Expression Kinetics of Regulatory Genes Involved in the Vesicle Trafficking Processes Operating in Tomato Flower Abscission Zone Cells during Pedicel Abscission
Abstract
1. Introduction
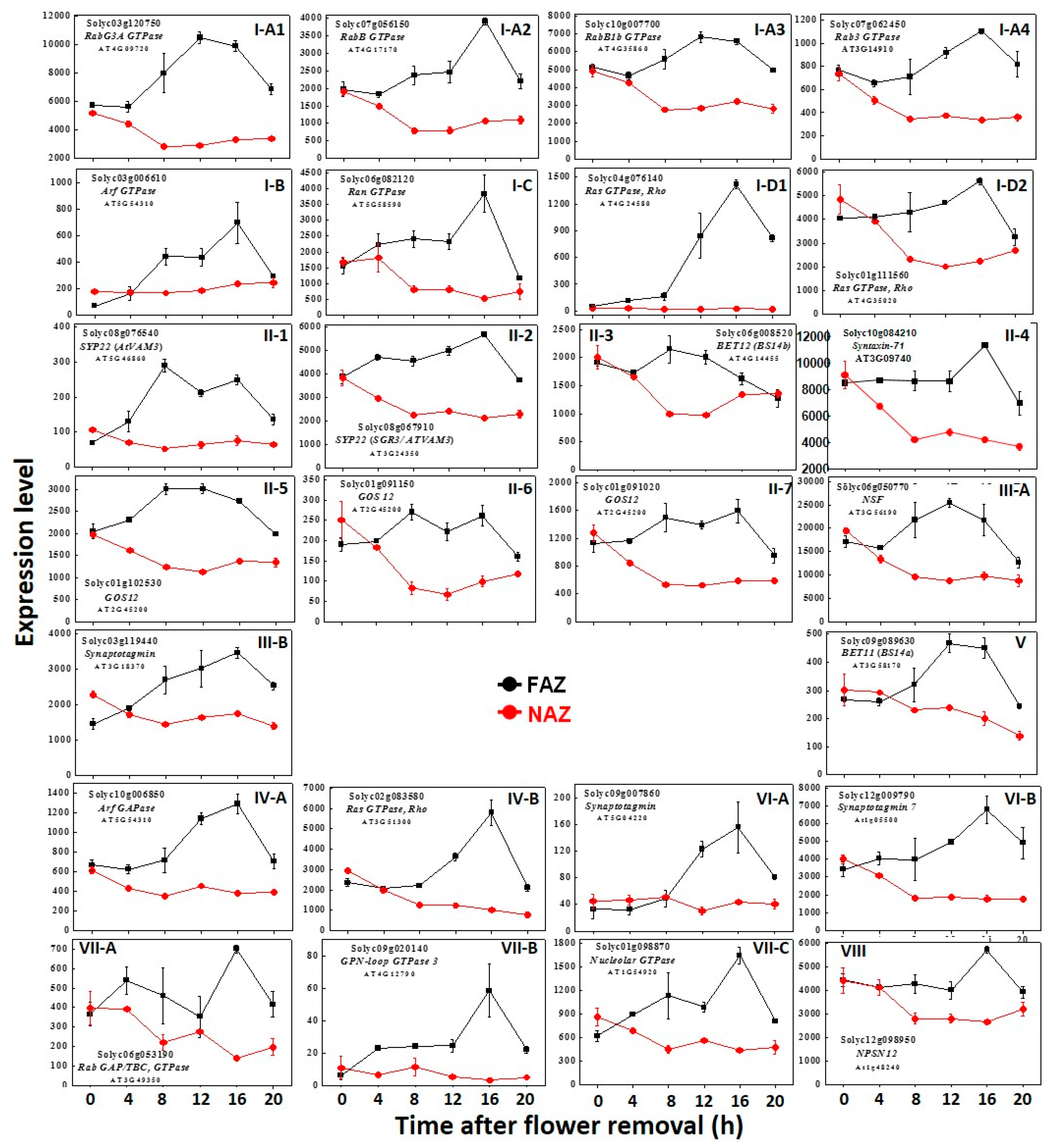
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Abscission Induction Treatments
3.2. RNA Extraction and Microarray Assays
3.3. Gene Expression Validation by Quantitative Real-Time PCR (qRT-PCR)
3.4. Sequence Deposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osborne, D.J. Abscission. Critic. Rev. Plant Sci. 1989, 8, 103–129. [Google Scholar] [CrossRef]
- Lewis, M.W.; Leslie, M.E.; Liljegren, S.J. Plant separation: 50 ways to leave your mother. Curr. Opin. Plant Biol. 2006, 9, 59–65. [Google Scholar] [CrossRef]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Four shades of detachment: Regulation of floral organ abscission. Plant Signal. Behav. 2014, 9, e976154. [Google Scholar] [CrossRef] [PubMed]
- Merelo, P.; Agustí, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gómez-Cadenas, A.; Coimbra, S.; Gómez, M.D.; Pérez-Amador, M.A.; Domingo, C.; et al. Cell wall remodeling in abscission zone cells during ethylene-promoted fruit abscission in Citrus. Front. Plant Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Del Campillo, E.; Lewis, L.N. Identification and kinetics of accumulation of proteins induced by ethylene in bean abscission zones. Plant Physiol. 1992, 98, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, V.K.S.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Identification of defense-related genes newly-associated with tomato flower abscission. Plant Signal. Behav. 2011, 6, 590–593. [Google Scholar] [CrossRef]
- Gonzalez-Carranza, Z.H.; Shahid, A.A.; Zhang, L.; Liu, Y.; Ninsuwan, U.; Roberts, J.A. A novel approach to dissect the abscission process in Arabidopsis. Plant Physiol. 2012, 160, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef]
- Kim, J.; Sundaresan, S.; Philosoph-Hadas, S.; Yang, R.; Meir, S.; Tucker, M.L. Examination of the abscission-associated transcriptomes for soybean, tomato, and Arabidopsis highlights the conserved biosynthesis of an extensible extracellular matrix and boundary layer. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Agusti, J.; Gimeno, J.; Merelo, P.; Serrano, R.; Cercos, M.; Conesa, A.; Talon, M.; Tadeo, F.R. Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: Involvement of CitbHLH1. J. Exp. Bot. 2012, 63, 6079–6091. [Google Scholar] [CrossRef]
