1. Introduction
Limb loss profoundly impacts autonomy, employability, and mental health. This disability severely impairs human mobility and physical function, imposing a significant global health economic burden. Despite decades of research on robotic prosthetic hands (RPHs) leading to breakthroughs in robotics, a relevant paradox persists: most amputees still rely on technologies that have barely evolved in half a century. This apparent disparity is less surprising when it is considered the difficulty of replicating the human hand. The hand holds a large sensory representation in the brain, and its grasping function is the most sophisticated coordination task. Additionally, the limb, which possesses both the highest density of mechanoreceptors and the most Degrees of Freedom (DoF), enables humans to both perceive and manipulate [
1]. Over the past two decades, engineering and clinical research have converged to create neuromuscular robotic prostheses—artificial limbs that sense the user’s residual neuromuscular activity, translate it into multi-degree-of-freedom (DoF) motion, and increasingly return haptic or proprioceptive feedback.
1.1. Rationale and Scope of This Review
Previous surveys have treated myoelectric control, hybrid interfaces, or specific actuation technologies separately. The present review adopts an interdisciplinary perspective, integrating recent advances in sensor technologies (sEMG and nerve cuffs); signal processing; actuation and human–machine interfaces (targeted muscle reinnervation, osseo-integrated gateways, brain–computer interfaces). By providing both foundational concepts and state-of-the-art insights, this review aims to present a comprehensive understanding of the current state of neuromuscular robotic prostheses and their potential for future development, thereby maximizing their real-world impact.
While several prior reviews have surveyed individual subdomains, for example, the mechanical design and kinematic performance of upper-limb prostheses, the electrophysiology and acquisition methods for myoelectric control, or machine-learning approaches to signal decoding, relatively few works synthesize these topics in a single review. This manuscript fills that gap by integrating hardware design, neuromuscular and implanted sensing modalities, control, and machine-learning architectures for real-time decoding and surgical/clinical adjuncts (e.g., TMR and osseointegration) that affect device integration.
The research question of this paper is: which hardware and algorithmic bottlenecks, across sensing, actuation, and control, currently limit neuromuscular robotic prostheses, and which integrated solutions show the most promise to mitigate those bottlenecks?
As a working hypothesis, it is proposed that the greatest near-term gains will come from co-designed solutions that trade off sensing modality stability, control complexity, and actuator energy density, rather than incremental improvements confined to a single subsystem.
1.2. Socio-Economic Relevance
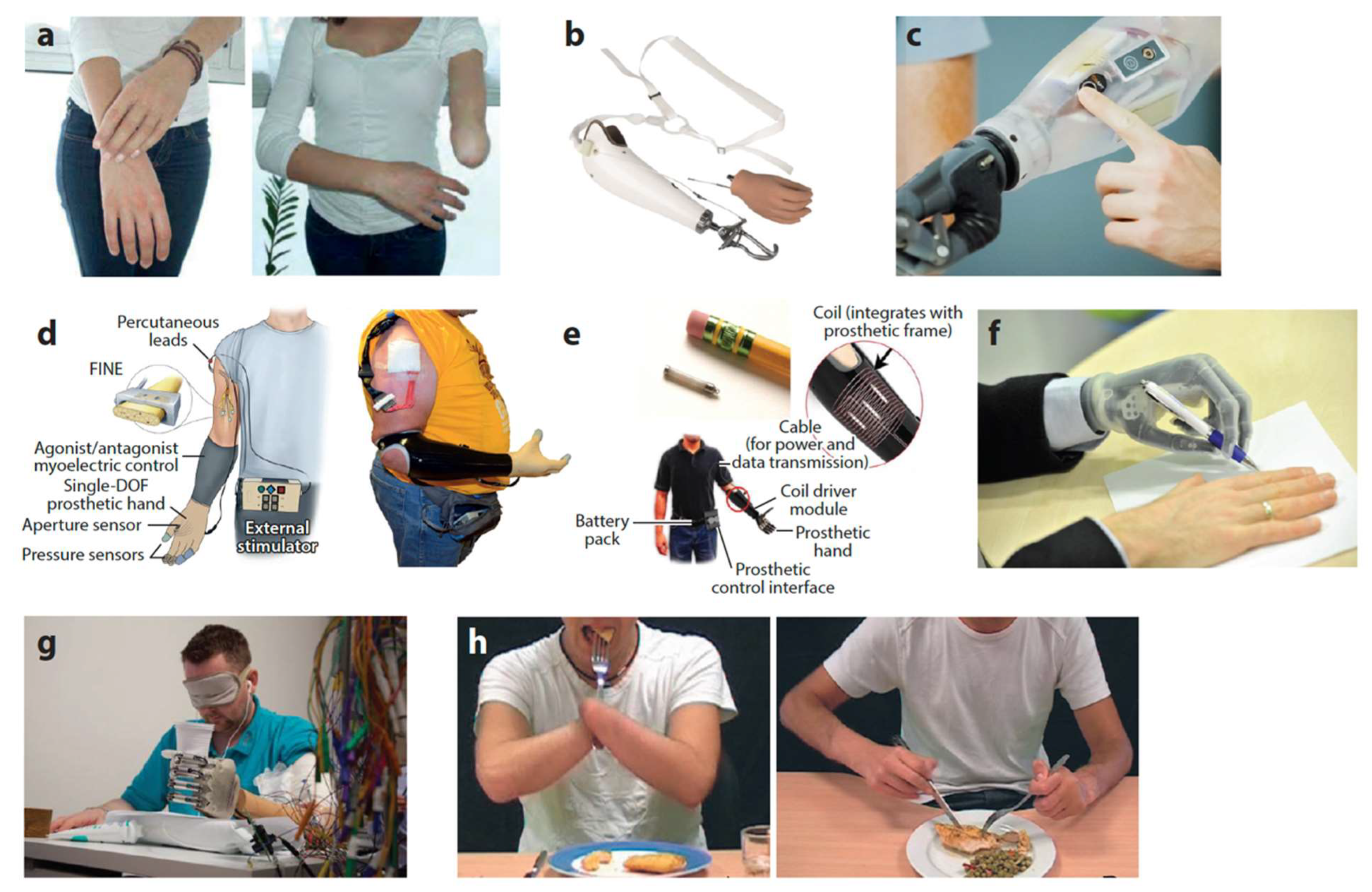
Multifunctional robotic hands (e.g., i-Limb Quantum, bebionic, Michelangelo) have been increasingly adopted worldwide since their introduction. Nevertheless, factors such as cost, weight, battery life, and limited robustness continue to constrain their use, particularly in low-resource settings (
Figure 1) [
1]. Some clinical trials have shown that when myoelectric prostheses are coupled with pattern-recognition control, users report significant gains in satisfaction measures, with a clear preference for pattern recognition control over conventional direct control during home use [
2]. The humanitarian and economic incentive for further innovation in this field is therefore clear.
1.3. Clarification of Terminology
In the context of this review, several terms are used to describe different yet interconnected aspects of assistive technologies for limb replacement. The term “robotic prosthetics” is a general term sometimes used to describe robotic technologies applied to prosthetic limbs, regardless of the control method. When associated with the term “neuromuscular,” it refers specifically to robotic prosthetic systems that are controlled via neuromuscular signals, typically captured through electromyography. These systems integrate sensors, actuators, and intelligent algorithms to interpret user intention from muscle activity. The expression “neuromuscular interfaces” designates the interface layer between the human neuromuscular system and the prosthesis, encompassing signal acquisition methods, signal processing algorithms, and the pathways used to relay control commands and feedback. Finally, “robotic prosthetic devices” refers to the physical electromechanical constructs of the prosthetic limbs themselves, including structural components, actuators, and embedded electronics, independent of the control strategy.
Although the term “neuromuscular prostheses” may suggest applications across all limb types, this review focuses specifically on upper limb prostheses. This is because the biomechanical and functional complexity of the human hand necessitates a far more sophisticated engineering approach than that required for lower-limb prostheses. While lower-limb prostheses primarily address simple tasks (locomotion and weight-bearing), upper-limb devices must replicate fine motor control, multi-degree-of-freedom movements, and intuitive interaction. Consequently, advanced neuromuscular technologies are almost exclusively developed and implemented in upper-limb prostheses.
2. Literature-Search Strategy
This review on neuromuscular robotic prostheses was conducted as a narrative state-of-the-art analysis. The methodology comprised a literature search of relevant publications to elucidate the anatomical, physiological, and technological foundations underpinning advanced prostheses. The review primarily focused on the following:
Literature Search Strategy: A detailed search was performed across scientific databases like PubMed, IEEE Xplore, Scopus, ScienceDirect, and SpringerLink using the following terms: “neuromuscular prostheses”, “robotic prostheses”, “surface electromyography”, “myoelectric control”, “biomechanical design”, and “sensor integration”. The literature search encompassed publications from January 2000 to July 2025 to ensure an up-to-date perspective.
Selection Criteria: Publications were selected based on their relevance to the current challenges and technological trends. Emphasis was placed on studies addressing the integration of sensor technologies, the underlying anatomophysiological principles, and innovative design approaches. Both experimental studies and review articles were considered, provided they contributed critical insights into the field.
Data Extraction and Synthesis: Key data such as methodological approaches, technological innovations, and challenges in the design and control of neuromuscular prostheses were extracted from the selected articles. The extracted information was organized into thematic sections reflecting the major aspects of the state-of-the-art discussion, such as anatomical fundamentals, physiological mechanisms, signal processing techniques, and emerging actuator technologies.
