Combined Environment Simulator for Low-Dose-Rate Radiation and Partial Gravity of Moon and Mars
Abstract
1. Introduction
2. Materials and Methods
2.1. System Descriptions
2.2. Neutron Source
= (0.693 ÷ (8.3 × 107 s)) × (15.4 × 10−8 g ÷ 252 g mol−1) × 6.0 × 1023 mol−1
= 3.1 × 106 s−1 = 3.1 × 106 Bq = 3.1 MBq
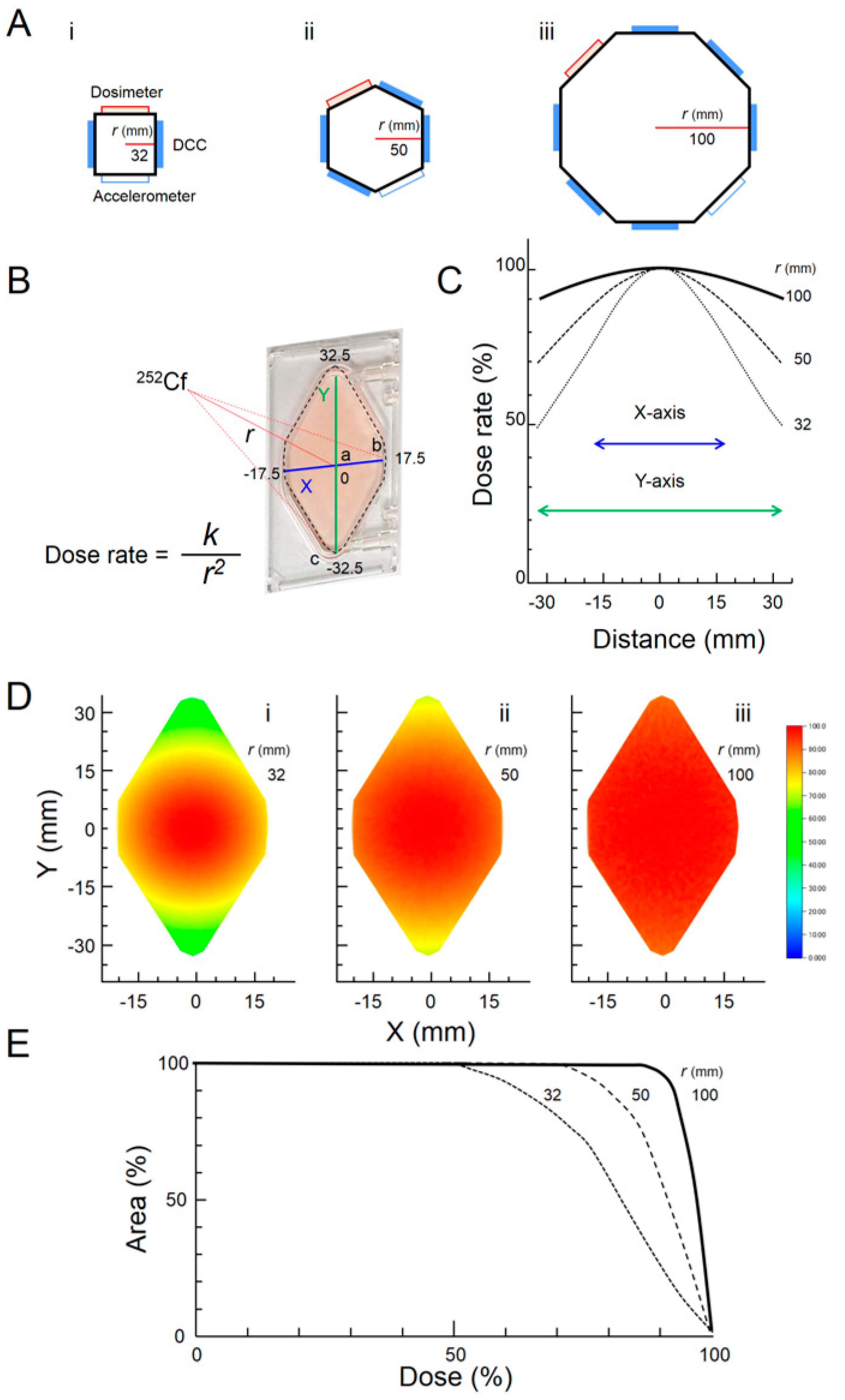
2.3. Calculation of Dose Distribution
2.4. Measurement of Dose Rate
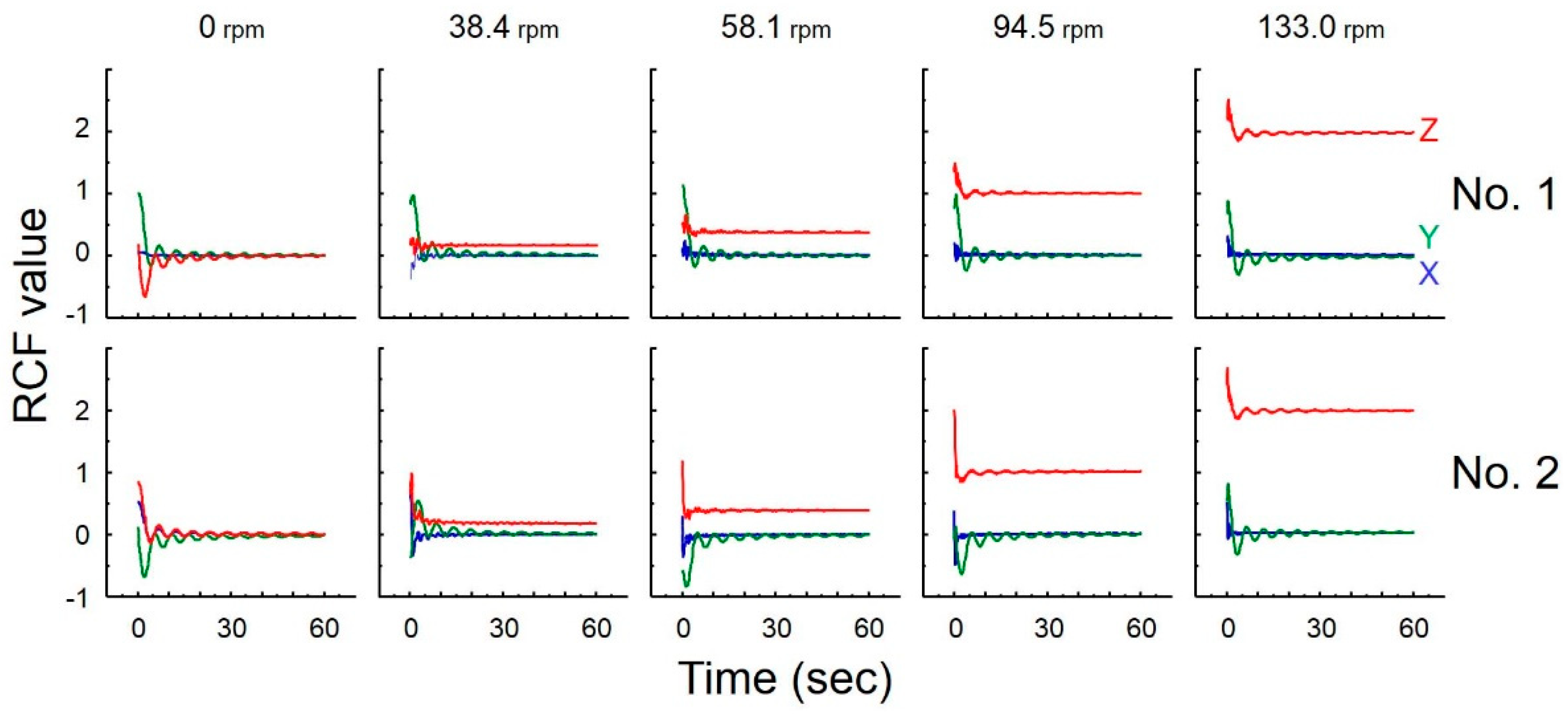
2.5. Measurement of Gravity Using Accelerometer
2.6. Measurement of Temperature
2.7. Statistical Analysis
3. Results
3.1. Simulation of Flatness and Symmetry in the Irradiation Fields
3.2. Measurement of Dose Rate
3.3. Simulation of Flatness and Symmetry in the Gravity Fields
3.4. Measurement of Gravity
3.5. Measurement of Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, A.; Ikeda, H.; Yoshida, Y. Role of high-linear energy transfer radiobiology in space radiation exposure risk. Int. J. Part. Ther. 2018, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space radiation biology for “Living in Space”. BioMed Res. Int. 2020, 2020, 4703286. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef] [PubMed]
- Borak, T.B.; Heilbronn, L.H.; Krumland, N.; Weil, M.M. Design and dosimetry of a facility to study health effects following exposures to fission neutrons at low dose rates for long durations. Int. J. Radiat. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Norbury, J.; Slaba, T. Space radiation accelerator experiments: The role of neutrons and light ions. Life Sci. Space Res. 2014, 3, 90–94. [Google Scholar] [CrossRef]
- Nelson, G.A. Space radiation and human exposures, a primer. Radiat. Res. 2016, 185, 349–358. [Google Scholar] [CrossRef]
- Chatterjee, A.; Borak, T.H. Physical and biological studies with protons and HZE particles in a NASA supported research center in radiation health. Phys. Med. 2001, 17, 59–66. [Google Scholar]