- Corbacho, J.; Romojaro, F.; Pech, J.C.; Latché, L.; Gomez-Jimenez, M.C. Transcriptomic events involved in melon mature-fruit abscission comprise the sequential induction of cell-wall degrading genes coupled to a stimulation of endo and exocytosis. PLoS ONE 2013, 8, e58363. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.; Hall, J.L. Fine structure and cytochemistry of the abscission zone cells of phaseolus leaves. I. Ultrastructural changes occurring during abscission. Ann. Bot. 1974, 38, 849–854. [Google Scholar] [CrossRef]
- Sexton, R.; Jamieson, G.G.; Allan, M.H. An ultrastructural study of abscission zone cells with special reference to the mechanism of enzyme secretion. Protoplasma 1977, 91, 369–387. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2018, 69, 21–37. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- de la Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; Clemente, H.S.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant-microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bar-Dror, T.; Dermastia, M.; Kladnik, A.; Žnidaric-Tušek, M.; Novak, M.P.; Meir, S.; Burd, S.; Philosoph-Hadas, S.; Ori, N.; Sonego, L.; et al. Programmed cell death occurs asymmetrically during abscission in tomato. Plant Cell 2011, 23, 4146–4163. [Google Scholar] [CrossRef]
- Dermastia, M.; Kladnik, A.; Bar-Dror, T.; Lers, A. Endoreduplication preferentially occurs at the proximal side of the abscission zone during abscission of tomato leaf. Plant Signal. Behav. 2012, 7, 1106–1109. [Google Scholar] [CrossRef][Green Version]
- Chersicola, M.; Kladnik, A.; Tušek-Žnidaricˇ, M.; Mrak, T.; Gruden, K.; Dermastia, M. 1-Aminocyclopropane-1-Carboxylate Oxidase induction in tomato flower pedicel phloem and abscission related processes are differentially sensitive to ethylene. Front. Plant Sci. 2017, 8, 464. [Google Scholar] [CrossRef]
- Vitale, A.; Denecke, J. The endoplasmic reticulum-gateway of the secretory pathway. Plant Cell 1999, 11, 615–628. [Google Scholar]
- Richter, S.; Voss, U.; Jurgens, G. Post-Golgi traffic in plants. Traffic 2009, 10, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Robinson, D.G. Transport vesicle formation in plant cells. Curr. Opin. Plant Biol. 2009, 12, 660–669. [Google Scholar] [CrossRef]
- Lycett, G. The role of Rab GTPases in cell wall metabolism. J. Exp. Bot. 2008, 59, 4061–4074. [Google Scholar] [CrossRef]
- Saito, C.; Ueda, T. Functions of RAB and SNARE proteins in plant life. Int. Rev. Cell Mol. Biol. 2009, 274, 183–233. [Google Scholar] [CrossRef]
