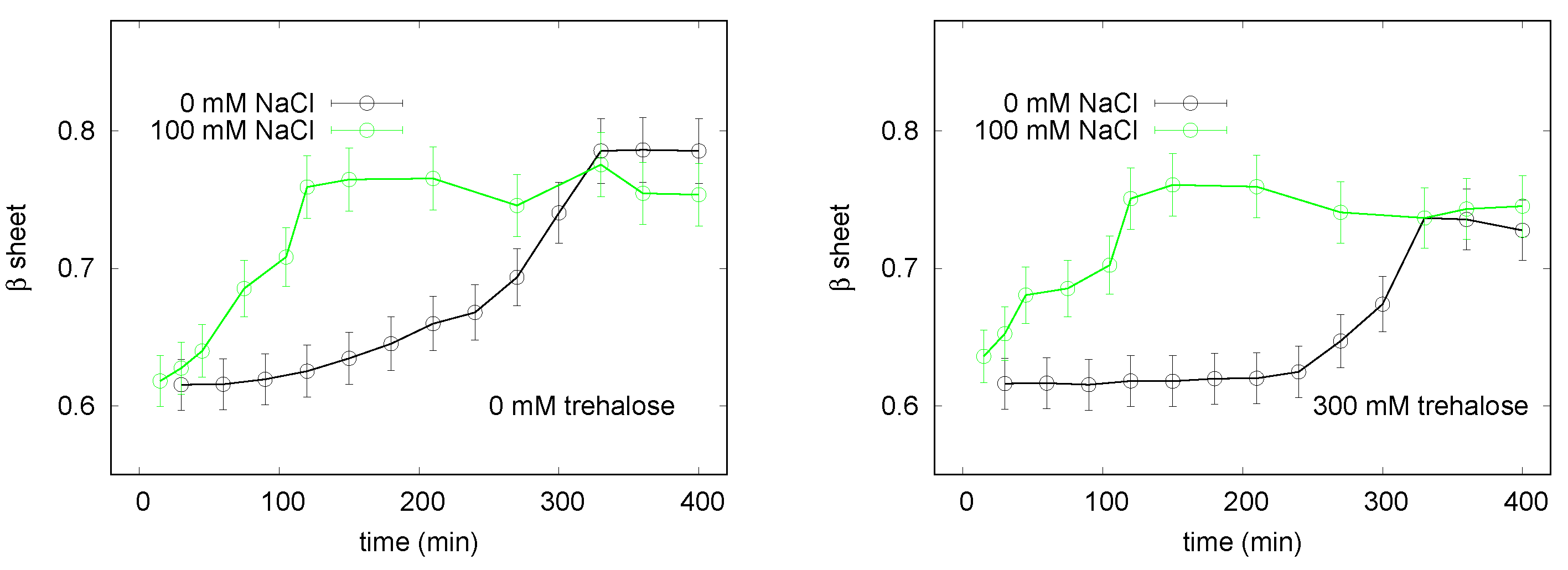
3.1. LYSOZYME
3.1.1. Spectroscopy Results
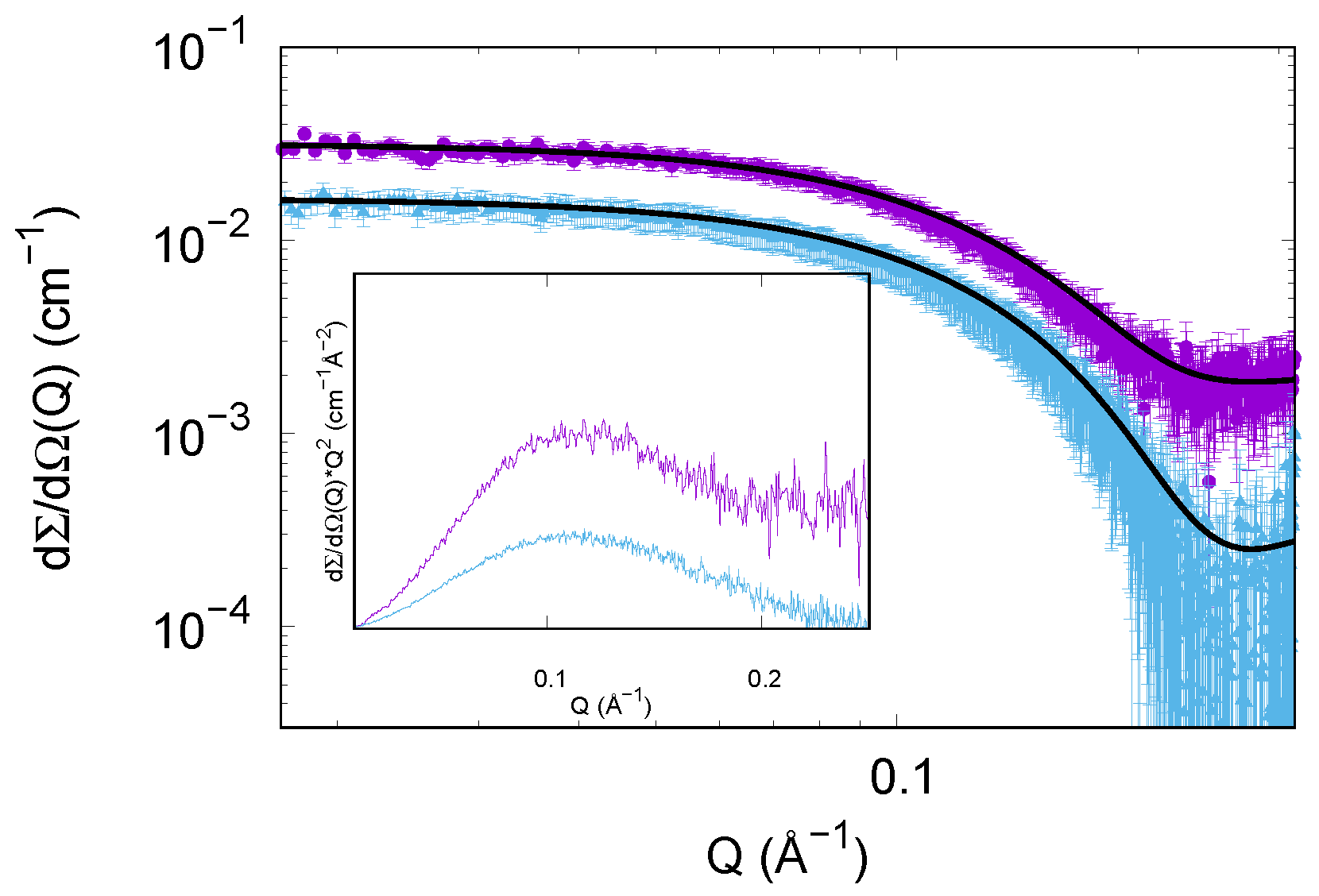
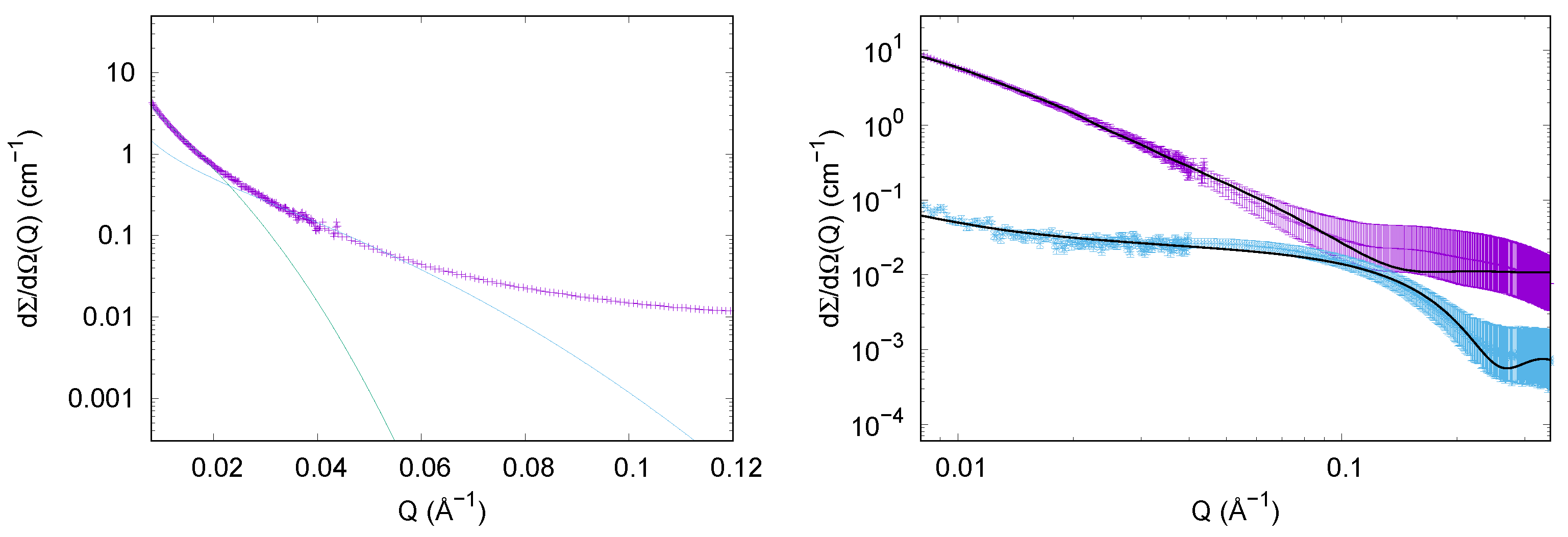
3.1.2. SAXS Results
3.2. INSULIN
3.2.1. Spectroscopy Results
3.2.2. SAXS Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| SAXS | Small Angle X-ray Scattering |
| CD | circular dichroism |
References
- Dyson, H.J.; Wright, P.E.; Scheraga, H.A. The role of hydrophobic interactions in initiation and propagation of protein folding. Proc. Natl. Acad. Sci. USA 2006, 103, 13057–13061. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Proteins Biochemistry and Biotechnology; Wiley: Hoboken, NJ, USA, 2014; pp. 1–23. [Google Scholar]
- Foder, V.; Cataldo, S.; Librizzi, F.; Pignataro, B.; Spiccia, P.; Leone, M. Self-Organization Pathways and Spatial Heterogeneity in Insulin Amyloid Fibril Formation. J. Phys. Chem. B 2009, 113, 10830–10837. [Google Scholar] [CrossRef] [PubMed]
- Manno, M.; Craparo, E.F.; Martorana, V.; Bulone, D.; Biagio, P.L.S. Kinetics of Insulin Aggregation: Disentanglement of Amyloid Fibrillation from Large-Size Cluster Formation. Biophys. J. 2006, 90, 4585–4591. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.S.; Chandra, N. Lysozyme: A model protein for amyloid research. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 84, pp. 63–111. [Google Scholar] [CrossRef]
- Fernãndez, A.; de las Mercedes Boland, M. Solvent environment conducive to protein aggregation. FEBS Lett. 2002, 529, 298–302. [Google Scholar] [CrossRef]
- Lee, E.; Choi, J.H.; Cho, M. The effect of Hofmeister anions on water structure at protein surfaces. Phys. Chem. Chem. Phys. 2017, 19, 20008–20015. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.S.; Flink, J.M.; Dikov, D.; Otzen, D.E. Sulfates dramatically stabilize a salt-dependent type of glucagon fibrils. Biophys. J. 2006, 90, 4181–4194. [Google Scholar] [CrossRef]
- Munishkina, L.A.; Henriques, J.; Uversky, V.N.; Fink, A.L. Role of Protein-Water Interactions and Electrostatics in α-Synuclein Fibril Formation. Biochemistry 2004, 43, 3289–3300. [Google Scholar] [CrossRef]