- Manzano, A.; Herranz, R.; den Toom, L.A.; Te Slaa, S.; Borst, G.; Visser, M.; Medina, F.J.; van Loon, J.J.W.A. Novel, Moon and Mars, partial gravity simulation paradigms and their effects on the balance between cell growth and cell proliferation during early plant development. NPJ Microgravity 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Risin, D.; Pellis, N.R. Modeled microgravity inhibits apoptosis in peripheral blood lymphocytes. Vitr. Cell Dev. Biol. Anim. 2001, 37, 66–72. [Google Scholar] [CrossRef]
- Canova, A.; Fiorasi, F.; Mognato, M.; Grifalconi, M.; Reddi, E.; Russo, A.; Celotti, L. Modeled microgravity affects cell response to ionizing radiation and increases genomic damage. Radiat. Res. 2005, 163, 191–199. [Google Scholar] [CrossRef]
- Mognato, M.; Celotti, L. Modeled microgravity affects cell survival and HPRT mutant frequency, but not the expression of DNA repair genes in human lymphocytes irradiated with ionising radiation. Mutat. Res. 2005, 578, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Mognato, M.; Girardi, C.; Fabris, S.; Celotti, L. DNA repair in modeled microgravity: Double strand break rejoining activity in human lymphocytes irradiated with γ-rays. Mutat. Res. 2009, 663, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Yang, Y.; Zhang, E.; Li, W.; Mi, X.; Meng, Y.; Yan, S.; Wang, Z.; Wei, W.; Shao, C.; et al. Simulated microgravity increases heavy ion radiation-induced apoptosis in human B lymphoblasts. Life Sci. 2014, 97, 123–128. [Google Scholar] [CrossRef]
- Girardi, C.; De Pittà, C.; Casara, S.; Sales, G.; Lanfranchi, G.; Celotti, L.; Mognato, M. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PLoS ONE 2012, 7, e31293. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Moreels, M.; Quintens, R.; Abou-El-Ardat, K.; El-Saghire, H.; Tabury, K.; Michaux, A.; Janssen, A.; Neefs, M.; Van Oostveldt, P.; et al. Chronic exposure to simulated space conditions predominantly affects cytoskeleton remodeling and oxidative stress response in mouse fetal fibroblasts. Int. J. Mol. Med. 2014, 34, 606–615. [Google Scholar] [CrossRef]