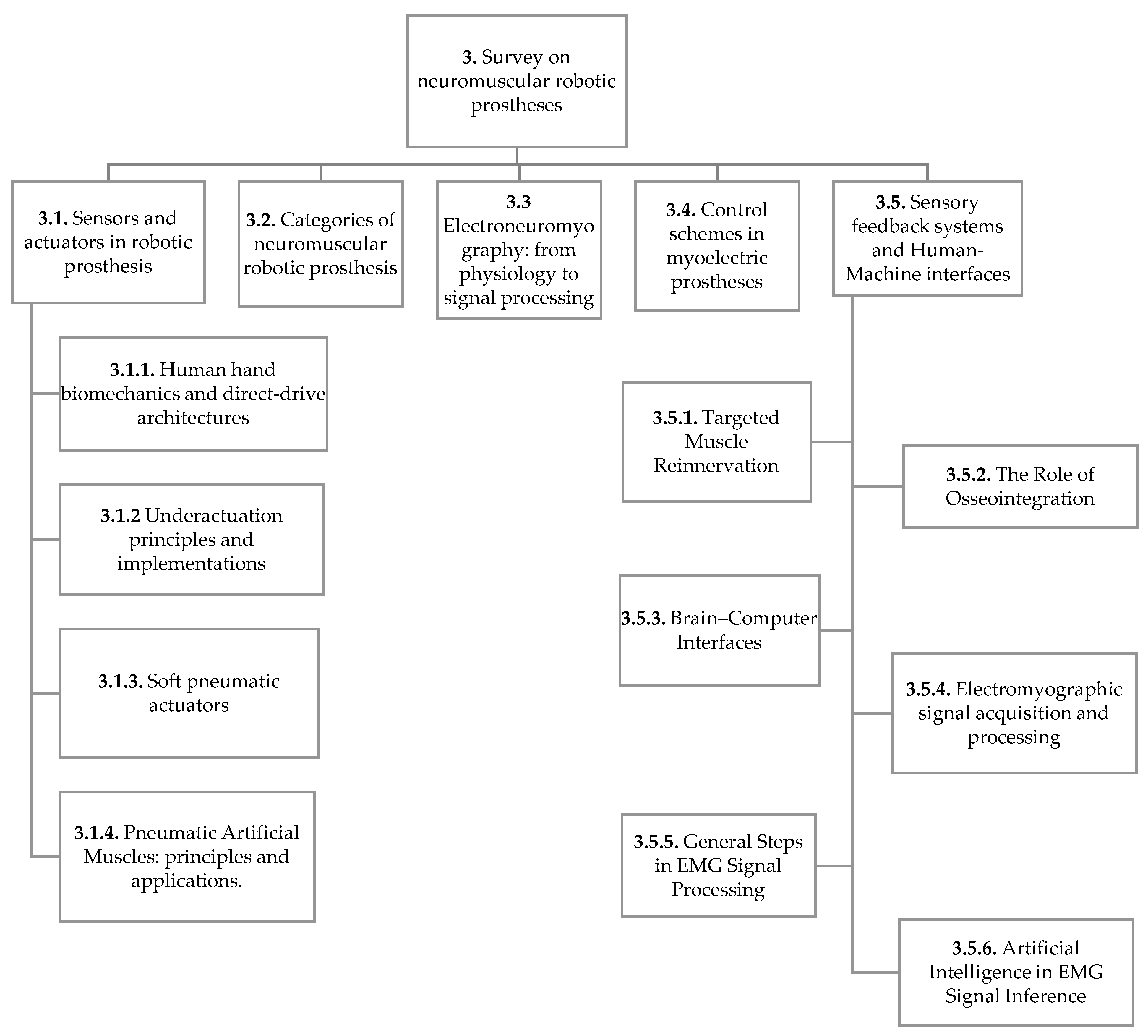
This structured approach enabled a thorough examination of scientific literature, forming the basis for the subsequent discussion on current trends and future directions in the development of robotic prosthetic devices. Given the layered structure of the following section, a flowchart is provided in
Figure 2 to summarize the hierarchical organization of the content of the manuscript.
3. Survey on Neuromuscular Robotic Prostheses
This section presents a detailed synthesis of the key findings derived from the state-of-the-art analysis of neuromuscular robotic prostheses. The results are organized into thematic subsections that capture the critical technological advancements, their interpretation, and the conclusions drawn from the literature review. In practice, neuromuscular prostheses are primarily developed for the upper limb, where precision, dexterity, and multifunctional control are essential. In contrast, lower-limb prostheses typically rely on simpler control schemes focused on gait stabilization.
3.1. Sensors and Actuators in Robotic Prostheses
3.1.1. Human Hand Biomechanics and Direct-Drive Architectures
Recreating the biomechanics of a human hand is a difficult challenge. Prosthetic hands rely on various actuators and actuation methods to mimic the movement and configurations of human hands. The various possible movements of the fingers allow for a range of configurations or patterns, enabling adaptation to the objects being grasped. Although the possible postures are infinite, some are regularly used in daily life activities. Hand configurations for grasping objects can be categorized as grips or pinches. The main pinches are referred to as digit-to-digit pinches, which involve two fingers, with the thumb opposing the index or any other finger and can be further divided into force pinches and precision pinches. There are also tridigital pinches used for writing. Grips, on the other hand, are a method of grasping objects, more robust compared to pinches, since they allow bigger contact areas and the use of all fingers. A detailed description of grasp patterns is given by [
3].
Early prototypes sought to replicate the skeletal structure of the fingers, but only recently has it become feasible to effectively implement biomimetic actuation using muscle-like actuators. One major challenge is integrating actuators, sensors, and electronic components into a prosthesis that maintains the dimensions and weight of a natural hand. Significant systems integration and miniaturization are essential before such devices can be effectively employed by amputee patients. Concerning sensory feedback, one current objective is to simulate the mechanoreceptors found in human skin, each sensitive to different stimuli and responsible for distinct sensory functions.
Actuators are the components responsible for generating motion in fingers and applying force according to the kinematics of articulating parts. Often, numerous joints will be coupled to form a finger. Knowing the actuator position, the position of all joints in that finger can be determined. Therefore, actuator position is a parameter or a degree of freedom (DOF) that controls a set of configurations [
4]. In this case, there is a direct drive, where each finger or even a joint has its own actuator, allowing independent movement and high dexterity. The most common configuration of prosthetic hands has six DOFs. One for each finger (flexion/extension), and the thumb usually has two DOFs, using two independent actuators, one to control flexion/extension and another to control circumduction. The circumduction rotation along the circumduction axis is required to alternate between a lateral grasp and a power or precision grasp [
5].
3.1.2. Underactuation Principles and Implementations
The underactuation method uses a single actuator to control several independent degrees of freedom [
6]. In this sense, the single actuator parameter cannot be used to describe the position of the joints since they are dependent on the contact state of each finger link with the object. These mechanisms use differential systems since they allow the other fingers to continue their movement when one of the fingers is stopped by the grasping object.
Underactuation is a widely adopted strategy to simplify the mechanical design of prosthetic devices while preserving functional dexterity. An underactuated system is characterized by having fewer actuation degrees (DOA—degree of actuation) than DOFs. In passive DOFs (non-actuated), the system exploits the capacity to adapt to the contacting surface, enabling a self-adjusting grip without controlling each joint individually. Such systems reduce the number of required motors, thereby simplifying complexity, weight, and cost [
6]. Several commercial robotic prosthetic solutions employ underactuated mechanisms. These include the Michelangelo prosthesis (Ottobock, Germany), the i-Limb Ultra (Össur, Iceland), the bebionic (Ottobock), and the VINCENTevolution 3 (Vincent Systems, Germany) [
1].
3.1.3. Soft Materials, 3D Printing, and High-Resolution Tactile Sensing
The most common actuator used in prostheses today is a direct current (DC) motor. These motors are small and lightweight and can be packaged in the hand or forearm. To reduce the speed and increase the limited torque from these devices, gearing, lead screws, and even harmonic drives may be used. Cables are also usually used to transmit force from actuators to fingers, enabling compact designs [
7]. Among recent solutions are the soft pneumatic actuators (SPAs), which consist of rubbery structures composed of one or more interconnected and physically constrained chambers that, when pressurized pneumatically or hydraulically, perform flexural or torsional movements [
8].
Currently, various research groups are developing innovative solutions such as 3D printing with biomaterials. These soft materials and flexible tactile sensors can replicate the mechanoreceptor responses that take place in human skin, offering enhanced sensory feedback. Some researchers have demonstrated a fully 3D-printed biomimetic skin where interdigitated capacitive electrodes and piezoelectric structures are co-printed within elastomeric layers (achieving both static and dynamic tactile sensing) and interfaced with a myoelectric band for closed-loop force feedback in prosthetic hands [
9,
10]. There are also examples of hybrid hands with a rigid endoskeleton and soft robotic joints equipped with independent neuromorphic tactile sensing layers. Tactile sensor designs can be categorized as capacitive, resistive, piezoresistive, piezoelectric, triboelectric, and optical. Flexible tactile sensors based on triboelectric, piezoelectric, and capacitive mechanisms using nanomaterials achieve good spatial resolutions and low detection thresholds for high-fidelity human-scale touch reproduction [
11]. Closed-loop prosthetic systems integrate capacitive tactile sensing with feedback actuation for grip force regulation, enabling naturalistic sensory experiences through dynamic modulation of applied force, as exemplified by wearable devices such as Tacsac [
12]. When combined with neuromorphic tactile sensors, in which spiking neural activity can be converted into targeted nerve stimulation, these systems may further enhance the naturalness of sensory experiences in the future.
Neuromorphic tactile sensing is a relatively recent technology that has demonstrated the ability of fine textures discrimination. For example, optical neuromorphic sensors encode tactile events as spike trains using spatial and temporal coding schemes, achieving robust classification across variations in contact force [
13]. Machine learning algorithms can process these patterns to modulate grasp force and respond adaptively. Such approaches support more naturalistic and responsive tactile feedback loops in prosthetic control systems.
A major barrier is the lack of standardized protocols for evaluating sensitivity, linearity, hysteresis, and longevity. Unified benchmarks are needed to compare disparate designs objectively. Durability remains critical, with cyclic loading and environmental factors degrading sensor performance. Open-source platforms like BioIn-Tacto could democratize sensor development, enhance reproducibility, and accelerate innovation [
14].
Bringing together the tactile perception capabilities outlined earlier with motor control is a crucial step in enabling their application to robotic functions such as grasping and object manipulation. By providing tactile feedback with both high sensitivity and rapid response, neuromorphic tactile sensors make it possible for robots to modulate grip force dynamically [
13]. Most neuroprosthetic devices currently encode neural stimulation patterns as artificial pulse waveforms. While effective, such non-biomimetic signals often produce perceptual experiences that feel unnatural to the user. Neuromorphic tactile systems offer a significant shift in approach by delivering sensory feedback through naturally occurring spike trains, providing a more faithful, biomimetic representation of touch. This capability enables prosthetic hands equipped with neuromorphic sensors to reinstate neuromuscular reflexes and markedly enhance performance in fine-motor tasks. For example, integrating these sensors has been shown to restore reflexive control in amputees, improving precision grasping and delicate manipulation [
13].
By using biomimetic signal encoding to stimulate either the user’s peripheral nerves or the somatosensory cortex, neuromorphic systems have the potential to reproduce complex somatosensory experiences. These capabilities can also improve grasp stability and coordinated motor control [
13]. Furthermore, innovations involving real-time neural decoding and adaptive feedback frameworks, powered by advanced deep learning models, will continue to drive and accelerate these advances in the years to come.