- Okur, H.I.; Hladalkova, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, R.; Ortore, M.G.; Spinozzi, F.; Carsughi, F.; Frielinghaus, H.; Cinelli, S.; Onori, G.; Mariani, P. Preferential hydration of lysozyme in water/glycerol mixtures: A small-angle neutron scattering study. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef]
- Spinozzi, F.; Ortore, M.G.; Sinibaldi, R.; Mariani, P.; Esposito, A.; Cinelli, S.; Onori, G. Microcalorimetric study of thermal unfolding of lysozyme in water/glycerol mixtures: An analysis by solvent exchange model. J. Chem. Phys. 2008, 129, 35101–35109. [Google Scholar] [CrossRef]
- Gabel, F.; Ringkjøbing Jensen, M.; Zaccai, G.; Blackledge, M. Quantitative Modelfree Analysis of Urea Binding to Unfolded Ubiquitin Using a Combination of Small Angle X-ray and Neutron Scattering. J. Am. Chem. Soc. 2009, 131, 8769–8771. [Google Scholar] [CrossRef] [PubMed]
- Ortore, M.G.; Sinibaldi, R.; Spinozzi, F.; Carsughi, F.; Clemens, D.; Bonincontro, A.; Mariani, P. New insights into urea action on proteins: SANS and Zeta Potential study of lysozyme. J. Phys. Chem. B 2008, 112, 12881–12887. [Google Scholar] [CrossRef] [PubMed]
- Chron, N.; Naepels, M.; Pluharov, E.; Laage, D. Protein Preferential Solvation in Water: Glycerol Mixtures. J. Phys. Chem. B 2020, 124, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N.; Yancey, P.H. Osmolytes and Cell-Volume Regulation: Physiological and Evolutionary Principles; American Cancer Society: Atlanta, GA, USA, 2011; pp. 441–484. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stöveken, N.; Richter, A.A.; Höppner, A.; Smits, S.H.; Heider, J.; Bremer, E. Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: Genetics, phylogenomics, biochemistry, and structural analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.; Anbu Rajan, L.; Anandan, R. Protective effect of betaine on protein, glycoproteins and amino acids in isoprenaline-induced myocardial infarction in albino rats. Biomed. Prev. Nutr. 2014, 4, 403–409. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997, 64, 25. [Google Scholar] [CrossRef]
- Enzo, E. Can Trehalose Prevent Neurodegeneration? Insights from Experimental Studies. Curr. Drug Targets 2014, 15, 551–557. [Google Scholar] [CrossRef]
- Zaman, M.; Khan, A.N.; Wahiduzzaman; Zakariya, S.M.; Khan, R.H. Protein misfolding, aggregation and mechanism of amyloid cytotoxicity: An overview and therapeutic strategies to inhibit aggregation. Int. J. Biol. Macromol. 2019, 134, 1022–1037. [Google Scholar] [CrossRef]