- Ikeda, H.; Souda, H.; Puspitasari, A.; Held, K.D.; Hidema, J.; Nikawa, T.; Yoshida, Y.; Kanai, T.; Takahashi, A. A new system for three-dimensional clinostat synchronized X-irradiation with a high-speed shutter for space radiation research. Biol. Sci. Space 2016, 30, 8–16. [Google Scholar] [CrossRef]
- Ikeda, H.; Souda, H.; Puspitasari, A.; Held, K.D.; Hidema, J.; Nikawa, T.; Yoshida, Y.; Kanai, T.; Takahashi, A. Development and performance evaluation of a three-dimensional clinostat synchronized heavy-ion irradiation system. Life Sci. Space Res. 2017, 12, 51–60. [Google Scholar] [CrossRef]
- Shen-Miller, J.; Hinchman, R.; Gordon, S.A. Thresholds for georesponse to acceleration in gravity-compensated Avena seedlings. Plant Physiol. 1968, 43, 338–344. [Google Scholar] [CrossRef]
- Lyon, C.J. Lateral transport of auxin mediated by gravity in the absence of special georeceptor tissue. Plant Physiol. 1971, 48, 642–644. [Google Scholar] [CrossRef][Green Version]
- Brown, A.H.; Dahl, A.O.; Chapman, D.K. Limitation on the use of the horizontal clinostat as a gravity compensator. Plant Physiol. 1976, 58, 4. [Google Scholar] [CrossRef] [PubMed]
- Laurinavicius, R.; Svegzdiene, D.; Buchen, B.; Sievers, A. Determination of the threshold acceleration for the gravitropic stimulation of cress roots and hypocotyls. Adv. Space Res. 1998, 21, 1203–1207. [Google Scholar] [CrossRef]
- Galland, P.; Finger, H.; Wallacher, Y. Gravitropism in Phycomyces: Threshold determination on a clinostat centrifuge. J. Plant Physiol. 2004, 161, 733–739. [Google Scholar] [CrossRef]