3.1.4. Soft Pneumatic Actuators
Recent investigations have shown that soft manipulators can achieve performance levels comparable to those of rigid systems in various applications. Research in this area addresses not only the materials involved but also the mechanical structure and integrated sensory capabilities of prosthetic devices, particularly for applications requiring delicate object manipulation. Flexible sensors and soft robotic components have contributed to safer and more effective human–prosthesis interaction, thereby improving usability and mitigating injury risks. However, further research is needed to evaluate whether soft architecture represents a viable solution for more widespread use.
The mechanical characterization of thermoplastic polyurethane, a flexible polymer widely used in soft robotics, reveals that it is suitable for the design and manufacturing of industrial and agricultural soft grippers [
15]. The Fin Ray effect is a biomimetic structural principle in which a flexible structure with angled internal cross-beams bends toward an applied force, allowing it to wrap around and adapt to the shape of an object for stable grasping. A soft robotic gripper inspired by this effect demonstrates improved mechanical performance, adaptability, and reduced structural failures under testing conditions [
16]. Further computational optimization of a Fin Ray effect soft robotic finger achieves a better balance between gripping force and flexibility [
17]. In parallel, advances in low-dimensional carbon-based flexible sensors have enabled their integration into wearable networks for environmental and health monitoring, with porous carbonaceous materials providing enhanced sensitivity and durability for human–machine interfaces [
18].
Emerging designs include prosthetic hands controlled by magnetically actuated muscles implanted in the forearm, providing intuitive and near-natural motion through thought-based commands. These designs represent a shift toward reducing external hardware dependence while improving user satisfaction and control fidelity. Innovative actuation systems, such as McKibben pneumatic artificial muscles (PAMs), offer the advantage of integrating structure with sensing, thereby enhancing safety and efficiency. McKibben PAMs have emerged as a promising alternative for actuation in prostheses, offering a unique blend of advantages such as lightweight design, flexibility, substantial force generation, and a remarkable similarity to the structure and function of biological muscles [
19,
20].
3.1.5. Pneumatic Artificial Muscles: Principles and Applications
PAMs are pneumatic actuators inspired by biological muscles. They consist of an elastomeric tube surrounded by a braided mesh of inextensible fibers (such as Kevlar or nylon) [
19]. When the tube is inflated with compressed air, it expands radially and contracts axially, generating force and movement analogous to human muscle contraction. The force generated is directly proportional to the air pressure and the muscle’s cross-sectional area, allowing for a wide range of actuation (from delicate movements to the generation of significant force). These features make PAMs ideal for a variety of applications in robotic prostheses. For upper limbs, they can be used to actuate fingers, hands, and arms, enabling grasping, object manipulation, and the performance of daily tasks with dexterity and natural movement. The integration of pressure sensors and tactile feedback can further enhance the precision and sensitivity of these prostheses. In lower limbs, PAMs can simulate the action of leg, ankle, and foot muscles, offering a more natural and efficient gait adaptable to various terrains and activities. Originally created for prosthetic limbs in the 1950s and 60s, the McKibben pneumatic artificial muscle has recently been commercialized and re-engineered for use in robotics and skeletal models. It is essentially a tube with a braided mesh shell. When air pressure is applied to the inside, the tube shortens and creates tension, acting like a spring. This design provides several key advantages: it is flexible, lightweight, and has a unique spring-like behavior with variable stiffness [
20].
The use of robots for rehabilitation is also gaining attention, with the use of multi-joint robotic arms in physical therapy programs, as these are more effective than single-joint machines for recreating natural human movement. A key concern for such robots is safety, which is addressed by using PAMs that are inherently compliant due to their use of compressed air [
21]. Their ability to generate force smoothly and adaptively makes them ideal for amplifying muscle strength and reducing fatigue. In pursuit of heightened human-like dexterity, researchers are employing a highly biomimetic design methodology to replicate the intricate manipulation capabilities of the human hand within robotic systems [
22].
Pneumatic muscle systems are distinguished from alternative actuators, such as electric motors or hydraulic systems, by their exceptionally high power-to-weight and power-to-volume ratios. These characteristics make them highly suitable for the design of human-centric exoskeleton systems intended to provide strength and mobility assistance [
23]. Despite these undeniable advantages, PAMs still face challenges for wide-spread application in robotic prostheses. Their inherently nonlinear dynamics require sophisticated control strategies, such as gain-scheduled or feedback-linearization controllers, to achieve accurate force and movement regulation [
23]. Additionally, the materials used in PAMs may experience wear and fatigue, particularly in applications demanding high frequency and intensity of actuation. Therefore, advances in material science are expected to have a significant impact on their durability and performance.
Ongoing research into new materials and manufacturing techniques may enhance durability and reduce maintenance needs. Moreover, integrating PAMs with EMG or neural signals could revolutionize the market for robotic prostheses, making them more intuitive and responsive to user commands. Recent efforts in prosthetic technologies focus on systems with compliant actuation and flexible architectures, enabling devices to deliver more natural and precise movements while remaining adaptable and accessible to a broad range of users.
Current actuation strategies in neuromuscular prostheses encompass a wide range of technologies, from conventional DC motors with gear reductions to pneumatic artificial muscles and soft actuators. Compact DC motors are often reported to provide relatively high fingertip forces and fast response, but this generally comes at the cost of weight and limited intrinsic compliance. Pneumatic artificial muscles, by contrast, tend to offer favorable specific power and biologically realistic compliance, though they are typically constrained by the need for external compressors and by reduced efficiency. Soft robotic actuators are frequently highlighted for their safety in human–robot interaction, but their force output and durability remain limited. Rather than enabling strict comparison, these examples illustrate the diversity of engineering trade-offs across actuation strategies.
A major hurdle for clinical use is the long-term reliability of these systems. Daily prosthesis uses subjects’ actuators to extensive cyclic loading, yet durability under clinically realistic conditions remains underexplored. Pneumatic artificial muscles are often reported to fail through bladder fatigue, while soft actuators are prone to material degradation. In contrast, geared DC motor drives tend to demonstrate greater longevity, although typically at the expense of increased wear in transmission components. Overall, durability assessments remain inconsistent across the literature, in part due to the lack of standardized testing protocols. This gap highlights the need for systematic evaluation under clinically realistic conditions before many emerging actuators can be considered viable for long-term clinical deployment.
3.2. Categories of Neuromuscular Robotic Prosthesis
Prosthetic devices represent an essential solution for restoring lost functionality in amputee patients. One of their primary contributions is the significant improvement in the quality of life. The diversity of these devices allows them to be classified based on multiple criteria, including the desired functionality, the activation mechanism, and the control method used.
In terms of functionality, prosthetic devices are divided into two main categories: passive and active prostheses. Passive prostheses are primarily designed to restore the physical appearance of the limb without providing active functional capabilities [
1]. They are valued mainly for their esthetic appeal, which helps the user achieve a more integrated and harmonious body image. The design of these devices focuses on visual appearance, comfort during use, and minimal weight. Their applicability spans all levels of amputation, as their purpose is restricted to enhancing visual appeal and psychological comfort.
Passive prostheses can be further divided into esthetic and functional types. Esthetic prostheses aim exclusively at the visual replacement of the missing limb, striving to achieve a high degree of realism. In contrast, functional prostheses are designed to perform specific tasks, adapting their appearance according to their intended function. Additionally, passive prostheses can be classified into two subcategories: static and adjustable. Static prostheses offer no movement, whereas adjustable prostheses incorporate mechanisms that allow adaptation to the user’s specific needs [
24].
Active prostheses, on the other hand, are defined by their ability to execute movements through internal forces, either by using mechanical energy generated by the user’s body or by actuators powered by external energy sources. These medical devices are recognized for providing mobility to the amputated limb and are categorized based on their activation system. Body-powered activation operates via a mechanical interface—such as cables attached to the patient’s shoulder—allowing specific movements to be translated into actions by the prosthesis. An example of this mechanism is the ability to open or close a prosthetic claw through arm extension, offering a simple and intuitive control interface [
24].
For electrically activated prostheses, control methods may include external button control, myoelectric control, or hybrid systems [
24]. Button-controlled prostheses require an external power source to drive the motors for articulated movements, allowing the user greater freedom by enabling individual movement control through buttons or pressure sensors. Sensor technology plays a crucial role in the accessibility and performance of myoelectric control systems. Affordable EMG electrodes are crucial in resource-constrained environments [
25]. Studies that focus on human–machine interfaces for upper-limb prostheses reveal a growing trend toward integrating various control strategies, including EMG-based, hybrid, and advanced signal processing approaches, to enhance user comfort, device precision, and overall usability [
26].
Feedback related to command execution is crucial for prosthetic device users, as it facilitates interaction with the device. This feedback can take several forms, vibrational, tactile, electrical, or somatosensory, each providing a distinct method to simulate the sensation of touch or interaction with the environment. In vibrational feedback, sensors incorporated into the prosthesis extremities activate vibratory devices on the user’s skin upon contact with an object, providing an indirect perception of touch that significantly enhances functionality and control in myoelectric prostheses, where capturing electromyographic signals is critical. Alternatively, tactile feedback applies a slight pressure on the user’s skin instead of vibration, simulating a more direct physical sensation of touch [
27].
For electrical feedback, low-intensity current pulses are used to simulate the sensation of touch when applied to the skin. Despite its potential to enhance prosthetic capabilities, electrical feedback may interfere with the acquisition of myoelectric signals. Finally, somatosensory feedback involves the direct application of electrical stimuli to the nervous system to replicate the natural sensation of force and proprioception derived from sensors. Although this approach is at the forefront of research, its implementation faces significant challenges, such as the need for precise neural mapping to effectively and selectively activate the appropriate areas [
27].
Each feedback method possesses unique characteristics and is at varying stages of development and research. Nonetheless, a shared objective across all approaches is the enhancement of prosthetic functionality and user experience, fostering greater integration and more natural interaction with the surrounding environment.
3.3. Electroneuromyography: From Physiology to Signal Processing
Locomotion is a fundamental human characteristic, largely driven by muscles acting as biological motors that generate force upon contraction. Electromyography uses electrodes to evaluate the electrical activity of nerves and muscles [
28]. Originally applied for diagnosing neuromuscular pathologies—from congenital myopathies to adult-onset disorders—EMG has been crucial since the early 20th-century cellular studies of muscle physiology. Two main approaches exist: classical neurological EMG (where electrically stimulated responses are measured under static conditions) and kinesiological EMG [
28,
29]. The kinesiological electromyography focuses on voluntary muscle activation during functional tasks, making it valuable in clinical assessment, rehabilitation, sports training, and in the development of robotic prostheses.