- Aprile, F.A.; Sormanni, P.; Perni, M.; Arosio, P.; Linse, S.; Knowles, T.P.J.; Dobson, C.M.; Vendruscolo, M. Selective targeting of primary and secondary nucleation pathways in Aβ42 aggregation using a rational antibody scanning method. Sci. Adv. 2017, 3, e1700488. [Google Scholar] [CrossRef]
- Battisti, A.; Palumbo Piccionello, A.; Sgarbossa, A.; Vilasi, S.; Ricci, C.; Ghetti, F.; Spinozzi, F.; Marino Gammazza, A.; Giacalone, V.; Martorana, A.; et al. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017, 7, 31714–31724. [Google Scholar] [CrossRef]
- Ricci, C.; Spinozzi, F.; Mariani, P.; Ortore, M.; Grazia Ortore, M. Protein Amyloidogenesis Investigated by Small Angle Scattering. Curr. Pharm. Des. 2016, 22, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, R.; Laudadio, E.; Falconi, E.; Massaccesi, L.; Ercolani, L.; Mobbili, G.; Minnelli, C.; Sciré, A.; Cianfruglia, L.; Armeni, T. Proteinprotein interactions of human glyoxalase II: Findings of a reliable docking protocol. Org. Biomol. Chem. 2018, 16, 5167–5177. [Google Scholar] [CrossRef] [PubMed]
- Elia, F.; Cantini, F.; Chiti, F.; Dobson, C.M.; Bemporad, F. Direct Conversion of an Enzyme from Native-like to Amyloid-like Aggregates within Inclusion Bodies. Biophys. J. 2017, 112, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Jain, V.; Yadav, D. Osmolytes: A Possible Therapeutic Molecule for Ameliorating the Neurodegeneration Caused by Protein Misfolding and Aggregation. Biomolecules 2020, 10, 132. [Google Scholar] [CrossRef]
- Uversky, V.N.; Finkelstein, A.V. Life in Phases: Intra- and Inter- Molecular Phase Transitions in Protein Solutions. Biomolecules 2019, 9, 842. [Google Scholar] [CrossRef]
- Kuehner, D.E.; Engmann, J.; Fergg, F.; Wernick, M.; Blanch, H.W.; Prausnitz, J.M. Lysozyme Net Charge and Ion Binding in Concentrated Aqueous Electrolyte Solutions. J. Phys. Chem. B 1999, 103, 1368–1374. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Jain, S.; Udgaonkar, J.B. Salt-induced modulation of the pathway of amyloid fibril formation by the mouse prion protein. Biochemistry 2010, 49, 7615–7624. [Google Scholar] [CrossRef]