- Tanigawa, N.; Yano, S.; Higashibata, A.; Tsuchiya, Y.; Tanii, H.; Ando, N.; Kubota, K.; Nagase, M. Development and validation of a closed chamber for cell culture experiments in space. Biol. Sci. Space 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Stoddard, D.H.; Hootman, H.E. 252Cf Shielding Guide, DP-1246; Savannah River Laboratory: Aiken, SC, USA, 1971.
- Iwamoto, Y.; Sato, T.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.; Kai, T.; Matsuda, N.; Hosoyamada, R.; Niita, K. Benchmark study of the recent version of the PHITS code. J. Nucl. Sci. Technol. 2017, 54, 617–635. [Google Scholar] [CrossRef]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.; Kai, T.; Tsai, P.-E.; Matsuda, N.; Iwase, H.; et al. Features of Particle and Heavy Ion Transport code System (PHITS) version 3.02. J. Nucl. Sci. Technol. 2018, 55, 684–690. [Google Scholar] [CrossRef]
- Valentin, J. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR): ICRP Publication 92. Ann. ICRP 2003, 33, 1–121. [Google Scholar]
- Acharya, M.M.; Baulch, J.E.; Klein, P.M.; Baddour, A.A.D.; Apodaca, L.A.; Kramár, E.A.; Alikhani, L.; Garcia, C., Jr.; Angulo, M.C.; Batra, R.S.; et al. New concerns for neurocognitive function during deep space exposures to chronic, low dose-rate, neutron radiation. eNeuro 2019, 6, ENEURO.0094-19.2019. [Google Scholar] [CrossRef]
- Britten, R.A.; Duncan, V.D.; Fesshaye, A.S.; Wellman, L.L.; Fallgren, C.M.; Sanford, L.D. Sleep fragmentation exacerbates executive function impairments induced by protracted low dose rate neutron exposure. Int. J. Radiat. Biol. 2019. [Google Scholar] [CrossRef]
- Perez, R.E.; Younger, S.; Bertheau, E.; Fallgren, C.M.; Weil, M.M.; Raber, J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice. Behav. Brain Res. 2020, 379, 112377. [Google Scholar] [CrossRef]
- Ikeda, H.; Muratani, M.; Hidema, J.; Hada, M.; Fujiwara, K.; Souda, H.; Yoshida, Y.; Takahashi, A. Expression profile of cell cycle-related genes in human fibroblasts exposed simultaneously to radiation and simulated microgravity. Int. J. Mol. Sci. 2019, 20, 4791. [Google Scholar] [CrossRef]