- Ebine, K.; Ueda, T. Roles of membrane trafficking in plant cell wall dynamics. Front. Plant Sci. 2005, 6, 878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Brandizzi, F. The plant secretory pathway: An essential factory for building the plant cell wall. Plant Cell Physiol. 2014, 55, 687–693. [Google Scholar] [CrossRef] [PubMed]
- van de Meene, A.M.L.; Doblin, M.S.; Bacic, A. The plant secretory pathway seen through the lens of the cell wall. Protoplasma 2007, 254, 75–94. [Google Scholar] [CrossRef]
- van Oostende, T.C.; Guillet, D.; Triplet, T.; Pandzic, E.; Wiseman, P.W.; Geitmann, A. Vesicle dynamics during plant cell cytokinesis reveals distinct developmental phases. Plant Physiol. 2017, 174, 1544–1558. [Google Scholar] [CrossRef]
- Lipka, V.; Kwon, C.; Panstruga, R. SNARE-Ware: The role of SNARE-domain proteins in plant biology. Annu. Rev. Cell Dev. Biol. 2007, 23, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Cai, H.; Karin, K.; Ferro-Novick, S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Develop. Cell 2007, 12, 671–682. [Google Scholar] [CrossRef]
- Gerst, J.G. SNARE regulators: Matchmakers and match breakers. Biochim. Biophys. Acta 2003, 1641, 99–110. [Google Scholar] [CrossRef]
- Hong, W.J.; Lev, S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014, 24, 36–43. [Google Scholar] [CrossRef]
- Santos, L. Molecular mechanisms of the AAA proteins in plants. In Advances in Agricultural and Food Biotechnology; Guevara-González, R.G., Torres-Pacheco, I., Eds.; Research Signpost: Kerala, India, 2006; Volume 1, pp. 1–15. ISBN 81-7736-269-0. [Google Scholar]
- Gil-Amado, J.A.; Gomez-Jimenez, M.C. Transcriptome analysis of mature fruit abscission control in olive. Plant Cell Physiol. 2013, 54, 244–269. [Google Scholar] [CrossRef]
- Briegas, B.; Corbacho, J.; Parra-Lobato, M.C.; Paredes, M.A.; Labrador, J.; Gallardo, M.; Gomez-Jimenez, M.C. Transcriptome and hormone analyses revealed insights into hormonal and vesicle trafficking regulation among Olea europaea fruit tissues in late development. Int. J. Mol. Sci. 2020, 21, 4819. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Leslie, M.E.; Darnielle, L.; Lewis, M.W.; Taylor, S.M.; Luo, R.; Geldner, N.; Chory, J.; Randazzo, P.A.; Yanofsky, M.F.; et al. Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development 2009, 136, 1909–1918. [Google Scholar] [CrossRef]
- Leslie, M.E.; Lewis, M.W.; Youn, J.Y.; Daniels, M.J.; Liljegren, S.J. The EVERSHED receptor-like kinase modulates floral organ shedding in Arabidopsis. Development 2010, 137, 467–476. [Google Scholar] [CrossRef]