- Bye, J.W.; Falconer, R.J. Thermal stability of lysozyme as a function of ion concentration: A reappraisal of the relationship between the Hofmeister series and protein stability. Protein Sci. 2013, 22, 1563–1570. [Google Scholar] [CrossRef]
- Poniková, S.; Antošová, A.; Demjén, E.; Sedláková, D.; Marek, J.; Varhač, R.; Gažová, Z.; Sedlák, E. Lysozyme stability and amyloid fibrillization dependence on Hofmeister anions in acidic pH. J. Biol. Inorg. Chem. 2015, 20, 921–933. [Google Scholar] [CrossRef]
- Muzaffar, M.; Ahmad, A. The mechanism of enhanced insulin amyloid fibril formation by NaCl is better explained by a conformational change model. PLoS ONE 2011, 6, e27906. [Google Scholar] [CrossRef]
- Soper, A.K.; Ricci, M.A.; Bruni, F.; Rhys, N.H.; McLain, S.E. Trehalose in Water Revisited. J. Phys. Chem. B 2018, 122, 7365–7374. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, N.; Sakhaee, S.; Takallou, A.; Mobaraki, A.; Maddah, M.; Moshrefi, R. Hydrodynamic volume of trehalose and its water uptake mechanism. Biophys. Chem. 2019, 249. [Google Scholar] [CrossRef]
- Ajito, S.; Hirai, M.; Iwase, H.; Shimizu, N.; Igarashi, N.; Ohta, N. Protective action of trehalose and glucose on protein hydration shell clarified by using X-ray and neutron scattering. Phys. B Condens. Matter 2018, 551, 249–255. [Google Scholar] [CrossRef]
- Chatani, E.; Imamura, H.; Yamamoto, N.; Kato, M. Stepwise Organization of the -Structure Identifies Key Regions Essential for the Propagation and Cytotoxicity of Insulin Amyloid Fibrils. J. Biol. Chem. 2014, 289, 10399–10410. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kishore, N.; Hosur, R.V. Inhibition of insulin fibrillation by osmolytes: Mechanistic Insights. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Chatani, E.; Inoue, R.; Imamura, H.; Sugiyama, M.; Kato, M.; Yamamoto, M.; Nishida, K.; Kanaya, T. Early aggregation preceding the nucleation of insulin amyloid fibrils as monitored by small angle X-ray scattering. Sci. Rep. 2015, 5, 15485. [Google Scholar] [CrossRef]
- Frid, P.; Anisimov, S.V.; Popovic, N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007, 53, 135–160. [Google Scholar] [CrossRef]
- Khurana, R.; Uversky, V.N.; Nielsen, L.; Fink, A.L. Is Congo Red an Amyloid-specific Dye? J. Biol. Chem. 2001, 276, 22715–22721. [Google Scholar] [CrossRef]