- Todd, P. Physical effects at the cellular level under altered gravity conditions. Adv. Space Res. 1992, 12, 43–49. [Google Scholar] [CrossRef]
- Hada, M.; Ikeda, H.; Rhone, J.R.; Beitman, A.J.; Plante, I.; Souda, H.; Yoshida, Y.; Held, K.D.; Fujiwara, K.; Saganti, P.B.; et al. Increased chromosome aberrations in cells exposed simultaneously to simulated microgravity and radiation. Int. J. Mol. Sci. 2019, 20, 43. [Google Scholar] [CrossRef]
- Yamanouchi, S.; Rhone, J.R.; Mao, J.-H.; Fujiwara, K.; Saganti, P.B.; Takahashi, A.; Hada, M. Simultaneous exposure of cultured human lymphoblastic cells to simulated microgravity and radiation increases chromosome aberrations. Life 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Takahashi, A.; Okaichi, K.; Ohnishi, K.; Matsumoto, H.; Takahashi, S.; Yamanaka, H.; Nakano, T.; Nagaoka, S. Cell growth and morphology of Dictyostelium discoideum in space environment. Biol. Sci. Space 1997, 11, 29–34. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, K.; Fukui, M.; Nakano, T.; Yamaguchi, K.; Nagaoka, S.; Ohnishi, T. Mutation frequency of Dictyostelium discoideum spores exposed to the space environment. Biol. Sci. Space 1997, 11, 81–86. [Google Scholar] [CrossRef][Green Version]
- Takahashi, A.; Ohnishi, K.; Takahashi, S.; Masukawa, M.; Sekikawa, K.; Amano, T.; Nakano, T.; Nagaoka, S.; Ohnishi, T. Differentiation of Dictyostelium discoideum vegetative cells into spores during Earth orbit in space. Adv. Space Res. 2001, 28, 549–553. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, K.; Takahashi, S.; Masukawa, M.; Sekikawa, K.; Amano, T.; Nakano, T.; Nagaoka, S.; Ohnishi, T. The effects of microgravity on induced mutation in Escherichia coli and Saccharomyces cerevisiae. Adv. Space Res. 2001, 28, 555–561. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, K.; Yokota, A.; Kumagai, T.; Nakano, T.; Ohnishi, T. Mutation frequency of plasmid DNA and Escherichia coli following long-term space flight on Mir. J. Radiat. Res. 2002, 43, S137–S140. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef]