- Liljegren, S.J. Organ abscission: Exit strategies require signals and moving traffic. Curr. Opin. Plant Biol. 2012, 15, 670–676. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, Y.; Sun, X.; Fan, H.; Gao, J.; Chao, Y.; Liu, A.; Yu, X.; Cai, Y.; Lin, Y. Genome-wide identification of differentially expressed miRNAs induced by ethephon treatment in abscission layer cells of cotton (Gossypium hirsutum). Gene 2018, 676, 263–268. [Google Scholar] [CrossRef]
- Merelo, P.; Agustí, J.; Ventimilla, D.; Talón, M.; Tadeo, F.R. Vesicular trafficking in abscission zone cells during ethylene-promoted fruit abscission in citrus. Acta Hortic. 2019, 1230, 41–48. [Google Scholar] [CrossRef]
- Sundaresan, S.; Philosoph-Hadas, S.; Ma, C.; Jiang, C.Z.; Riov, J.; Mugasimangalam, R.; Kochanek, B.; Salim, S.; Reid, M.S.; Meir, S. Tomato Hybrid Proline-Rich Protein regulates the abscission zone competence to respond to ethylene signals. Hort. Res. 2018, 5, 28. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, K.S.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol. 2010, 154, 1929–1956. [Google Scholar] [CrossRef]
- Sundaresan, S.; Philosoph-Hadas, S.; Riov, J.; Mugasimangalam, R.; Kuravadi, N.A.; Kochanek, B.; Salim, S.; Tucker, M.L.; Meir, S. De novo transcriptome sequencing and development of abscission zone-specific microarray as a new molecular tool for analysis of tomato organ abscission. Front. Plant Sci. 2016, 6, 1258. [Google Scholar] [CrossRef]
- Paul, P.; Simm, S.; Mirus, O.; Scharf, K.D.; Fragkostefanakis, S.; Schleiff, E. The complexity of vesicle transport factors in plants examined by orthology search. PLoS ONE 2014, 5, e97745. [Google Scholar] [CrossRef]
- Uemura, T.; Ueda, T.; Ohniwa, R.L.; Nakano, A.; Takeyasu, K.; Sato, M.H. Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 2004, 29, 49–65. [Google Scholar] [CrossRef]
- Enami, K.; Ichikawa, M.; Uemura, T.; Kutsuna, N.; Hasezawa, S.; Nakagawa, T.; Nakano, A.; Sato, M.H. Differential expression control and polarized distribution of plasma membrane-resident SYP1 SNAREs in Arabidopsis thaliana. Plant Cell Physiol. 2009, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Neu, C.; Pajonk, S.; Yun, H.S.; Lipka, U.; Humphry, M.; Bau, S.; Straus, M.; Kwaaitaal, M.; Rampelt, H.; et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008, 451, 835–840. [Google Scholar] [CrossRef]
