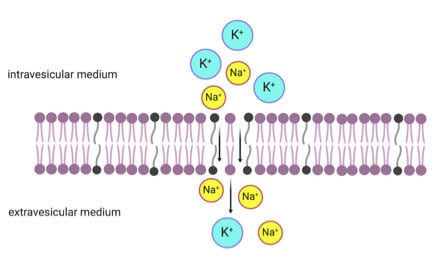
Semipermeable Mixed Phospholipid-Fatty Acid Membranes Exhibit K+/Na+ Selectivity in the Absence of Proteins
Abstract
1. Introduction
2. Materials and Methods
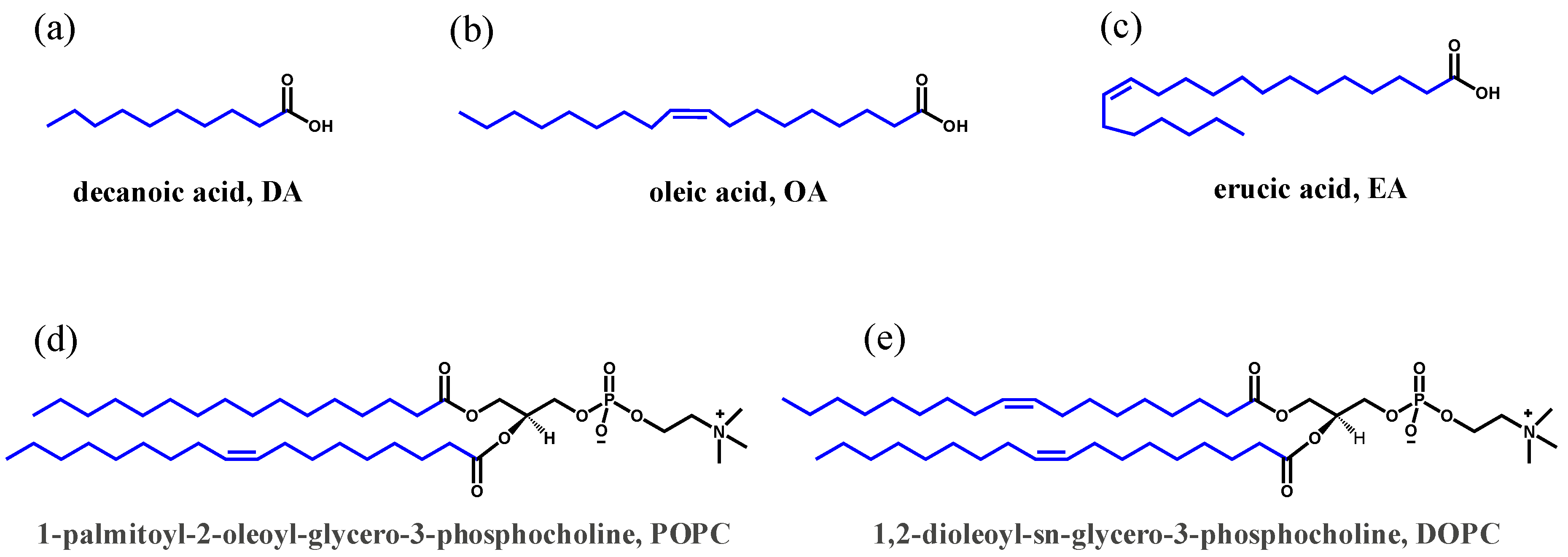
2.1. Materials
2.2. Vesicle Preparation and Characterization
2.3. Calcein Leakage Assay
2.4. K+ and Na+ Calibration and Measurements
2.5. K+ and Na+ Permeability Experiments
3. Results
3.1. Calcein Leakage Assay
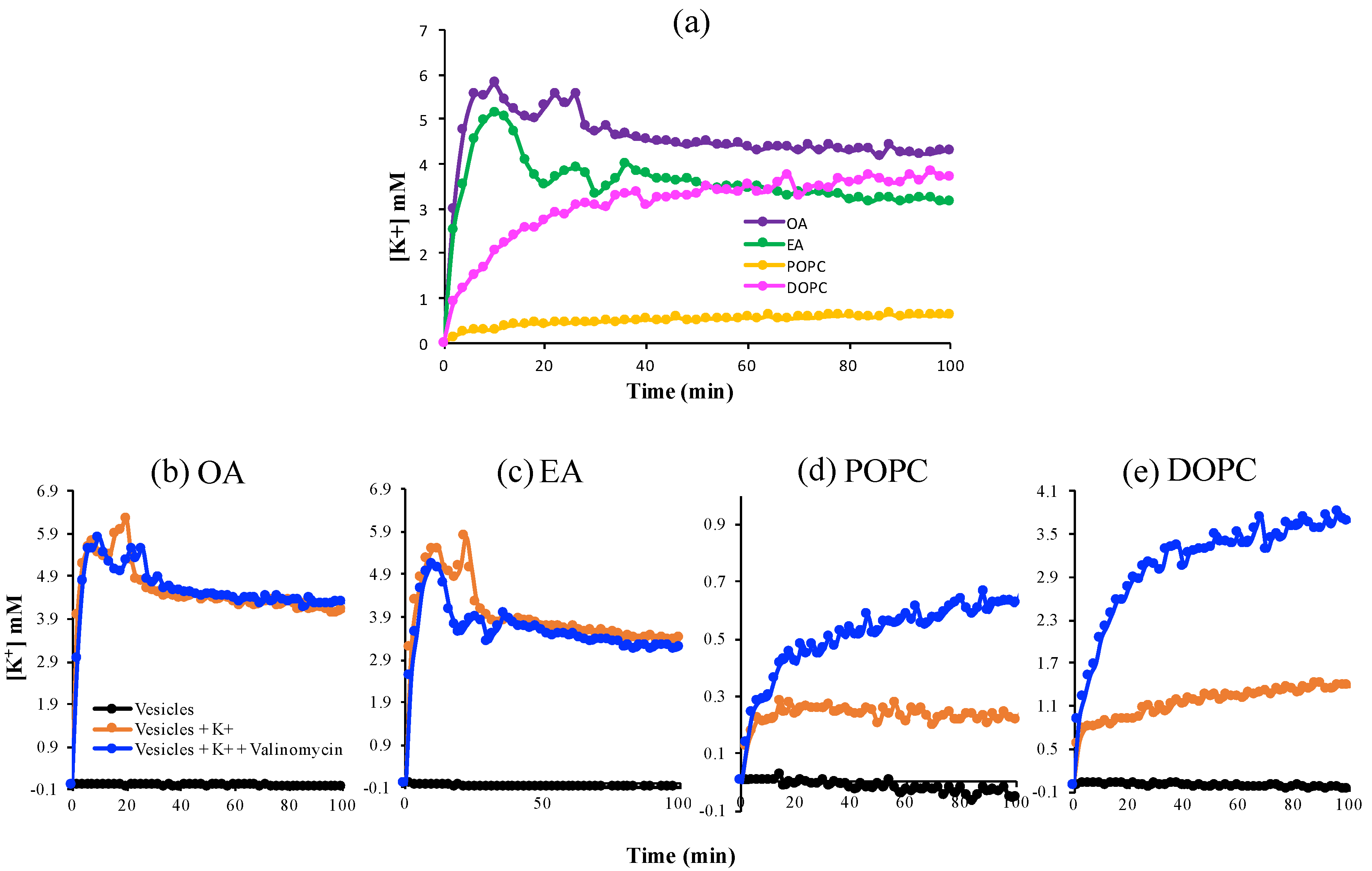
3.2. K+ Permeability of Pure Fatty Acid and Pure Phospholipid Membranes
3.3. K+ and Na+ Permeability of Mixed Fatty Acid-Phospholipid Membranes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmgren, M.; Wagg, J.; Bezanilla, F.; Rakowski, R.F.; De Weer, P.; Gadsby, D.C. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature 2000, 403, 898–901. [Google Scholar] [CrossRef]
- Cannon, S.C. Paying the price at the pump: Dystonia from mutations in a Na+/K+-ATPase. Neuron 2004, 43, 153–154. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Macallum, A.B. The paleochemistry of the body fluids and tissues. Physiol. Rev. 1926, 6, 316–357. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Nat. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef]
- Pohorille, A.; Deamer, D. Self-assembly and function of primitive cell membranes. Res. Microbiol. 2009, 160, 449–456. [Google Scholar] [CrossRef]
- Kee, T.P.; Monnard, P.-A. On the emergence of a proto-metabolism and the assembly of early protocells. Elements 2016, 12, 419–424. [Google Scholar] [CrossRef]
- Deamer, D.W.; Pashley, R. Amphiphilic components of the Murchison carbonaceous chondrite: Surface properties and membrane formation. Orig. Life Evol. Biosph. 1989, 19, 21–38. [Google Scholar] [CrossRef]
- Komiya, M.; Shimoyama, A.; Harada, K. Examination of organic compounds from insoluble organic matter isolated from some Antarctic carbonaceous chondrites by heating experiments. Geochim. Cosmochim. Acta 1993, 57, 907–914. [Google Scholar] [CrossRef]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 C. Orig. Life Evol. Biosph. 2001, 31, 103–118. [Google Scholar] [CrossRef]