During muscle contraction, electrical signals are produced by the sequential activation of many muscle fibers. These signals are complex and chaotic due to the superposition of multiple action potentials and are captured using multi-channel amplifiers, data acquisition systems, and specialized processing software [
28,
29]. The amplitude and frequency of the EMG signal increase with muscle force, and the basic functional unit, the motor unit (comprising a motor neuron and its innervated fibers), ensures that all fibers contract synchronously. With a broad frequency range and amplitudes, EMG signals offer distinct advantages over other bio-signals such as ECG or EEG [
28]. Key signal characteristics include amplitude (the potential difference between positive and negative peaks), phase, rise time, and overall duration. These features are essential for understanding motor unit action potentials (MUAPs), which represent the summation of individual fiber action potentials. Analyzing MUAPs provides insights into motor unit recruitment—both spatially (adding more motor units) and temporally (increasing firing rates)—and helps diagnose neuropathic or myopathic conditions [
28].
EMG signals can be obtained invasively or noninvasively (
Table 1). Invasive techniques, such as needle electrodes, provide localized, high-resolution recordings but are more uncomfortable for patients. In contrast, sEMG uses electrodes placed on the skin to capture the aggregated activity of numerous motor units, making it especially useful for movement analysis and control. The quality of sEMG recordings depends critically on electrode placement and skin preparation. Guidelines like those from the SENIAM project help standardize electrode dimensions and inter-electrode distances to ensure reliable and comparable results. Proper skin cleaning (e.g., with alcohol) minimizes artifacts, and using multiple sensors can improve the decoding of movement intentions [
30].
The core EMG signal is the result of electrical activity produced by skeletal muscles. This myoelectric data, stemming from motor neurons in the central nervous system, is interpreted through electrical readings [
31]. Implantable sensors receive power and commands from an external telemetry controller that activates a coil integrated into a sleeve housing the prosthesis. Each implant functions as an independent differential amplifier connected to two electrodes, one at each end, to detect the muscle contraction signal. The primary drawback of these implantable myoelectric sensors (IMES) is their requirement for surgical implantation along with prolonged rehabilitation and training, which delays rapid results. Needle electrodes form another category, ideal for detecting changes in motor unit size and internal structure and for recording spontaneous fibrillation potentials. Although widely used, needle electrodes are perceived as more painful and yield less favorable results during forceful contractions. They are classified as monopolar, bipolar, or concentric. Monopolar electrodes tend to be cheaper and less painful, but they are electrically less stable and require a separate surface reference, unlike concentric designs [
31,
32,
33,
34].
Neural interfaces remain a long-standing challenge in achieving effective communication between prosthetic devices and the nervous system. Various approaches have been developed to both record and stimulate nerve fiber activity, with the primary differences being the degree of invasiveness and selectivity. A balance must be struck between increased invasiveness for higher selectivity and reduced invasiveness to minimize tissue damage and ensure chronic stability [
35]. For viable restoration of motor and sensory functions, interfaces must provide both topographical and functional selectivity, which is aided by understanding the fascicular organization of peripheral nerves. Current invasive neural interfaces are divided into extraneural (e.g., cuff electrodes that wrap around nerves) and intraneural (intrafascicular) types. Cuff electrodes, while useful for activating or recording from groups of nerve fibers, offer reduced selectivity since they engage superficial, myelinated fibers. Innovative cuff designs may improve this, but often require multiple cuffs to achieve adequate selectivity, thereby increasing implantation risks [
35].
Implantable flexible nerve electrodes (IFNEs) have been applied in neurological disorders for recording and stimulating nerve signals. However, mechanical mismatches between rigid electrodes and soft tissues can lead to chronic inflammation and perineural fibrosis. Advances in electrode design have also led to improved selectivity for targeting specific axon populations. For example, innovative structures such as spiral or flat interface nerve electrodes (FINE) have been developed. FINEs use a non-conductive cuff containing multiple conductive elements to reshape the nerve under controlled pressure without compromising blood flow, thus enhancing stimulation, or recording selectivity. Designs integrating embedded multiplexers further reduce the number of wires needed, lowering the risk of neurovascular damage while maintaining a flexible, conformal interface with the nerve. Intrafascicular electrodes, such as the Longitudinal Intrafascicular Electrode (LIFE) and the Transverse Intrafascicular Multichannel Electrode (TIME), are implanted directly within nerve fascicles, offering higher signal-to-noise ratios, lower stimulation thresholds, and superior selectivity. While LIFEs are widely used, TIMEs—capable of interacting with multiple fascicles—have demonstrated improved target muscle selectivity with lower stimulation currents [
35].
A recent innovation is the Neurotassel, an implantable neural probe developed in neuroscience to adapt effectively to brain tissue. It consists of a matrix of ultra-flexible microelectrodes with a high aspect ratio (i.e., very thin compared to their length). Notably, these microelectrodes self-organize into a fine fiber, causing minimal neuronal loss during chronic implantation and reducing inflammatory responses. Furthermore, the Neurotassel’s scalable design allows its matrix to comprise multiple micro-filaments, forming a fine fiber that serves as a powerful and minimally invasive tool for recording neural activity [
36].
In the broader technological context, flexible sensors have emerged as a significant innovation. By emulating human touch, these sensors enable intelligent systems to acquire detailed information on pressure, temperature, and texture. When integrated into wearable devices, they allow real-time monitoring of vital signs and physical activities, a crucial asset for healthcare, sports performance, and early disease detection. In robotics, flexible sensors enhance environmental awareness, enabling robots to handle delicate objects or navigate complex environments. Flexible sensors improve human–machine interfaces by providing intuitive tactile feedback that helps users control grip force and pressure precisely. In biomedical applications, they are used in implantable devices for continuous vital sign monitoring, although challenges remain regarding durability, sensitivity, and large-scale integration [
35,
36].
Non-invasive surface electrodes are typically made of conductive materials and are fixed to the skin. Gel electrodes are commonly used due to their lower impedance and noise, although they can dry out over time and may cause inflammatory reactions. In contrast, solid metal electrodes can be used over longer periods without signal degradation, though they are more expensive and may be more susceptible to movement artifacts. Electrodes may be passive (transmitting the signal for external amplification) or active (including built-in pre-amplifiers to reduce noise). Furthermore, EMG signals can be recorded in monopolar, bipolar, or multipolar configurations. Monopolar setups use a distant reference electrode and are suited for small muscles, while bipolar configurations capture differential signals from two nearby sites, reducing common noise. Accurate electrode placement requires anatomical knowledge since aspects such as amputation type or previous surgeries can affect the underlying anatomy and compromise pattern recognition [
30,
31].
3.4. Control Schemes in Myoelectric Prostheses
The ultimate goal in controlling robotic prostheses is to replicate the intuitive control of a natural hand, enabling uniform finger and wrist movements. Increasing degrees of freedom and achieving proportional control can enhance dexterity but may compromise the robustness of signal decoding [
24]. One promising solution is to integrate robotic automation and shared control strategies, wherein user commands are combined with automated adjustments for grip force and grasp patterns [
37,
38,
39].
Some studies highlight advancements in human-like robotic hands due to integration of tactile and force/torque sensors to enable stable grasping, dexterous manipulation and expressive gestures like playing musical instruments. Innovative designs range from adaptive actuation mechanisms and compliant low-cost servo hands to multimodal EMG-vision fusion for prosthetic control. These studies point out the urgent need for continued research and collaboration in developing advanced robotic and prosthetic hand technologies [
40,
41].
For transradial amputees, decoding peripheral nerve signals can enable highly accurate analysis of various grasp types. Advanced regenerative interfaces that reconnect severed nerves with muscle grafts act as bioamplifiers, enhancing both the specificity and long-term stability of the captured EMG signals. Some studies also research the promising benefits of new robust framework that integrates EMG and force myography (FMG) signals to enhance grasp type classification during dynamic arm position changes. Unlike EMG-only systems, the combined EMG-FMG approach demonstrates stable performance across varying window sizes, proving more robust than either signal alone. Overall, this framework has the potential to significantly enhance the robustness of natural grasp classification under dynamic conditions, offering a promising solution to bridge the gap between academic research and practical prosthetic applications [
42].
Neural electrodes are an emerging technology that aims to mimic natural sensory processing at both cortical and peripheral levels. The current focus is on evolving from basic feedback to the induction of more complex, natural sensations through direct nerve stimulation [
42,
43,
44,
45]. The integration of additional sensors is increasingly important in bidirectional prosthetic interfaces. For example, the Mia hand developed by Controzzi incorporates force sensors at the fingertips to measure normal and tangential forces. Myoelectric control systems have significantly advanced, focusing on restoring motor function in amputees and individuals with motor impairments, though each control modality presents its advantages and limitations [
24].
Real-life scenarios underline the importance of system robustness. For instance, the “hot coffee” problem illustrates that even a good laboratory success rate in grasp detection may not suffice when a slip could result in injury. Studies also indicate that a considerable proportion of both child and adult amputees abandon prostheses due to functional dissatisfaction. Control strategies for prostheses are generally classified into sequential and simultaneous approaches. Most upper-limb prostheses today use sequential control, which is further divided into pattern recognition (using classifiers to distinguish EMG signal patterns) and non-pattern recognition (predefined command sequences). While simultaneous control offers the potential for more natural multi-degree-of-freedom movement, it faces substantial challenges in integration and reliability [
37,
38].
A typical EMG control flow involves several key stages that enable effective signal processing and motor control (
Table 2). The process begins with data segmentation, where raw EMG signals are divided into manageable segments for analysis, allowing relevant information to be isolated for further processing. Next, Finite State Machine (FSM) control is employed, transitioning between different states (such as opening or closing a prosthetic hand) once predefined signal thresholds are reached. This ensures accurate and context-appropriate responses to muscle activity [
24].
Following this, pattern recognition algorithms identify specific movement patterns within the segmented data, allowing the system to interpret user intent and facilitate intuitive control over assistive devices. The system then utilizes regression control, mapping signal amplitude continuously to motor output. This enables proportional control, where motor responses are directly correlated with the intensity of the user’s muscle contractions. Additionally, postural control and ON/OFF strategies are applied using fixed signal thresholds to either maintain or initiate movement, ensuring that actions are triggered or halted as necessary. Finally, proportional control ensures that motor output is directly proportional to the detected signal amplitude, providing motor responses that are finely tuned to the user’s muscle activity [
43,
44].