- Honsbein, A.; Blatt, M.R.; Grefen, C. A molecular framework for coupling cellular volume and osmotic solute transport control. J. Exp. Bot. 2011, 62, 2363–2370. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Riov, J.; Tucker, M.L.; Patterson, S.E.; Roberts, J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J. Exp. Bot. 2019, 70, 1461–1467. [Google Scholar] [CrossRef]
- Drakakaki, G.; van de Ven, W.; Pan, S.; Miao, Y.; Wang, J.; Keinath, N.F.; Weatherly, B.; Jiang, L.; Schumacher, K.; Hicks, G.; et al. Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res. 2012, 22, 413–424. [Google Scholar] [CrossRef]
- Parsons, H.T.; Drakakaki, G.; Heazlewood, J.L. Proteomic dissection of the Arabidopsis Golgi and trans-Golgi network. Front. Plant Sci. 2013, 3, 298. [Google Scholar] [CrossRef]
- Park, E.; Drakakaki, G. Proteomics of endosomal compartments from plants case study: Isolation of trans-Golgi network vesicles. In Plant Endosomes: Methods and Protocols, Methods in Molecular Biology; Otegui, M.S., Ed.; Springer Science + Business Media: New York, NY, USA, 2014; Volume 1209, pp. 179–187. [Google Scholar] [CrossRef]
- Heard, W.; Sklenár, J.; Tomé, D.F.; Robatzek, S.; Jones, A.M. Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol. Cell. Proteom. 2015, 14, 1796–1813. [Google Scholar] [CrossRef]
- Wilkop, T.; Pattathil, S.; Ren, G.; Davis, J.D.; Bao, W.; Duan, D.; Peralta, G.A.; Domozych, S.D.; Hahn, G.M.; Drakakaki, G. A hybrid approach enabling large-scale glycomic analysis of post-Golgi vesicles reveals a transport route for polysaccharides. Plant Cell 2019, 31, 627–644. [Google Scholar] [CrossRef]
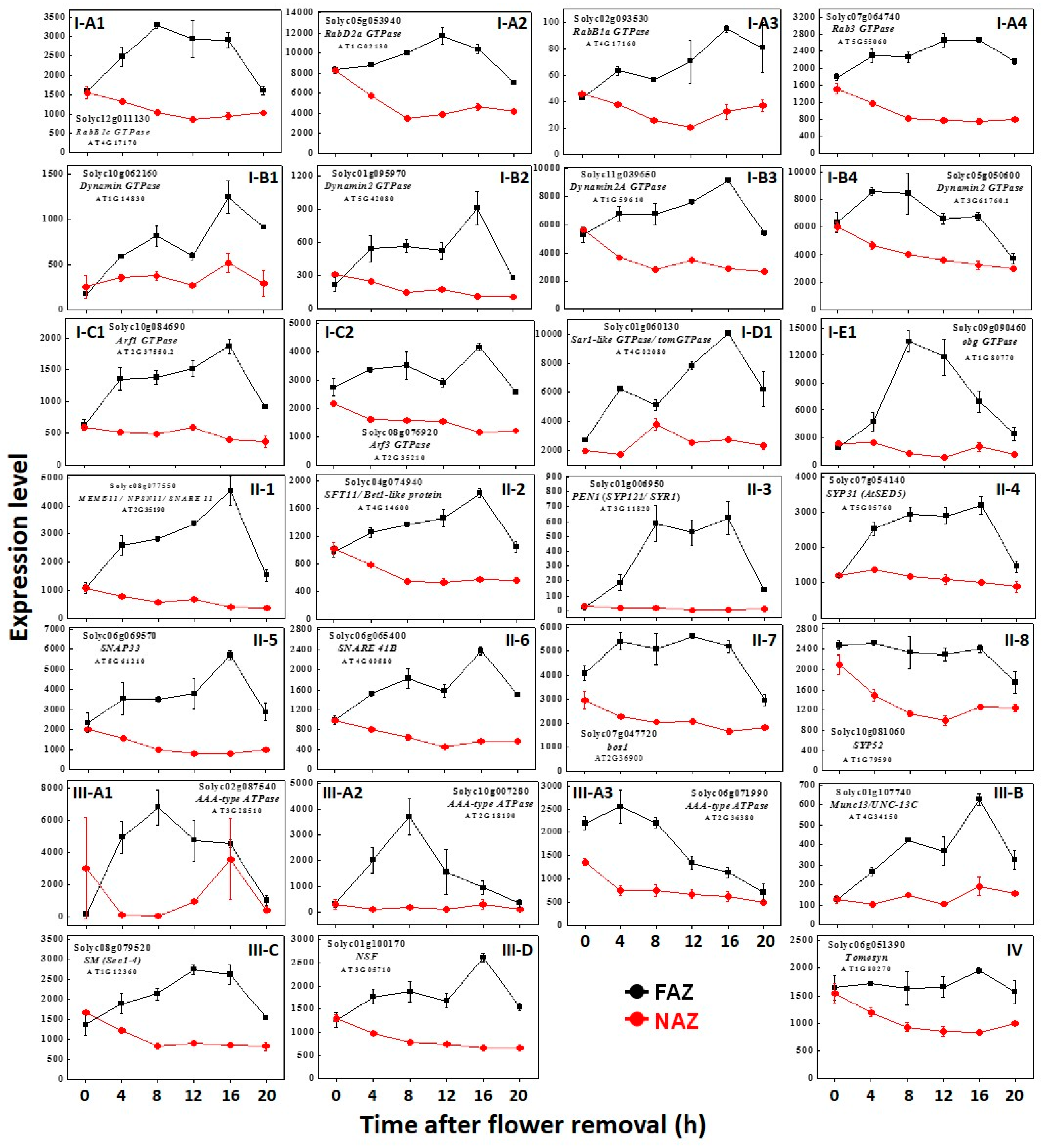
| Function | GTPases | SNAREs | SNARE Regulators |
|---|---|---|---|
| Vesicle Budding | Arf1 GTPase Solyc10g084690 ↑ Arf3 GTPase Solyc08g076920 ↑ Dynamin2 GTPase Solyc01g095970 ↑ Dynamin2 GTPase Solyc05g050600 ↑ Dynamin GTPase Solyc10g062160 ↑ Dynamin2A GTPase Solyc11g039650 ↑ | Matchmakers NSF Solyc01g100170 ↑ AAA-type ATPase Solyc06g071990 ↑ AAA-type ATPase Solyc02g087540 ↑ AAA-type ATPase Solyc10g007280 ↑ Munc13/UNC-13C Solyc01g107740 ↑ Sec1-4/Munc (SM) Solyc08g079520 ↑ | |
| ER→ cis Golgi | RabB1c GTPase Solyc12g011130 ↑ RabB1a GTPase Solyc02g093530 ↑ RabD2a GTPase Solyc05g053940 → Sar1 GTPase Solyc01g060130 ↑ | bos1—v-SNARE, Qb-SNARE Solyc07g047720 ↑ | |
| Golgi Apparatus | Bet1 - MEMB11* t-SNARE, Qc- SNARE Solyc04g074940 ↑ SNARE 11—SFT11* t-SNARE, Qb-SNARE Solyc08g077550 ↑ SNARE 41b trans membrane Solyc06g065400 ↑ SYP31—Syntaxin 32 t-SNARE- Qa SNARE Solyc07g054140 ↑ | ||
| Trans Golgi Network (TGN) | |||