- Amenitsch, H.; Rappolt, M.; Kriechbaum, M.; Mio, H.; Laggner, P.; Bernstorff, S. First performance assessment of the small-angle X-ray scattering beamline at ELETTRA. J. Synchrotron Radiat. 1998, 5, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, A.P.; Brown, K.; Burmeister, W.; Claustre, L.; Gonzalez, A.; McSweeney, S.; Mitchell, E.; Moy, J.P.; Svensson, S.O.; Thompson, A.W. Calibration and application of an X-ray image intensifier/charge-coupled device detector for monochromatic macromolecular crystallography. J. Synchrotron Radiat. 1997, 4, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, E.; Svergun, D. Accuracy of molecular mass determination of proteins in solution by small-angle X-ray scattering. J. Appl. Cryst. 2007, 40, s245–s249. [Google Scholar] [CrossRef]
- Foderà, V.; Zaccone, A.; Lattuada, M.; Donald, A.M. Electrostatics Controls the Formation of Amyloid Superstructures in Protein Aggregation. Phys. Rev. Lett. 2013, 111, 108105. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhu, X.; Ni, S.; Su, Z.; Zhou, H.M. Conformational changes of lysozyme refolding intermediates and implications for aggregation and renaturation. Int. J. Biochem. Cell Biol. 2004, 36, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Liu, K.N.; How, S.C.; Chen, W.A.; Lai, C.M.; Liu, H.S.; Hu, C.J.; Wang, S.S.S. Carnosine’s Effect on Amyloid Fibril Formation and Induced Cytotoxicity of Lysozyme. PLoS ONE 2013, 8, e81982. [Google Scholar] [CrossRef]
- Glatter, O. Fourier Transformation and Deconvolution; North-Holland: Amsterdam, The Netherlands, 2002; pp. 103–124. [Google Scholar]
- Spinozzi, F.; Ferrero, C.; Ortore, M.G.; De Maria Antolinos, A.; Mariani, P. GENFIT: Software for the analysis of small-angle X-ray and neutron scattering data of macro-molecules in solution. J. Appl. Crystallogr. 2014, 47, 1132–1139. [Google Scholar] [CrossRef]
- Ortore, M.G.; Spinozzi, F.; Mariani, P.; Paciaroni, A.; Barbosa, L.R.S.; Amenitsch, H.; Steinhart, M.; Ollivier, J.; Russo, D. Combining structure and dynamics: Non-denaturing high-pressure effect on lysozyme in solution. J. R. Soc. Interface 2009, 6 (Suppl. S5), S619–S634. [Google Scholar] [CrossRef]
- Hill, S.E.; Robinson, J.; Matthews, G.; Muschol, M. Amyloid Protofibrils of Lysozyme Nucleate and Grow Via Oligomer Fusion. Biophys. J. 2009, 96, 3781–3790. [Google Scholar] [CrossRef]