Pattern recognition methods analyze short-duration signal segments by extracting time-domain, frequency-domain, or time–frequency features. The classification process involves pre-processing (using techniques like PCA, LDA, or MDA), classification (via neural networks, Bayesian systems, or fuzzy logic), and post-processing to refine outputs. However, real-world variability, such as stress, electrode displacement, or changes in muscle morphology, can challenge the transfer of laboratory results to clinical practice [
43].
Non-pattern recognition strategies are simpler to implement but offer limited functionality. Often, a hybrid approach that combines both methods yields the best results. For example, proportional myoelectric control directly correlates EMG amplitude with motor output, allowing for continuous and intuitive control, especially in systems requiring fine movements. In contrast, FSM control uses a limited number of predefined states and is effective for a fixed set of movements but lacks flexibility. Threshold-based control, which activates responses once a signal surpasses a set level, is also common but may not provide the smooth adjustment seen with proportional methods. Simultaneous control is regarded as the most natural approach, enabling the prosthesis to perform multiple functions at once. Algorithms based on regression can decode concurrent commands, such as differentiating between wrist rotation and hand opening, to enhance control flexibility. Hybrid control systems may also integrate additional sensor inputs, such as pressure sensors, accelerometers, EEG signals, or computer vision, to further improve accuracy and robustness [
24,
41].
Commercial systems often use a reduced number of electrodes and predominantly sequential control strategies, sometimes augmented with external control methods (
Table 2). Examples include the i-limb Quantum, which uses gesture control, and the Bebionic system, which incorporates finger-position encoders to prevent object drops. Some manufacturers are exploring the simultaneous activation of two electrodes to switch between different grasp types, as seen with the Michelangelo hand by Ottobock, although this method can be less intuitive. Direct control via pattern recognition is employed in systems like the Coapt and Ottobock Myo Plus, which achieve high classification rates with 2–12 sEMG electrodes [
1,
24,
37,
38].
Conventional approaches such as FSM and pattern recognition have been enhanced by machine learning techniques, particularly HD-sEMG paired with deep learning algorithms. Recent studies using sequential temporal regression and reinforcement learning have enabled natural control of wrist and finger movements, increasing system responsiveness and dexterity. Incorporating incremental learning strategies allows control systems to adapt to long-term variations in EMG signal patterns, supporting sustained, user-specific calibration over time. Hybrid control strategies that combine EMG, force, and motion sensors are increasingly being tested in real-time scenarios, enhancing system robustness and reducing command misclassification in dynamic conditions [
43,
44].
Advancements in prosthetic technology are continuously striving to create devices that are more intuitive, functional, and comfortable for the user. A key focus is on improving the human–machine interface, which allows for more natural and seamless control of the prosthetic limb. One promising approach involves the surgical creation of electro-neuromuscular constructs, where nerves and muscles are restructured to create new control signals that can be more easily interpreted by the prosthetic device. This method aims to provide a more direct and reliable link between the user’s brain and the artificial limb [
45].
Researchers are exploring various sensor technologies to enhance the control of lower prosthetics. For instance, studies have investigated using brain signals, specifically those from electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS), to detect a user’s intent to move [
46]. This approach allows for a direct neural connection to the prosthetic, bypassing the need for muscle signals entirely. In an equivalent way, other research has focused on the use of continuous neural control to restore a more biomimetic gait after amputation, enabling the prosthetic to move in a way that closely mimics natural human walking [
47]. Furthermore, preserving the biomechanical relationship between opposing muscle groups, known as agonist–antagonist muscle strain, in the residual limb is crucial for maintaining motor control and a sense of limb perception [
48].
Another critical area of development is the integration of advanced sensors directly into prosthetic devices and their interfaces. Flexible and stretchable sensors are becoming increasingly important for creating more comfortable and functional prosthetic skins [
49]. The application of flexible sensors in human–machine interfaces is a rapidly growing field, with these sensors being used to detect various inputs such as bending, pressure, and tactile feedback [
50,
51,
52,
53]. For example, wearable sensors can be used to recognize a user’s intent for adaptive control of intelligent ankle–foot prosthetics, allowing the device to adjust its function in real-time based on the wearer’s movements [
51]. The use of artificial intelligence (AI) is also enabling more intuitive control by processing complex nerve signals in real-time, translating a user’s intent into precise prosthetic movements [
54].
3.5. Sensory Feedback Systems and Human–Machine Interfaces
Overall, decades of research in robotic prostheses have yielded both invasive and non-invasive solutions integrated with the human body. The efficacy of these interfaces is primarily measured by their selectivity, signal quality, and stability, with ongoing research focusing on improving electrode impedance and filtering methods.
There is still no consensus on the best non-invasive approaches for providing sensory feedback in prostheses. Transcutaneous electrical nerve stimulation (TENS) has been explored as a non-invasive option to elicit natural-like sensations in amputees, but implantable electrodes, especially intraneural interfaces, have shown markedly superior outcomes. These invasive methods not only improve the functionality (e.g., enhanced texture and shape discrimination) but also reduce the cognitive load for the user, delivering a feedback experience that feels nearly natural [
42].
The challenges in the motor and sensory domains are distinct. On the motor side, robust signal decoding (often utilizing deep learning techniques) is essential, whereas in the sensory domain, implantable electrodes are emerging as the most promising solution, even though advances have primarily focused on tactile feedback. Thermal perception, mediated by fast-conducting Aδ fibers and slower C fibers, remains particularly challenging; current electrodes cannot effectively target these minute fibers. Future developments may involve higher-selectivity electrodes or sensory remapping techniques to simulate temperature feedback [
40,
47].
Recent advances in surgically embedded systems led to the development of the Agonist-Antagonist Myoneural Interface (AMI). This interface enables natural neural feedback and improved proprioception through physiological muscle reconnection in the residual limb, facilitating more adaptive gait and balance control in lower-limb amputees [
40]. Flexible and stretchable sensors continue to play a leading role in human–machine interfaces, particularly for continuous monitoring and adaptive control.
Recent clinical trials have demonstrated that patients with below-knee amputations who undergo AMI surgery walk on average 41% faster and navigate stairs and obstacles with reduced cognitive load compared to conventional amputees [
48]. The foundational AMI framework, covering conceptual design, surgical technique, and preclinical efficacy, has highlighted the improvements in pain mitigation, phantom-limb range of motion, and controllability through preserved fascicle dynamics and central proprioceptive pathways. By specifically preserving agonist–antagonist muscle relationships, the AMI maintains spindle-fiber and Golgi-tendon-organ afferents that underpin natural proprioception. Neuroimaging studies further confirm that AMI recipients exhibit proprioceptive response strengths in the primary sensorimotor cortex comparable to intact-limb controls when proprioceptors are passively stimulated [
48].
Concurrently, materials innovations have produced stretchable, self-powered sensors that conform to residual-limb anatomies. Triboelectric nanogenerators can harvest biomechanical energy to power continuous monitoring without batteries, converting gait-induced motions into electrical signals with high efficiency [
49,
50,
51]. Aerosol jet-printed silver strain gauges on low-melting-point plastic substrates enable custom geometries on non-planar surfaces, achieving sub-millimeter resolution and robust cycling performance [
52]. Artificial intelligence–assisted mechanoluminescent films integrate deep-learning color-decoding pipelines directly on flexible substrates, transducing complex deformation patterns in real time for haptic feedback [
50,
51]. High-density, dry-electrode EMG arrays fabricated on flexible PCBs map muscle activity across broad areas with signal quality matching rigid grids, facilitating both clinical gait analyses and at-home training [
52,
53,
54].
In summary, advanced control architectures fuse multimodal biosignals with machine learning to deliver intuitive and adaptive behavior. Continuous EMG controllers modulate ankle and knee actuators in real time, achieving smooth postural adjustments and load-transfer improvements substantiated in direct-control studies. Hybrid EEG–fNIRS brain–computer interfaces decode cortical engagement to adjust assistance levels dynamically, enhancing user experience and reducing mental workload. At the peripheral-nerve level, recurrent neural network decoders translate multichannel nerve recordings into movement commands with high accuracy and stable performance.
3.5.1. Targeted Muscle Reinnervation
Targeted muscle reinnervation (TMR) is an innovative neural interface procedure designed to improve control of upper-limb prostheses. In TMR, nerves that once controlled the amputated limb are surgically transferred to remaining intact muscles. After reinnervation, these muscles generate electrical signals in response to the original motor commands, enabling a more intuitive and natural control of the prosthesis while enhancing sensory feedback [
55,
56]. Severed nerves can remain viable for regeneration even years after amputation. Ensuring complete denervation of the target muscle is crucial so that the resulting EMG signals originate solely from the transferred nerves, thereby avoiding mixed signals that could complicate prosthetic control. For optimal independent control of various movements, reinnervated muscle segments must produce distinct EMG signals; minimizing the subcutaneous fat layer can further enhance signal amplitude and differentiation [
57,
58,
59,
60].
This surgical procedure has rapidly evolved into a multifaceted neural-interface strategy that not only enhances intuitive myoelectric control of upper-limb prostheses but also provides durable relief from neuroma and phantom-limb pain. Originally developed to reroute residual peripheral nerves into expendable muscles, TMR creates new myoelectric signal sources that correspond directly to motor intentions, yielding markedly improved prosthetic dexterity and user satisfaction. Over the years, indications for TMR have broadened in cases of neuroma pain after primary amputation, with a stable pain reduction and functional gains postoperatively [
61,
62,
63].
At its core, TMR entails coapting a transected mixed motor or sensory nerve into the motor branch of a denervated target muscle, harnessing the muscle’s trophic milieu to guide axonal regrowth and reestablish afferent–efferent loops [
60]. Ideal donor nerves include those that previously controlled wrist or finger movement; common recipients are segments of the pectoralis or biceps in transhumeral amputations and the brachi-oradialis or flexor digitorum in more distal levels [
60]. Precise dissection and intraoperative nerve stimulation ensure complete denervation of the target muscle, preventing signal contamination by residual native fibers and maximizing the specificity of the regenerated EMG signals.