| TGN→ PM | SNAP33—t-SNARE, Qb+c-SNARE Solyc06g069570 ↑ PEN1—(SYP121/SYR1) Syntaxin t-SNARE—Qa SNARE Solyc01g006950 ↑ | Matchbreakers Tomosyn Solyc06g051390 → | |
| Golgi→Vacuole | SYP52—t- SNARE Syntaxin-51, Qc-SNARE Solyc10g081060 → | ||
| Unknown Function | obg GTPase Solyc09g090460 ↑ | ||
| Exocytosis General | Rab3 GTPase Solyc07g064740 ↑ |
| Function | GTPases | SNAREs | SNARE Regulators |
|---|---|---|---|
| Vesicle Budding | Arf1 GTPase Solyc10g084690 → Arf3 GTPase Solyc08g076920 → Dynamin2 GTPase Solyc01g095970 → Dynamin2 GTPase Solyc05g050600 → Dynamin GTPase Solyc10g062160 ↑ Dynamin2A GTPase Solyc11g039650 → Arf GTPase Solyc03g006610 ↑ | Matchmakers NSF Solyc01g100170 → AAA-type ATPase Solyc06g071990 → AAA-type ATPase Solyc02g087540 ↑ AAA-type ATPase Solyc10g007280 ↑ Munc13/UNC-13C Solyc01g107740 ↑ Sec1-4/Munc (SM) Solyc08g079520 → NSF Solyc06g050770 ↑ Synaptotagmin Solyc03g119440 ↑ | |
| ER→cis Golgi | RabB1c GTPase Solyc12g011130 ↑ RabB1a GTPase Solyc02g093530 → RabD2a GTPase Solyc05g053940 ↑ Sar1 GTPase Solyc01g060130 → RabB GTPase Solyc07g056150 ↑ RabB1b GTPase Solyc10g007700 ↑ | Bos1—v-SNARE, Qb-SNARE Solyc07g047720 → Bet12—t-SNARE, Qc-SNARE Solyc06g008520 → SYP71—t- SNARE Syntaxin- Qc-SNARESolyc10g084210 → | |
| Golgi Apparatus, Trans Golgi Network (TGN) | Bet1—MEMB11* t-SNARE, Qc- SNARE Solyc04g074940 → SNARE 11—SFT11* t-SNARE, Qb-SNARE Solyc08g077550 → SNARE41b trans membrane Solyc06g065400 ↑ SYP31—Syntaxin 32 t-SNARE- Qa SNARE Solyc07g054140 ↑ GOS12-v-SNARE-C-Qb-SNAR Solyc01g091020 ↑ Solyc01g091150 ↑ Solyc01g102530 ↑ SYP 22 -t-SNARE Syntaxin, Qa-SNARE Solyc08g076540 ↑ SYP22-t-SNARE Syntaxin 32- Qa-SNARE Solyc08g067910→ | ||
| TGN→PM | SNAP33-t-SNARE, Qb+c-SNARE Solyc06g069570 → PEN1-(SYP121/SYR1) Syntaxin t-SNARE-Qa SNARE Solyc01g006950 ↑ | Matchbreakers Tomosyn Solyc06g051390 → | |
| Golgi→Vacuole | RabG3A GTPase Solyc03g120750 ↑ | SYP52-t- SNARE Syntaxin-51, Qc-SNARE Solyc10g081060 → | |
| Cytoskeleton and Vesicular Trafficking | Ras GTPase, Rho Solyc04g076140 ↑ Ras GTPase, Rho Solyc01g111560↑ | ||
| Nuclear Transport | Ran GTPase Solyc06g082120 → | ||
| Unknown Function | obg GTPase Solyc09g090460 ↑ | ||
| Exocytosis General | Rab3 GTPase Solyc07g064740 → Rab3 GTPase Solyc07g062450 → |
| Function | GTPases | SNAREs | SNARE Regulators |
|---|---|---|---|