- Foustoukos, D.I.; Seyfried, W.E. Hydrocarbons in hydrothermal vent fluids: The role of chromium-bearing catalysts. Science 2004, 304, 1002–1005. [Google Scholar] [CrossRef]
- Dalai, P.; Kaddour, H.; Sahai, N. Incubating life: Prebiotic sources of organics for the origin of life. Elements 2016, 12, 401–406. [Google Scholar] [CrossRef]
- Dalai, P.; Ustriyana, P.; Sahai, N. Aqueous magnesium as an environmental selection pressure in the evolution of phospholipid membranes on early earth. Geochim. Cosmochim. Acta 2018, 223, 216–228. [Google Scholar] [CrossRef]
- Dalai, P.; Sahai, N. Protocell emergence and evolution. In Handbook of Astrobiology; Kolb, V., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 491–520. [Google Scholar]
- Hargreaves, W.; Mulvihill, S.; Deamer, D. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78. [Google Scholar] [CrossRef]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of phosphatidylcholine under possible primitive Earth conditions. J. Mol. Evol. 1982, 18, 196–202. [Google Scholar] [CrossRef]
- Maheen, G.; Tian, G.; Wang, Y.; He, C.; Shi, Z.; Yuan, H.; Feng, S. Resolving the enigma of prebiotic C-O-P bond formation: Prebiotic hydrothermal synthesis of important biological phosphate esters. Heteroat. Chem. 2010, 21, 161–167. [Google Scholar] [CrossRef]
- Albertsen, A.N.; Duffy, C.; Sutherland, J.D.; Monnard, P.-A. Self-assembly of phosphate amphiphiles in mixtures of prebiotically plausible surfactants. Astrobiology 2014, 14, 462–472. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar]
- Jin, L.; Kamat, N.P.; Jena, S.; Szostak, J.W. Fatty acid/phospholipid blended membranes: A potential intermediate state in protocellular evolution. Small 2018, 14, 1704077. [Google Scholar] [CrossRef]