- Wagner, E.B.; Granzella, N.P.; Saito, H.; Newman, D.J.; Young, L.R.; Bouxsein, M.L. Partial weight suspension: A novel murine model for investigating adaptation to reduced musculoskeletal loading. J. Appl. Physiol. 2010, 109, 350–357. [Google Scholar] [CrossRef]
- Wilson, J.M.; Krigsfeld, G.S.; Sanzari, J.K.; Wagner, E.B.; Mick, R.; Kennedy, A.R. Comparison of hindlimb unloading and partial weight suspension models for spaceflight-type condition induced effects on white blood cells. Adv. Space Res. 2012, 49, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Ellman, R.; Spatz, J.; Cloutier, A.; Palme, R.; Christiansen, B.A.; Bouxsein, M.L. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J. Bone Miner. Res. 2013, 28, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Mortreux, M.; Ko, F.C.; Riveros, D.; Bouxsein, M.L.; Rutkove, S.B. Longitudinal time course of muscle impairments during partial weight-bearing in rats. NPJ Microgravity 2019, 5, 20. [Google Scholar] [CrossRef]
- Ishioka, N.; Suzuki, H.; Asashima, M.; Kamisaka, S.; Mogami, Y.; Ochiai, T.; Aizawa-Yano, S.; Higashibata, A.; Ando, N.; Nagase, M.; et al. Development and verification of hardware for life science experiments in the Japanese Experiment Module “Kibo” on the International Space Station. J. Gravit. Physiol. 2004, 11, 81–91. [Google Scholar]
- Shiba, D.; Mizuno, H.; Yumoto, A.; Shimomura, M.; Kobayashi, H.; Morita, H.; Shimbo, M.; Hamada, M.; Kudo, T.; Shinohara, M.; et al. Development of new experimental platform ‘MARS’-Multiple Artificial-gravity Research System-to elucidate the impacts of micro/partial gravity on mice. Sci. Rep. 2017, 7, 10837. [Google Scholar] [CrossRef]
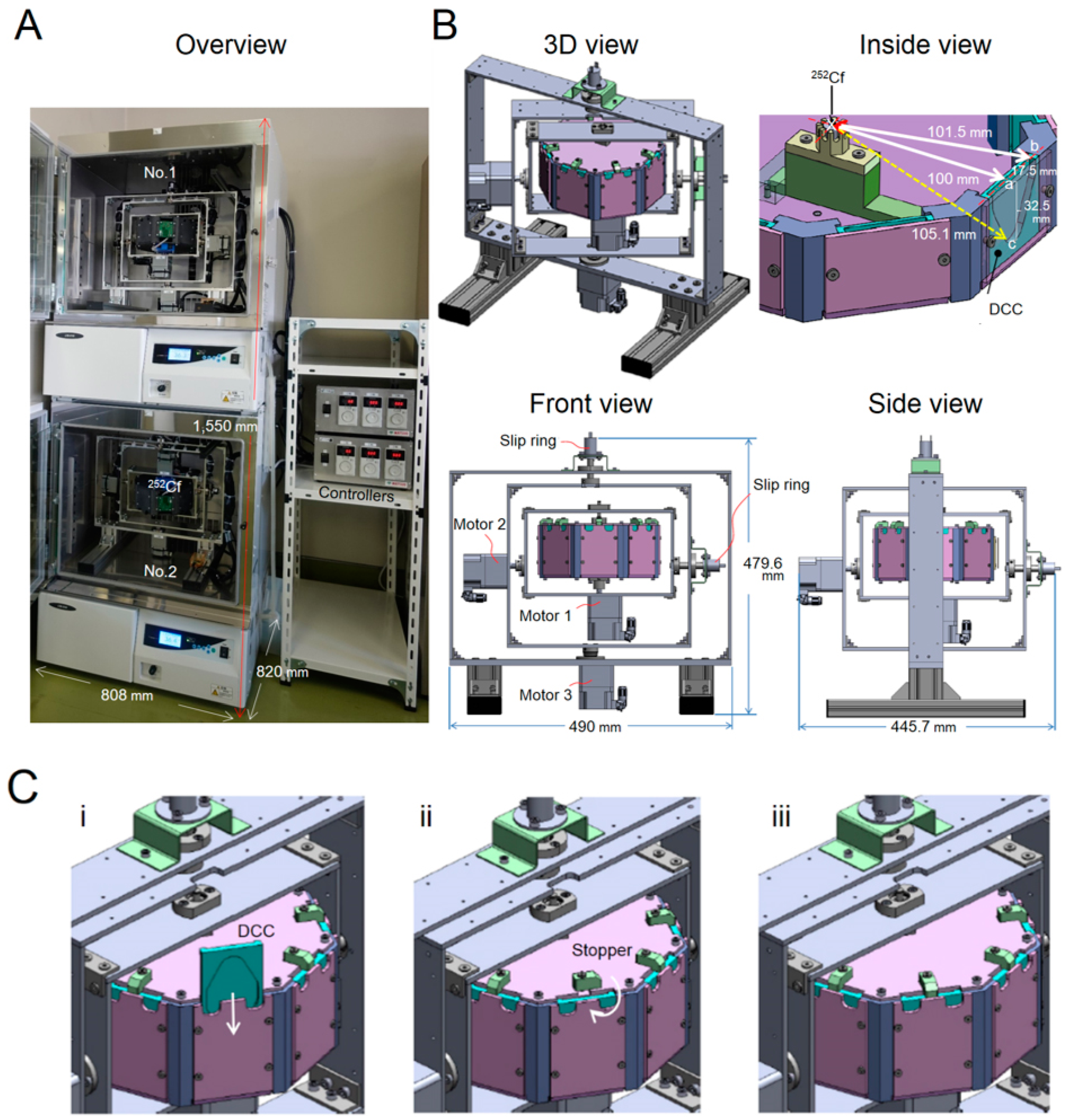
| Subsystem | Specifications |
|---|---|