| Vesicle Budding | Arf1 GTPase Solyc10g084690 → Arf3 GTPase Solyc08g076920 → Dynamin2 GTPase Solyc01g095970 → Dynamin2 GTPase Solyc05g050600 → Dynamin GTPase Solyc10g062160 → Dynamin2A GTPase Solyc11g039650 → Arf GTPase Solyc03g006610 → Arf-GAP Solyc10g006850 ↑ | Matchmakers NSF Solyc01g100170 → AAA-type ATPase Solyc06g071990 ↓ AAA-type ATPase Solyc02g087540 ↓ AAA-type ATPase Solyc10g007280 ↓ Munc13/UNC-13C Solyc01g107740 → Sec1-4/Munc (SM) Solyc08g079520 ↑ NSF Solyc06g050770 ↑ Synaptotagmin Solyc03g119440 → Synaptotagmin Solyc09g007860 ↑ Synaptotagmin 7 Solyc09g007860 → | |
| ER→cis Golgi | RabB1c GTPase Solyc12g011130 → RabB1a GTPase Solyc02g093530 → RabD2a GTPase Solyc05g053940 ↑ Sar1 GTPase Solyc01g060130 ↑ RabB GTPase Solyc07g056150 → RabB1b GTPase Solyc10g007700 ↑ | Bos1—v-SNARE, Qb-SNARE Solyc07g047720 → Bet12—t-SNARE, Qc-SNARE Solyc06g008520 → SYP71-t- SNARE Syntaxin- Qc-SNARE Solyc10g084210 → BET11—t-SNARE, Qc-SNARE Solyc09g089630 ↑ | |
| Golgi Apparatus, Trans Golgi Network (TGN) | Bet1—MEMB11 t-SNARE, Qc- SNARE Solyc04g074940 → SNARE 11—SFT11 t-SNARE, Qb-SNARE Solyc08g077550 → SNARE41b trans membrane Solyc06g065400 ↑ SYP31—Syntaxin 32 t-SNARE- Qa SNARE Solyc07g054140 → GOS12 -v-SNARE-C-Qb-SNAR Solyc01g091020 → Solyc01g091150 → Solyc01g102530 → SYP32—t-SNARE Syntaxin 32- Qa-SNARE Solyc08g067910 → SYP 22-t-SNARE Syntaxin, Qa-SNARE Solyc08g076540 ↓ | ||
| TGN→PM | SNAP33—t-SNARE, Qb+c-SNARE Solyc06g069570 → PEN1—(SYP121/SYR1) Syntaxin t-SNARE—Qa SNARE Solyc01g006950 → | Matchbreakers Tomosyn Solyc06g051390 → | |
| Golgi→Vacuole | RabG3A GTPase Solyc03g120750 ↑ | SYP52—t- SNARE Syntaxin-51, Qc-SNARE Solyc10g081060 → | |
| Cytoskeleton and Vesicular Trafficking | Ras GTPase, Rho Solyc04g076140 ↑ Ras GTPase, Rho Solyc01g111560 → Ras GTPase, Rho Solyc02g083580 ↑ | ||
| Nuclear Transport | Ran GTPase Solyc06g082120 → | ||
| Unknown Function | obg GTPase Solyc09g090460 → | ||
| Exocytosis General | Rab3 GTPase Solyc07g064740 ↑ Rab3 GTPase Solyc07g062450 ↑ |
| Function | GTPases | SNAREs | SNARE Regulators |
|---|---|---|---|
| Vesicle Budding | Arf1 GTPase Solyc10g084690 ↑ Arf3 GTPase Solyc08g076920 ↑ Dynamin2 GTPase Solyc01g095970 ↑ Dynamin2 GTPase Solyc05g050600 → Dynamin GTPase Solyc10g062160 ↑ Dynamin2A GTPase Solyc11g039650 ↑ Arf GTPase Solyc03g006610 ↑ Arf-GAP Solyc10g006850 ↑ | Matchmakers NSF Solyc01g100170 ↑ AAA-type ATPase Solyc06g071990 ↓ AAA-type ATPase Solyc10g007280 ↓ Munc13/UNC-13C Solyc01g107740 ↑ Sec1-4/Munc (SM) Solyc08g079520 ↑ NSF Solyc06g050770 ↓ Synaptotagmin Solyc03g119440 → Synaptotagmin Solyc09g007860 → Synaptotagmin 7 Solyc09g007860 ↑ | |