- Budin, I.; Szostak, J.W. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Nat. Acad. Sci. USA 2011, 108, 5249–5254. [Google Scholar] [CrossRef]
- Kendall, D.A.; MacDonald, R.C. A fluorescence assay to monitor vesicle fusion and lysis. J. Biol.Chem. 1982, 257, 13892–13895. [Google Scholar]
- Venema, K.; Gibrat, R.; Grouzis, J.-P.; Grignon, C. Quantitative measurement of cationic fluxes, selectivity and membrane potential using liposomes multilabelled with fluorescent probes. Biochim. Biophys. Acta Biomembr. 1993, 1146, 87–96. [Google Scholar] [CrossRef]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobe, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [CrossRef]
- Costa, P.F.; Emilio, M.G.; Fernandes, P.L.; Ferreira, H.G.; Ferreira, K.G. Determination of ionic permeability coefficients of the plasma membrane of Xenopus laevis oocytes under voltage clamp. J. Physiol. 1989, 413, 199–211. [Google Scholar] [CrossRef]
- Lande, M.B.; Donovan, J.M.; Zeidel, M.L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995, 106, 67–84. [Google Scholar] [CrossRef]
- Paula, S.; Deamer, D.W. Membrane permeability barriers to ionic and polar solutes. Curr. Topics. Memb. 1999, 48, 77–95. [Google Scholar]
- Paula, S.; Volkov, A.; Van Hoek, A.; Haines, T.; Deamer, D.W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef]
- Wilson, M.A.; Pohorille, A. Mechanism of unassisted ion transport across membrane bilayers. J. Am. Chem. Soc. 1996, 118, 6580–6587. [Google Scholar] [CrossRef]
- Mancinelli, R.; Botti, A.; Bruni, F.; Ricci, M.A.; Soper, A.K. Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J. Phys. Chem. B 2007, 111, 13570–13577. [Google Scholar] [CrossRef]
- Degrève, L.; Vechi, S.M.; Junior, C.Q. The hydration structure of the Na+ and K+ ions and the selectivity of their ionic channels. Biochim. Biophys. Acta-Bioenerg. 1996, 1274, 149–156. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Factors governing the Na+ vs K+ selectivity in sodium ion channels. J. Am. Chem. Soc. 2010, 132, 2321–2332. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Ion selectivity strategies of sodium channel selectivity filters. Acc. Chem. Res. 2014, 47, 3580–3587. [Google Scholar] [CrossRef]