When an amputation occurs, the protective sheath of a nerve is damaged, causing the axons inside to grow chaotically into the surrounding scar tissue. This disorganized growth frequently results in the development of painful neuromas. To address this, reinnervation techniques such as TMR and Regenerative Peripheral Nerve Interface (RPNI) are employed. Both TMR and RPNI redirect the severed nerve into a segment of healthy, denervated muscle. This muscle segment acts as a conduit, providing a more organized pathway for the nerve to regrow and find new targets (motor end plates) for reinnervation. By preventing the nerve from sprouting randomly into fibrotic tissue, these procedures lower the risk of neuroma formation and help reduce post-amputation pain. They are both equally effective in pain relief, though TMR may confer superior control signals when ample muscle targets exist. Combining TMR with RPNI (“TMRp-ni”) may further optimize neuroma prevention, but more studies are needed [
64].
The spatially distinct activation patterns can be exploited by HD-sEMG for high-fidelity control. Unlike conventional systems, HD-sEMG sensors can be seamlessly integrated into clothing, creating a non-intrusive myoelectric interface that can be used throughout a user’s daily routine. A recent study introduced a user-friendly HD-sEMG device designed to interface with an 8-degree-of-freedom mobile manipulator. The research evaluated the use of real-time myoelectric gesture recognition to enable precise control over a range of self-care and household tasks. The study demonstrated the potential of this wearable system for controlling mobile manipulators within a home environment, highlighting its efficacy in physically assistive roles [
65]
Modern TMR-based controllers integrate EMG pattern recognition algorithms, such as support-vector machines and convolutional neural networks, to map complex activation patterns to movements in real time. Hybrid approaches that fuse EMG, inertial measurement unit (IMU), and pressure sensor data achieve >95% classification accuracy across multiple tasks. Inspired by multimodal strategies used in fatigue detection systems, where EMG and IMU signals are fused via deep learning architectures for robust real-time classification, emerging bidirectional interfaces incorporate afferent stimulation to the reinnervated muscles, closing the loop for sensory feedback and further refining motor control [
66].
Ideal TMR candidates are those with residual limb lengths permitting effective nerve transfer, typically mid-humeral or more proximal for upper-limb amputations, and sufficient muscle mass to host reinnervation sites. Each candidate is individually evaluated based on the quality of soft tissues, nerve function, and their commitment to the rehabilitation process. Younger patients often exhibit better outcomes due to the higher regenerative capacity of nerves. The surgical procedure is facilitated by flexible soft tissues and the absence of prior skin grafts, although previous surgeries do not automatically preclude TMR. Longer residual limbs allow for more extensive nerve transfers. Contraindications include proximal brachial plexus injuries or severe soft tissue scarring that precludes adequate nerve mobilization. Postoperative protocols begin with an immobilization period, followed by progressive physical rehabilitation training [
55,
56].
While generally safe, TMR carries surgical risks such as hematoma, infection, and transient sensory deficits in donor-nerve distribution [
61]. Surgical risks include hemorrhage, infection, paresthesia, loss of sensitivity, exacerbation of phantom pain, and possibly the need for additional interventions. Postoperative protocols typically require an initial prosthesis-free period to ensure proper wound healing, and patients are advised that full reinnervation may take several months. Often, amputation is combined with neurectomies to manage neuroma formation; physical examination, supported by simple radiographs, is critical to assess residual bone length and soft tissue conditions of the patient. Phantom pain may transiently worsen during the reinnervation phase but typically subsides as new afferent pathways mature over the following months [
57].
TMRpni addresses cases with limited local targets, ensuring no regenerating axon is left without a functional end organ. Additionally, fully implantable systems that combine intramuscular electrodes with external transmitters could, in the future, demonstrate stable signal quality and user acceptance [
61].
Ongoing advances aim to miniaturize implantable electronics, integrate direct neural recording from mixed sensory–motor fascicles, and leverage AI for adaptive signal decoding. As surgical techniques refine and closed-loop sensory feedback systems mature, TMR stands poised to deliver truly biomimetic experiences, restoring not just function but naturalistic embodiment.
3.5.2. The Role of Osseointegration
Amputation treatments are often performed under emergency conditions, prioritizing patient survival over long-term esthetics or functionality. Consequently, many patients are left with a residual stump that is both functionally poor and painful, which can compromise subsequent outcomes. In recent years, significant investments have been made in innovative prostheses and control strategies, making the functional preparation of the stump crucial for advanced robotic prostheses. Traditional surgical techniques aim to preserve as much limb length as possible to form a cushion for standard prosthetic sockets. However, such approaches result in a passive stump that is not ideal for the dynamic control required by robotic devices [
67,
68,
69].
Discomfort and poor socket fit are among the primary reasons for prosthetic device rejection. To mitigate these issues, the interface must provide a comfortable, stable, and individually tailored fit that protects the residual limb. Excessive and continuous pressure, as well as localized pressure peaks, can lead to pain, edema, eczema, and even neuroma formation. These factors adversely affect both control and overall quality of life [
68,
69]. Different fixation systems exist for prostheses, which can be categorized as invasive or non-invasive. Non-invasive methods include the use of sleeves or harnesses, vacuum systems, and systems like the BOA Fit, which offer precise, adjustable fittings. However, non-invasive systems may not always provide optimal comfort or stability. Osseointegration represents a more invasive but transformative approach that involves implanting a titanium screw into the residual bone, allowing the prosthesis to be directly affixed. The procedure typically requires two surgical stages: the first to insert the implant and the second to attach the percutaneous abutment. Although osseointegration offers better esthetics and a greater sense of security, it carries risks such as infection and may necessitate multiple interventions [
70].
The pioneering work of Ingvar Brånemark, who demonstrated stable titanium–bone integration in rabbit tibiae and later in dental implants, laid the foundation for modern osseointegration. Today, the technique is applied in hip arthroplasty, dental implants, craniofacial reconstruction, and the direct anchoring of prosthetic devices. This method not only eliminates the complications associated with conventional socket interfaces but also offers benefits such as reduced energy expenditure, improved range of motion, and indirect sensory feedback—referred to as osseoperception, which enhances the user’s ability to perceive vibratory sensations through bone [
68,
69,
70,
71]. The success of osseointegration depends on the bone’s capacity to incorporate a non-biological implant, initiating a process that starts with hematoma formation and progresses through lamellar bone formation and subsequent remodeling. Patient selection is critical; candidates must have adequate bone stock, adhere to rehabilitation protocols, and be in good overall health. Preoperative planning, often involving CT scans to assess bone quality and dimensions, is essential to minimize risks and ensure implant stability [
68,
69,
70,
71,
72].
Several osseointegrated systems have been developed for amputees. For example, the OPRA (Osseointegrated Prostheses for the Rehabilitation of Amputees) system, introduced in 1998 in Sweden, uses an intramedullary implant with an external abutment for prosthesis attachment. A modified version, e-OPRA, incorporates a bidirectional communication gateway between implanted neuromuscular electrodes and the external device, thereby enabling both motor control and sensory feedback [
69,
70,
71,
72]. Recent advances include both intramedullary and extramedullary designs. Extramedullary devices (
Figure 3), which fix the prosthesis to the external surface of the bone, may offer lower risks of bone resorption and infection, increased stability, and enhanced bone regeneration compared to their intramedullary counterparts [
68,
69].
Despite improvements in implant design, surgical techniques, and rehabilitation protocols, infection remains a major concern. Osseointegration surgery is highly demanding and should be performed only by experienced orthopedic surgeons, with rigorous patient selection based on clinical criteria. Studies indicate that osseointegration improves comfort, increases range of motion, and enhances mobility compared to conventional socket prostheses. A key benefit is osseoperception, the enhanced ability to discern vibratory sensations via bone conduction, which contributes to a more natural incorporation of the prosthesis into the user’s body image. This integration supports not only better motor control but also improved psychosocial well-being [
67,
68,
69].
3.5.3. Brain–Computer Interfaces
Brain–computer interfaces (BCIs) enable direct communication between the brain and external devices by translating neural activity into control commands. These systems are particularly valuable for individuals with neurological disorders, paralysis, or severe motor impairments, allowing them to operate assistive technologies or even participate in consumer applications through their brain signals. Most non-invasive BCIs acquire signals via electroencephalography (EEG), prized for its high temporal resolution, low cost, and safety. However, EEG is inherently nonstationary and prone to noise and artifacts, which demand rapid and precise extraction of relevant features for accurate decoding [
73,
74,
75].
A foundational paradigm in EEG-based BCIs is motor imagery, where users imagine specific movements without physical execution. The standard processing pipeline comprises five stages: acquisition, pre-processing, feature extraction, feature selection, and classification. Pre-processing tackles noise and artifacts, while feature extraction methods, such as wavelet transforms, short-time Fourier transform (STFT), common spatial pattern (CSP), and filter bank CSP (FBCSP), capture time, frequency, and spatial characteristics. Extracted features are then fed into machine learning classifiers, including convolutional neural networks (CNNs) and sparse autoencoders (SAE), to distinguish between imagined actions [
76].
Despite the effectiveness of this pipeline, conventional workflows rely heavily on manual preprocessing and handcrafted feature engineering, creating barriers to real-time and clinical applications. To address this, recent frameworks employ end-to-end CNN architectures that operate directly on raw EEG data. By learning hierarchical representations autonomously, these models classify multi-class motor imagery, such as hand, foot, or tongue movements, without explicit filtering, while integrating skip connections and adaptive pooling to improve resilience against inter- and intra-subject variability [
76]. Such generalized models not only maintain competitive accuracy across diverse datasets but also reduce computational overhead, facilitating deployment on embedded or mobile platforms.
In neurorehabilitation, EEG-BCI systems harness motor imagery combined with feedback modalities to drive neural plasticity and restore motor function. A growing body of research highlights promising outcomes for upper extremity recovery, often employing robotic orthoses or virtual training scenarios that translate neural commands into assisted movements. The integration of virtual reality (VR) technologies enhances patient engagement and adherence; while non-immersive VR setups dominate clinical studies, fully immersive environments show potential for even greater therapeutic efficacy by creating more compelling sensorimotor experiences [
76].
Lower-limb BCI applications are less prevalent but have demonstrated feasibility in tasks like foot movement control and wheelchair navigation. Innovative approaches incorporate haptic feedback to complete the sensory-motor loop, providing users with tactile cues that reinforce intended movements. To date, unified frameworks addressing both upper and lower extremities remain rare, representing a significant opportunity for future development in holistic rehabilitation solutions [
76].