| 3D clinostat | External size: W 490 mm × D 445.7 mm × H 479.6 mm |
| Rotational velocity: 66°/s and 78°/s | |
| Manufacturer: Matsuo Industries, Inc. (Aichi, Japan) | |
| Centrifuge | Rotor size: W 216 mm × D 216 mm × H 90 mm (octagonal type) |
| Speed control range: 27–133 rpm | |
| Manufacturer: Matsuo Industries, Inc. | |
| Radiation source | 252Cf (N-252CE, Japan Radioisotope Association, Tokyo, Japan) |
| External size: Diameter 9.4 mm × L 36.3 mm | |
| Dose-equivalent average energy: 2.3 MeV | |
| Dose-averaged LET: 68 keV/μm | |
| Half-life: 2.645 years | |
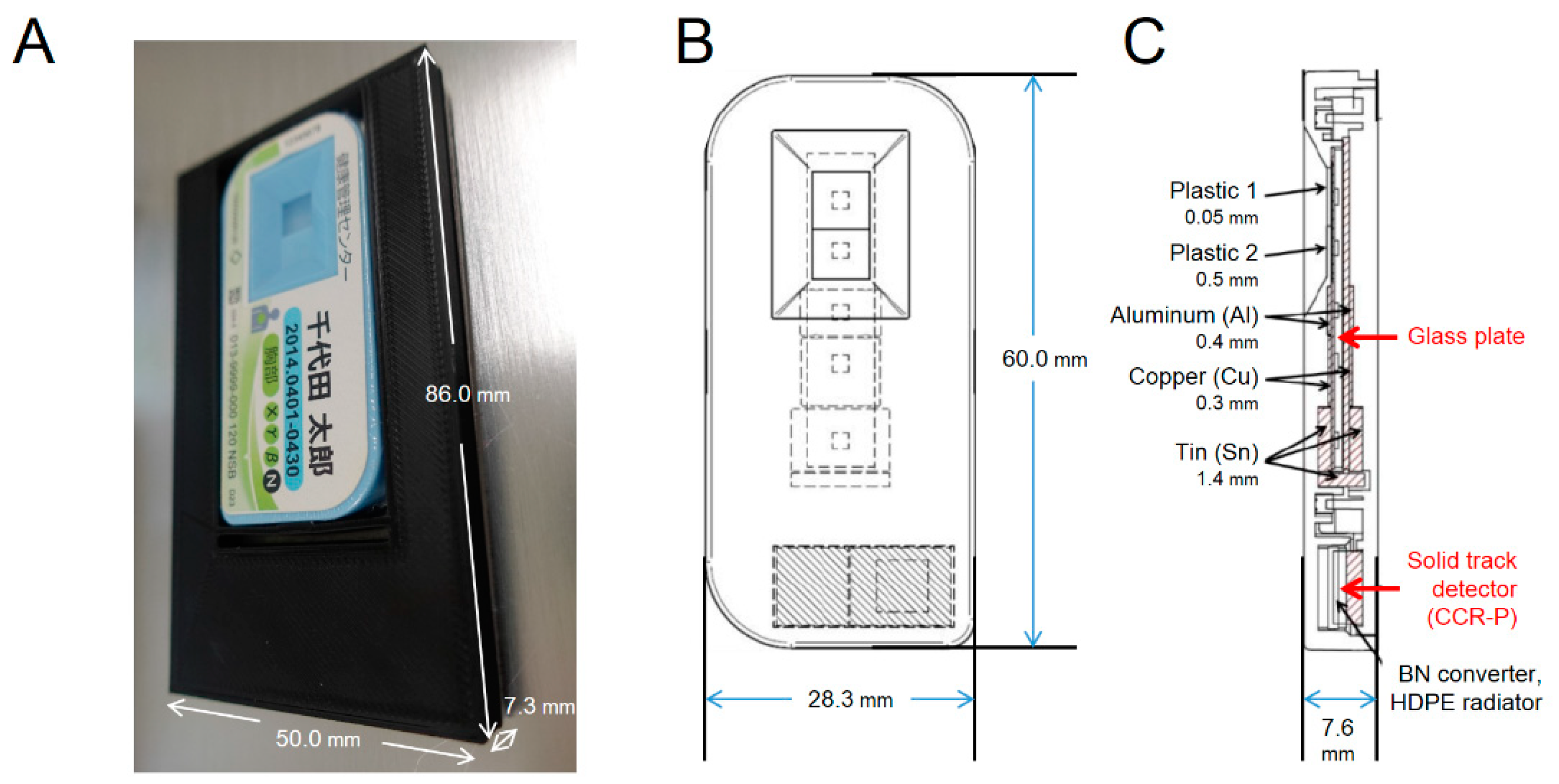
| Sample chamber | Disposable closed cell culture chamber (DCC, Chiyoda Co., Kanagawa, Japan) |
| External size: W 86.0 mm × D 50.0 mm × H 7.3 mm | |
| Cultivation area: 15.5 cm2 (W 65 mm × D 35 mm) | |
| Liquid depth: 3 mm | |
| Material: polystyrene (bottom thickness: 1 mm; top thickness: 50 µm) | |
| Six DCCs can be placed in the rotor | |
| Incubator | Low-temperature incubator without control of CO2 concentrations: LTE-510 (Tokyo Rikakikai Co., Tokyo, Japan) |
| Internal size: W 600 mm × D 500 mm × H 500 mm | |
| Temperature control range/accuracy: −10–60 °C/±0.2 °C |
| Position of DCC * in Octagonal Rotor | Distance from 252Cf | Theoretical Dose Rate † | Relative Value |
|---|---|---|---|
| a (center) | 100.0 mm | 1.23 ± 0.18 mGy/day | 100.0 |
| b (short end) | 101.5 mm | 1.19 ± 0.18 mGy/day | 97.1 |
| c (long end) | 105.1 mm | 1.11 ± 0.17 mGy/day | 90.5 |
| Control Samples | Irradiation Samples | |
|---|---|---|
| Incubator of SwiNG | No. 1 | No. 2 |
| Neutron * | ND | 10.8 ± 0.0 mSv |
| Total dose rate † | ND | 1.08 ± 0.00 mGy/day |
| Position | γ-ray | Neutron | Total |
|---|---|---|---|
| The surface of SwiNG | 1.5 µSv/h | 18.4 µSv/h | 19.9 µSv/h |
| 1 m distance from 252Cf | 0.3 µSv/h | 6.7 µSv/h | 7.0 µSv/h |
| 2 m distance from 252Cf | 0.2 µSv/h | 2.5 µSv/h | 2.7 µSv/h |