- Tang, C.Y.; Allen, H.C. Ionic Binding of Na+ versus K+ to the carboxylic acid headgroup of palmitic acid monolayers studied by vibrational sum frequency generation Spectroscopy. J. Phys. Chem. A 2009, 113, 7383–7393. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef]
- Sutherland, J.D. The origin of life—Out of the blue. Angew. Chem. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Deamer, D.W. The first living systems: A bioenergetic perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [CrossRef]
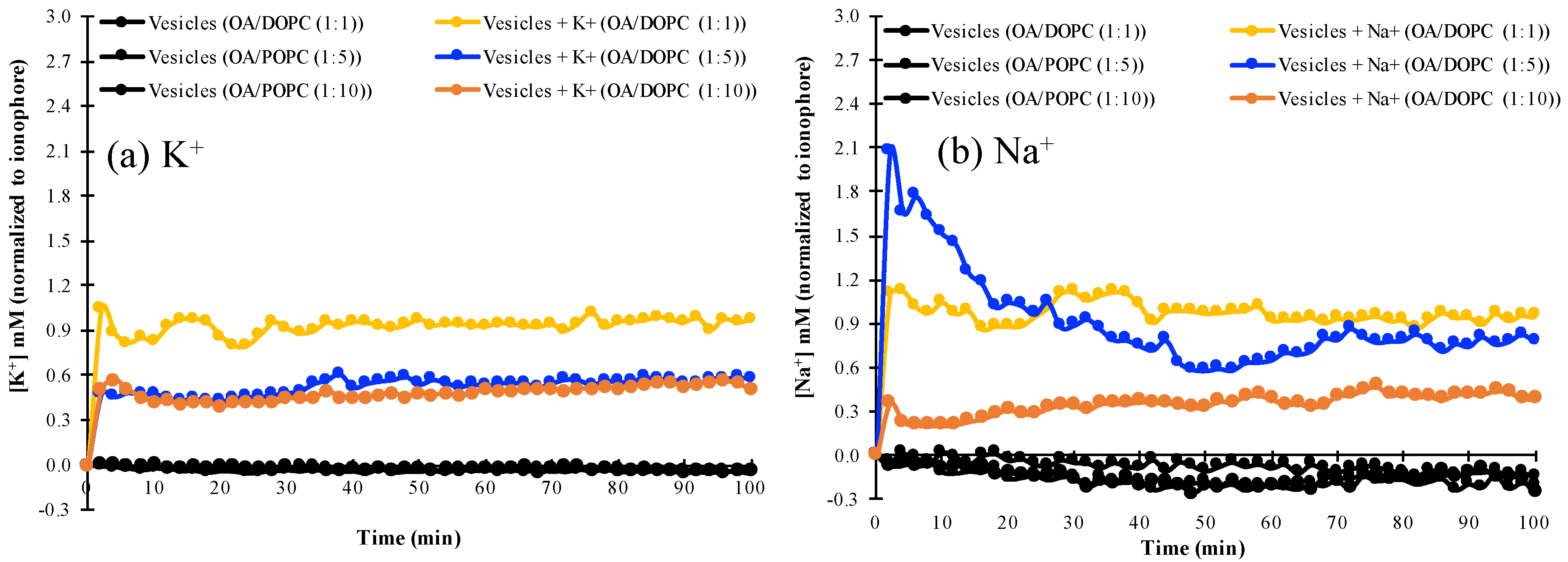
| Time (min) | OA/DOPC (1:1) (mM) | OA/DOPC (1:5) (mM) | OA/DOPC (1:10) (mM) | |||
|---|---|---|---|---|---|---|
| Na+ | K+ | Na+ | K+ | Na+ | K+ | |
| 2 | 1.1 | 1.05 | 2.07 | 0.48 | 0.36 | 0.5 |
| 10 | 1.05 | 0.83 | 1.52 | 0.48 | 0.21 | 0.42 |
| 24 | 0.95 | 0.8 | 0.97 | 0.46 | 0.29 | 0.42 |
| 60 | 0.92 | 0.93 | 0.65 | 0.53 | 0.39 | 0.5 |
| 100 | 1.02 | 0.97 | 0.78 | 0.58 | 0.39 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Dalai, P.; Sahai, N. Semipermeable Mixed Phospholipid-Fatty Acid Membranes Exhibit K+/Na+ Selectivity in the Absence of Proteins. Life 2020, 10, 39. https://doi.org/10.3390/life10040039
Zhou X, Dalai P, Sahai N. Semipermeable Mixed Phospholipid-Fatty Acid Membranes Exhibit K+/Na+ Selectivity in the Absence of Proteins. Life. 2020; 10(4):39. https://doi.org/10.3390/life10040039
Chicago/Turabian StyleZhou, Xianfeng, Punam Dalai, and Nita Sahai. 2020. "Semipermeable Mixed Phospholipid-Fatty Acid Membranes Exhibit K+/Na+ Selectivity in the Absence of Proteins" Life 10, no. 4: 39. https://doi.org/10.3390/life10040039
APA StyleZhou, X., Dalai, P., & Sahai, N. (2020). Semipermeable Mixed Phospholipid-Fatty Acid Membranes Exhibit K+/Na+ Selectivity in the Absence of Proteins. Life, 10(4), 39. https://doi.org/10.3390/life10040039