Despite these advances, widespread clinical adoption of EEG-BCIs faces several hurdles: a lack of standardized protocols, extensive calibration and training requirements, and limited validation across heterogeneous patient populations. Overcoming these challenges will depend on advancements in signal-processing algorithms, the creation of more affordable and user-friendly hardware, and the integration of BCIs with complementary rehabilitation technologies. For instance, coupling BCIs with functional electrical stimulation can enhance motor recovery by directly activating muscles in synchrony with neural intent. Moreover, adaptive BCI systems that personalize therapy based on real-time neural feedback promise to optimize rehabilitation outcomes and reduce the burden of manual adjustment [
76].
While EEG-based systems offer safety and convenience, they are limited in spatial resolution and signal fidelity. Invasive BCIs—using implanted microelectrode arrays and wireless systems—seek to overcome these constraints by providing high-resolution, stable recordings over extended periods. Biocompatible materials minimize immune responses and scar tissue formation, ensuring long-term device viability [
75,
76].
A leading example is Neuralink’s N1 chip, designed to capture high-bandwidth neural data through 3072 analog pixels. Each pixel contains a low-noise amplifier, achieving a system gain to match the resolution of on-chip Analog-to-Digital Converters (ADCs). Data from the amplifiers feed into ADCs, are multiplexed, and then transmitted serially. The SoC incorporates sophisticated power management strategies, backpressure buffering to alleviate data congestion and event-based sampling to conserve resources. A JTAG interface based on Boundary Scan and a dedicated test access port (TAP) controller enables configuration, diagnostics, and on-chip testing without invasive probing [
77]. This architecture exemplifies the balance between high-density neural recording, low power consumption and robust data handling required for next-generation BCI applications.
Together, these technological innovations, spanning non-invasive end-to-end decoding frameworks, immersive neurorehabilitation platforms and advanced implantable hardware, are driving a paradigm shift toward more accessible, scalable, and effective BCIs for both clinical and consumer markets.
3.5.4. Electromyographic Signal Acquisition and Processing
The use of EMG in robotic devices, especially in prostheses, has been a strong area of research, given that it is crucial for developing more natural and effective human–machine interfaces. In this type of device, the components’ size and portability have implications for their operational effectiveness and efficiency. As previously mentioned, the data captured by electromyographic electrodes vary depending on the nature of the muscle contraction, the size of the analyzed muscle, and other technical or methodological variables [
78].
One of the invasive electromyography techniques consists of inserting a needle electrode through the skin directly into the muscle. Although it can provide a localized and high-resolution description of the electrical activity of the muscle, it is a somewhat painful experience for the patient. The pick-up area of the needle electrode depends on its specific design and may include only one or two muscle fibers. The monopolar needle electrode requires a reference electrode placed away from the needle insertion point in an electrically neutral location (such as over a bone). The concentric needle electrode avoids the reference electrode by referencing the active surface of the electrode to the cannula of the needle. Both types of needle electrodes are used in clinical settings. By placing several needle electrodes in various locations, a more detailed description of the electrical activity of a larger muscle group can be obtained [
78].
An additional development of the surface electrode technique is the linear electrode array, designed to provide a spatial description of myoelectric activity. The resulting multichannel EMG signal from several electrodes placed equidistantly along the direction of the muscle allows studying the generation and extinction of action potentials and estimating the conduction velocity. This method can be recorded at lower sampling rates than needle EMG, as the intervening tissue between the motor units and the surface electrode acts as a low-pass filter of the electrical signal. It also has most of its spectral power below 400–500 Hz, which implies that a sampling rate of 1 kHz or higher is necessary. For needle EMG, the sampling rate should be chosen so that different waveforms of MUAPs, which can contain frequencies up to 10 kHz, are accurately reproduced; therefore, a sampling rate of 50 kHz is often used [
78].
In sEMG, the size of the electrode is determined by the conductive area and orientation relative to the muscle fibers. The dimensions of the electrode should remain within a range that preserves sensitivity to signal variations. Electrodes of varied sizes and shapes are commercially available, with circular and rectangular formats being the most common. The spacing between bipolar electrodes needs to be chosen according to the muscle being studied, since excessive separation can introduce instability. In smaller muscles, the distance between electrodes should not exceed one-quarter of the muscle fiber length, thus avoiding unstable readings caused by tendon effects. The material that constitutes the contact area with the skin must ensure good electrode–skin contact and consistent low impedance. Pre-gelled Ag/AgCl electrodes are generally the most recommended [
30,
31,
78].
Several steps must be followed when using sEMG. The first should be skin preparation with good electrode-skin contact to ensure the best signal acquisition, minimizing electrical artifacts and interference. It is recommended to clean the skin with alcohol, which disinfects and evaporates quickly, preparing the skin for sensor placement. The use of multiple EMG sensors to decode movement intentions is also vital. Traditionally, sensors were placed between the muscle innervation areas and the tendinous zones, which are known to offer better quality signals. However, the complexity of the overlapping muscles in the human forearms obscures the median muscle lines on the skin surface, which makes it difficult to capture electromyographic signals [
78].
Several studies demonstrate that small deviations of an electrode over the muscle innervation area can significantly alter the signal amplitude. Furthermore, the process of positioning the sensors requires careful customization, with significant inter-individual variability due to the anthropometric characteristics of each individual. Creating a functional myoelectric interface requires considerable adjustment effort, especially when increasing the number of sensors. The signal-to-noise ratio can be improved by positioning the electrodes as close as possible to the signal source. The reference electrode should be placed in a location where the risk of signal disturbances is minimal, preferably in electrically inactive tissues. Depending on the muscle under analysis, it is common to position the reference electrode on the wrist, elbow, chest, tibia, ankle, or the C7 vertebra [
78,
79,
80].
The manipulation capabilities of the human hand are governed by the activation patterns of the numerous muscles of the forearm and hand. Some of these muscles are superficial and easily accessible, but most are in deeper compartments. For this reason, there are only a small number of superficial forearm muscles from which sEMG signals can be reliably detected. In addition, the relatively small size and proximity of many muscles are also a concern. Once acquired, EMG signals undergo several processing steps: amplification, filtering, and rectification, to remove noise and extract useful features. Usual challenges include movement artifacts, electrocardiographic interference (especially in trunk or neck recordings), and electromagnetic interference from nearby devices. Advanced noise-cancelation and narrowband filtering techniques, like those used in ECG processing, are applied to preserve the EMG spectral content [
78,
79,
80,
81].
In terms of control, EMG signals from electrodes on the residual limb are analyzed to extract control commands. Simple controllers may use signal amplitude to differentiate functions (such as opening/closing a hand), while multifaceted prostheses combine inputs from multiple electrodes and employ advanced algorithms to capture transient signal patterns at the onset of rapid contractions. Real-time processing is critical, as response times must be short enough to prevent perceptible delays [
78,
79,
80,
81,
82]. The amplitude of the sEMG signal is a key parameter for clinical and control applications and can be estimated using statistical techniques (e.g., standard deviation or dispersion estimates) over sliding time windows. Due to the stochastic nature of sEMG (resulting from random motor unit recruitment, variable firing rates, and external interference), advanced statistical tools and models (often based on linear time-invariant, LTI, filter concepts) are employed. Although these models, such as the filtered noise model, simplify the complex relationship between neural activity and the EMG signal, they face limitations due to nonlinear tissue properties and variable electrode–tissue interfaces [
78,
79,
80,
81,
82].
3.5.5. General Steps in EMG Signal Processing
The acquisition and processing of electromyographic (EMG) signals for controlling a bionic hand involve several sequential stages that transform raw muscle signals into actionable commands. The process begins at the signal source, typically the forearm, where muscle contractions generate interpretable signals. Strategically placed electrodes capture these signals, which are then transmitted to a data acquisition device. Here, analog signals are converted into discrete digital values, a step that is crucial for accurate digital processing [
83,
84].
The digitized signals undergo pre-processing to remove noise and artifacts, ensuring that only high-quality, relevant data are analyzed. This includes segmenting the continuous signal into temporal windows, which is essential for subsequent feature extraction. In this phase, key metrics such as amplitude, frequency, and other parameters are extracted to differentiate between distinct types of muscular movements. These extracted features are then fed into classification algorithms that interpret the user’s intended movement and convert it into a specific command for the prosthetic hand. The movement controller receives this command, activates the corresponding motors via the servo driver, and simultaneously sends performance and position feedback back into the system for real-time adjustments, ensuring a more intuitive and responsive control experience [
83,
84].
3.5.6. Artificial Intelligence in EMG Signal Inference
Recent advances in EMG pattern recognition, bolstered by deep learning techniques, promise significant improvements in the functionality and versatility of control systems. Deep learning models enable the discrimination of a wide array of movement intentions, supporting the control of prostheses with multiple degrees of freedom. However, deploying these advanced techniques in real-world environments remains challenging due to individual variability and the dynamic nature of everyday activities. Understanding artificial intelligence (AI) in biomedical applications begins with grasping the fundamentals of deep learning.
Discriminative models classify input data into predefined labels by learning discriminative features through adaptive nonlinear transformations and probabilistic predictions [
85]. Classification is achieved using a softmax layer combined with one-hot label encoding. Common discriminative architectures include Multilayer Perceptrons (MLPs), Recurrent Neural Networks (RNNs), and Convolutional Neural Networks (CNNs), along with their variants [
85,
86,
87]. The softmax layer is an activation function used in the final layer of classification models. It converts output values (logits) into probabilities, ensuring each value lies between 0 and 1 and that their sum equals 1. During training, true labels are converted into one-hot vectors, where only the position corresponding to the true class is set to 1. This combination allows error calculation and network weight adjustments. Softmax ensures interpretable probabilities, while one-hot encoding enables direct numerical comparison between predictions and true labels, critical for gradient-based learning [
85,
86,
87].
One-hot encoding represents categorical labels as binary vectors, where only one position is “1” (active) and others are “0”. For example, categories 0, 1, 2, and 3 are encoded as (1, 0, 0, 0, 0), (0, 1, 0, 0), (0, 0, 1, 0), and (0, 0, 0, 1), respectively. This method avoids implicit ordinal relationships between categories. However, for large datasets, it increases dimensionality and storage inefficiency due to sparse vectors (e.g., 100 categories require 100-dimensional vectors) [
85,
86].
In contrast, representational models like autoencoders (AE), restricted Boltzmann machines (RBM) and deep belief networks (DBN) are used to autonomously extract the most salient features from the data without manual intervention. Although these models excel at capturing underlying data distributions, they often require a combination with discriminative techniques to perform classification tasks effectively. Generative models such as variational autoencoders (VAEs) and generative adversarial networks (GANs) can also synthesize new data samples, which is especially useful for augmenting limited training datasets [
85,
86].