| Rotary Speed of Motor 1 (Centrifuge) * | Theoretical RCF † (r = 100–101 mm) | Measured RCF ‡ | Simulation | |
|---|---|---|---|---|
| No. 1 | No. 2 | |||
| 0.0 rpm | ~µG | 0.01 ± 0.00G | 0.01 ± 0.00G | interplanetary space |
| 38.4 rpm | 0.165–0.167G | 0.17 ± 0.00G | 0.17 ± 0.00G | on the Moon |
| 58.1 rpm | 0.377–0.381G | 0.38 ± 0.00G | 0.39 ± 0.00G | on Mars |
| 94.5 rpm | 0.998–1.008G | 1.01 ± 0.00G | 1.01 ± 0.00G | on the Earth |
| 133.0 rpm | 1.978–1.997G | 1.98 ± 0.01G | 2.00 ± 0.00G | hypergravity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, A.; Yamanouchi, S.; Takeuchi, K.; Takahashi, S.; Tashiro, M.; Hidema, J.; Higashitani, A.; Adachi, T.; Zhang, S.; Guirguis, F.N.L.; et al. Combined Environment Simulator for Low-Dose-Rate Radiation and Partial Gravity of Moon and Mars. Life 2020, 10, 274. https://doi.org/10.3390/life10110274
Takahashi A, Yamanouchi S, Takeuchi K, Takahashi S, Tashiro M, Hidema J, Higashitani A, Adachi T, Zhang S, Guirguis FNL, et al. Combined Environment Simulator for Low-Dose-Rate Radiation and Partial Gravity of Moon and Mars. Life. 2020; 10(11):274. https://doi.org/10.3390/life10110274
Chicago/Turabian StyleTakahashi, Akihisa, Sakuya Yamanouchi, Kazuomi Takeuchi, Shogo Takahashi, Mutsumi Tashiro, Jun Hidema, Atsushi Higashitani, Takuya Adachi, Shenke Zhang, Fady Nagy Lotfy Guirguis, and et al. 2020. "Combined Environment Simulator for Low-Dose-Rate Radiation and Partial Gravity of Moon and Mars" Life 10, no. 11: 274. https://doi.org/10.3390/life10110274
APA StyleTakahashi, A., Yamanouchi, S., Takeuchi, K., Takahashi, S., Tashiro, M., Hidema, J., Higashitani, A., Adachi, T., Zhang, S., Guirguis, F. N. L., Yoshida, Y., Nagamatsu, A., Hada, M., Takeuchi, K., Takahashi, T., & Sekitomi, Y. (2020). Combined Environment Simulator for Low-Dose-Rate Radiation and Partial Gravity of Moon and Mars. Life, 10(11), 274. https://doi.org/10.3390/life10110274