- Ortore, M.G.; Spinozzi, F.; Vilasi, S.; Sirangelo, I.; Irace, G.; Shukla, A.; Narayanan, T.; Sinibaldi, R.; Mariani, P. Time-resolved small-angle X-ray scattering study of the early stage of amyloid formation of an apomyoglobin mutant. Phys. Rev. E 2011, 84, 061904. [Google Scholar] [CrossRef]
- Mangione, M.R.; Palumbo Piccionello, A.; Marino, C.; Ortore, M.G.; Picone, P.; Vilasi, S.; Di Carlo, M.; Buscemi, S.; Bulone, D.; San Biagio, P.L. Photo-inhibition of A[small beta] fibrillation mediated by a newly designed fluorinated oxadiazole. RSC Adv. 2015, 5, 16540–16548. [Google Scholar] [CrossRef]
- Piccirilli, F.; Plotegher, N.; Spinozzi, F.; Bubacco, L.; Mariani, P.; Beltramini, M.; Tessari, I.; Militello, V.; Perucchi, A.; Amenitsch, H.; et al. Pressure effects on α-synuclein amyloid fibrils: An experimental investigation on their dissociation and reversible nature. Arch. Biochem. Biophys. 2017, 627, 46–55. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Schurtenberger, P. Scattering Functions of Semiflexible Polymers with and without Excluded Volume Effects. Macromolecules 1996, 29, 7602–7612. [Google Scholar] [CrossRef]
- Bekard, I.B.; Dunstan, D.E. Tyrosine Autofluorescence as a Measure of Bovine Insulin Fibrillation. Biophys. J. 2009, 97, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Vilasi, S.; Iannuzzi, C.; Portaccio, M.; Irace, G.; Sirangelo, I. Effect of Trehalose on W7FW14F Apomyoglobin and Insulin Fibrillization: New Insight into Inhibition Activity. Biochemistry 2008, 47, 1789–1796. [Google Scholar] [CrossRef]
- Arora, A.; Ha, C.; Park, C.B. Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett. 2004, 564, 121–125. [Google Scholar] [CrossRef]
- Olsson, C.; Jansson, H.; Swenson, J. The role of trehalose for the stabilization of proteins. J. Phys. Chem. B 2016, 120, 4723–4731. [Google Scholar] [CrossRef]
- Guez, V.; Roux, P.; Navon, A.; Goldberg, M.E. Role of individual disulfide bonds in hen lysozyme early folding steps. Protein Sci. 2002, 11, 1136–1151. [Google Scholar] [CrossRef]