| ER→cis Golgi | RabB1c GTPase Solyc12g011130 → RabB1a GTPase Solyc02g093530 ↑ RabD2a GTPase Solyc05g053940 ↓ Sar1 GTPase Solyc01g060130 ↑ RabB GTPase Solyc07g056150 ↑ RabB1b GTPase Solyc10g007700 → | Bos1—v-SNARE, Qb-SNARE Solyc07g047720 → SYP71-t- SNARE Syntaxin- Qc-SNARE Solyc10g084210 ↑ BET11—t-SNARE, Qc-SNARE Solyc09g089630 → | |
| Golgi Apparatus, Trans Golgi Network (TGN) | Bet1—MEMB11* t-SNARE, Qc- SNARE Solyc04g074940 ↑ SNARE 11—SFT11* t-SNARE, Qb-SNARE Solyc08g077550 ↑ SNARE41b trans membrane Solyc06g065400 ↑ SYP31—Syntaxin 32 t-SNARE- Qa SNARE Solyc07g054140 → GOS12-v-SNARE-C-Qb-SNAR Solyc01g091020 → Solyc01g091150 → Solyc01g102530 ↓ SYP32—t-SNARE Syntaxin 32- Qa-SNARE Solyc08g067910 → SYP22-t-SNARE Syntaxin, Qa-SNARE Solyc08g076540 → | ||
| TGN→PM | SNAP33—t-SNARE, Qb+c-SNARE Solyc06g069570 ↑ PEN1—(SYP121/SYR1) Syntaxin t-SNARE-Qa SNARE Solyc01g006950 → NPSN12—t-SNARE Qb-SNARE Solyc12g098950 ↑ | Matchbreakers Tomosyn Solyc06g051390 ↑ | |
| Golgi→Vacuole | RabG3A GTPase Solyc03g120750 → | SYP52—t-SNARE Syntaxin-51, Qc-SNARE Solyc10g081060 → | |
| Cytoskeleton and Vesicular Trafficking | Ras GTPase, Rho Solyc04g076140 ↑ Ras GTPase, Rho Solyc01g111560 ↑ Ras GTPase, Rho Solyc02g083580 ↑ | ||
| Nuclear Transport | Ran GTPase Solyc06g082120 ↑ | ||
| Unknown Function | obg GTPase Solyc09g090460 ↓ | ||
| Exocytosis General | Rab3 GTPase Solyc07g064740 → Rab3 GTPase Solyc07g062450 ↑ | +3 new GTPases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaresan, S.; Philosoph-Hadas, S.; Riov, J.; Salim, S.; Meir, S. Expression Kinetics of Regulatory Genes Involved in the Vesicle Trafficking Processes Operating in Tomato Flower Abscission Zone Cells during Pedicel Abscission. Life 2020, 10, 273. https://doi.org/10.3390/life10110273
Sundaresan S, Philosoph-Hadas S, Riov J, Salim S, Meir S. Expression Kinetics of Regulatory Genes Involved in the Vesicle Trafficking Processes Operating in Tomato Flower Abscission Zone Cells during Pedicel Abscission. Life. 2020; 10(11):273. https://doi.org/10.3390/life10110273
Chicago/Turabian StyleSundaresan, Srivignesh, Sonia Philosoph-Hadas, Joseph Riov, Shoshana Salim, and Shimon Meir. 2020. "Expression Kinetics of Regulatory Genes Involved in the Vesicle Trafficking Processes Operating in Tomato Flower Abscission Zone Cells during Pedicel Abscission" Life 10, no. 11: 273. https://doi.org/10.3390/life10110273
APA StyleSundaresan, S., Philosoph-Hadas, S., Riov, J., Salim, S., & Meir, S. (2020). Expression Kinetics of Regulatory Genes Involved in the Vesicle Trafficking Processes Operating in Tomato Flower Abscission Zone Cells during Pedicel Abscission. Life, 10(11), 273. https://doi.org/10.3390/life10110273