- Kurouski, D.; Washington, J.; Ozbil, M.; Prabhakar, R.; Shekhtman, A.; Lednev, I.K. Disulfide Bridges Remain Intact while Native Insulin Converts into Amyloid Fibrils. PLoS ONE 2012, 7, e36989. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Cordone, L.; Cottone, G.; Cupane, A.; Emanuele, A.; Giuffrida, S.; Levantino, M. Proteins in Saccharides Matrices and the Trehalose Peculiarity: Biochemical and Biophysical Properties. Curr. Org. Chem. 2015, 19, 1684–1706. [Google Scholar] [CrossRef]
- Booth, D.R.; Sunde, M.; Bellotti, V.; Robinson, C.V.; Hutchinson, W.L.; Fraser, P.E.; Hawkins, P.N.; Dobson, C.M.; Radford, S.E.; Blake, C.C.F.; et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 1997, 385, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Visconti, L.; Malagrin, F.; Broggini, L.; De Luca, C.M.G.; Moda, F.; Gianni, S.; Ricagno, S.; Toto, A. Investigating the Molecular Basis of the Aggregation Propensity of the Pathological D76N Mutant of Beta-2 Microglobulin: Role of the Denatured State. Int. J. Mol. Sci. 2019, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Chan, B.P.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef]
- Santa-María, I.; Hernández, F.; Moreno, F.J.; Avila, J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-β-peptide aggregation. Neurosci. Lett. 2007, 429, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Bruni, F.; Ricci, M.A. Protection against Dehydration: A Neutron Diffraction Study on Aqueous Solutions of a Model Peptide and Trehalose. J. Phys. Chem. B 2018, 122, 10291–10295. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, E.; Ricci, C.; Pieraccini, S.; Spinozzi, F.; Mariani, P.; Ortore, M.G. Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils. Life 2020, 10, 60. https://doi.org/10.3390/life10050060
Mari E, Ricci C, Pieraccini S, Spinozzi F, Mariani P, Ortore MG. Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils. Life. 2020; 10(5):60. https://doi.org/10.3390/life10050060
Chicago/Turabian StyleMari, Eleonora, Caterina Ricci, Silvia Pieraccini, Francesco Spinozzi, Paolo Mariani, and Maria Grazia Ortore. 2020. "Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils" Life 10, no. 5: 60. https://doi.org/10.3390/life10050060
APA StyleMari, E., Ricci, C., Pieraccini, S., Spinozzi, F., Mariani, P., & Ortore, M. G. (2020). Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils. Life, 10(5), 60. https://doi.org/10.3390/life10050060