Hybrid deep learning models integrate both representational and discriminative approaches to harness the strengths of each, achieving robust and flexible performance in EMG signal interpretation. Notably, recent research using 3D Convolutional Neural Networks (3DCNNs) has shown promise in extracting muscle synergy features by converting one-dimensional sEMG signals into two-dimensional anatomical representations and then compiling these into three-dimensional segments. This approach captures both spatial and temporal components of muscle activity, outperforming traditional CNNs and autoencoders in terms of accuracy and efficiency while also reducing training times [
85,
86].
Representational deep learning models autonomously learn pure, high-level features from raw data without manual feature engineering. Unlike traditional machine learning, these models identify underlying patterns through hierarchical representations. Examples include autoencoders (AEs), Restricted Boltzmann Machines (RBMs) and Deep Belief Networks (DBNs). AEs compress input data into latent representations and reconstruct them, while RBMs and DBNs learn probability distributions and hierarchical features. However, these models focus solely on feature extraction, not classification. Generative models learn the joint probability distribution of input data and labels, enabling synthetic data generation. In biosignal processing, they are used to augment training datasets. Key examples include VAE (which learns probabilistic latent spaces to generate data) and GAN (which uses a generator-discriminator framework to produce realistic synthetic samples). Unlike conventional AEs, VAEs prioritize generation over reconstruction [
85,
86].
Hybrid models integrate multiple algorithm types, such as combining representational models for feature extraction with discriminative models for classification. A common example pairs DBNs with softmax layers for decision-making. True hybrid systems explicitly merge distinct paradigms (e.g., GANs with CNNs for image processing or VAEs with RNNs for sequential data). These models leverage the strengths of different approaches to tackle complex challenges [
85,
86].
Advanced algorithms and high-quality open-source datasets are enhancing EMG pattern recognition, attracting more researchers to the field. Deep learning techniques now outperform conventional methods in grasp classification and movement detection. However, the need for large training datasets remains a challenge. Studies using surface EMG (sEMG) to predict continuous wrist movements are particularly relevant for post-stroke rehabilitation, where intention prediction can personalize robotic orthosis assistance [
85,
86].
Recent studies propose 3DCNNs to extract muscle synergies from sEMG signals. These models transform 1D sEMG data into 2D anatomical maps, compiled into 3D segments capturing spatial-temporal dynamics. 3DCNNs outperform traditional CNNs and autoencoders in accuracy and efficiency while reducing training time. Limitations include validation gaps in complex movements and physically impaired populations. Future work aims to expand predictions to multi-degree-of-freedom motions and integrate biomechanical constraints [
87].
This integration of cutting-edge algorithms (
Figure 4) and open datasets is driving innovation in EMG-based rehabilitation technologies, paving the way for personalized and efficient therapeutic solutions.
4. Challenges in Robotic Hand Prostheses
The excessive cost of robotic prostheses remains a significant barrier to broader adoption. With most advanced solutions priced between USD 10,000 and USD 20,000, many researchers advocate for more affordable alternatives, especially for developing countries. Open-source prostheses present a promising alternative by drastically reducing development and distribution costs. Although rigid architecture currently prevails, there is growing interest in developing more flexible systems that facilitate safe interactions. These more compliant architectures combine simple actuation with the performance of a highly adaptable hand.
The advancements in neuromuscular robotic prostheses have been remarkable, integrating EMG signal processing, machine learning algorithms, and BCIs to enhance prosthetic control and functionality. The studies reviewed demonstrate significant progress in decoding neural and muscular signals to achieve more intuitive and precise movements. However, despite these advancements, several challenges remain.
One of the key findings is the necessity of robust signal acquisition and processing methods to ensure real-time and accurate control. Many studies highlight the importance of minimizing signal noise and improving electrode placement strategies to enhance the reliability of EMG-based control systems. Additionally, the integration of AI has shown great potential in adapting responses to user-specific needs, but further optimization is required to refine learning algorithms and reduce computational latency. Another critical aspect discussed in recent research is the bidirectional communication between prostheses and the user. Sensory feedback mechanisms, such as electrical stimulation and haptic interfaces, are being developed to restore a sense of touch and proprioception in amputees. While promising, these technologies still face hurdles in terms of biocompatibility, long-term stability, and user adaptation. Moreover, current designs of robotic prostheses are often constrained by power consumption and portability. The miniaturization of electronic components and the development of energy-efficient actuation systems are essential to improve the wearability and usability of these devices. Emerging energy harvesting techniques and low-power computing architectures could provide viable solutions for future prosthetic systems.
The recent advances in neuromuscular robotic prostheses illustrate a compelling integration of signal processing, sensor technology, and intelligent control systems. One of the major engineering challenges lies in the design of analog front ends and digital conversion circuits that can reliably amplify and convert low-amplitude bioelectrical signals, typically in the microvolt range, into robust digital data without introducing excessive noise. The selection of high-quality electrodes, proper amplifier design, and low-noise ADCs are critical to ensuring that even the faintest signals are preserved for further processing. Modern signal processing techniques have evolved to include sophisticated filtering, segmentation, and feature extraction algorithms that are implemented on embedded platforms. These methods not only remove artifacts from the raw signal but also extract meaningful parameters such as signal amplitude, frequency characteristics, and time-domain features. This is crucial for the subsequent steps where pattern recognition algorithms based on deep learning techniques interpret these features to infer user intention.
Control strategies have also evolved with the integration of both classic control systems and data-driven approaches that exploit the power of deep neural networks. For instance, CNNs are employed to decipher complex patterns from EMG data, enabling a more natural and multi-DOF control of prosthetic limbs. This shift toward incorporating AI has enabled prosthetic systems to continuously learn and adapt to the user’s specific muscular and neural signatures, thereby enhancing both precision and responsiveness. Another exciting development is the integration of BCIs with traditional EMG-based systems. This hybrid approach combines complementary data streams from peripheral muscles and central nervous system activities, which can significantly improve system robustness and response accuracy. However, achieving seamless, bidirectional communication between these diverse sensors remains a challenging task, particularly in terms of synchronizing high-bandwidth neural data with lower-frequency muscle signals. Addressing issues such as latency, data fusion, and real-time processing remains paramount for achieving the objective of natural and intuitive control.
The hardware and embedded system design aspects are equally critical. Engineers must optimize the trade-offs between power consumption, processing speed, and overall device miniaturization. Advances in semiconductor technology and low-power design have enabled the development of highly integrated SoCs that aggregate the necessary analog and digital functionalities into a compact form factor. Wireless communication modules, efficient battery management, and the integration of energy harvesting techniques are emerging as key enablers for the next generation of wearable prostheses.
From a clinical perspective, there is an increasing emphasis on the standardization of testing protocols and validation methods to facilitate the transition from laboratory research to real-world applications. Interdisciplinary collaboration between engineers, neuroscientists, and clinicians is crucial to ensuring that the solutions developed meet the practical needs of users. Future research directions should focus on improving the robustness of signal processing techniques, enhancing sensory feedback integration, and developing lightweight, energy-efficient prosthetic models. Furthermore, the adoption of hybrid control strategies may offer a more inclusive approach to prosthetic control, leading to a new generation of intelligent and adaptive robotic limbs.
5. Limitations of the Existing Works and Future Road-Map
After decades of research in the field of robotic prostheses, a paradoxical situation has emerged: although the development of new prostheses represents one of the most promising areas in robotics, most amputee patients still use technologies that have evolved little over the past decades. This paradox is even more striking when considering the colossal challenge of developing a prosthesis capable of replicating the sensory and motor functions of a human hand. The hand possesses one of the most complex motor and sensory representations in the human brain, making the execution of grasping tasks extremely complex.
Current challenges are vast and multifaceted, requiring a combination of advanced mechatronic solutions to develop highly sensorized robotic hand prostheses, along with novel approaches to establish robust and efficient interfaces with patients’ nervous systems. There is presently a diverse range of options for restoring hand function, from custom cosmetic solutions to state-of-the-art prostheses featuring sensory feedback and advanced control. Each solution offers its benefits and drawbacks, with the ideal choice depending on the patient’s individual needs and preferences. For instance, the custom cosmetic solution (
Figure 1a) provides a natural and realistic appearance that enhances the patient’s self-esteem and confidence. It can be personalized to match the skin tone, texture, and characteristics of the unaffected hand; however, it does not restore motor or sensory functions.
The body-powered prosthetic solution (
Figure 1b) harnesses the patient’s muscle strength to control the prosthesis, offering intuitive and direct control. It does not require an external power source and is both lightweight and portable, though it may demand more physical effort and offer more limited movement compared to myoelectric prostheses. In contrast, the surface electromyography (sEMG) pattern recognition system (
Figure 1c) analyzes myoelectric signals from the patient’s residual muscles to identify movement patterns. In this system, electromyographic patterns are translated into control commands for the prosthesis, enabling more complex and precise movements. Furthermore, with sEMG control combined with extracellular stimulation via an implanted FINE (flat interface nerve electrode) electrode (
Figure 1d), there is a merging of myoelectric control and neural stimulation to provide sensory feedback to the prosthetic hand. The implanted FINE electrode stimulates sensory nerves by transmitting information about the external environment, thereby offering a more natural and intuitive sensory experience.
In fully implanted myoelectric sensor systems (
Figure 1e), the need for external electrodes is eliminated, resulting in a more natural and discreet appearance. These sensors capture myoelectric signals directly from the muscles, thereby reducing noise and interference. However, they require surgical implantation and may have a limited lifespan. The prosthesis designated as i-Limb Ultra (
Figure 1f) operates using sEMG control. Additionally, in systems where sensory feedback is provided by intraneural TIMEs (Transverse Intrafascicular Multichannel Electrode) electrodes (
Figure 1g), direct stimulation of sensory nerves within the peripheral nerve delivers high-resolution feedback. These electrodes represent a recent technological advancement with the potential to revolutionize sensory feedback in prostheses [
1].
Table 3 shows a qualitative evidence map regarding the technological approaches discussed throughout this review and the associated limitations.
Table 4 provides an evidence map of neuromuscular prosthesis technologies, covering the domains of sensing, control, actuation, feedback, and BCI. This table includes typical capabilities, known limitations, and readiness levels.
This analysis highlights the technological improvements and ongoing challenges in the design of neuromuscular robotic prostheses, with particular focus on signal acquisition technologies, control strategies, and actuator typologies. The inherent complexity of replicating human hand function, especially in terms of tactile sensitivity, proprioception, and multi-joint control, has driven the development of increasingly integrated solutions. The diversity of control approaches available today reflects the trade-offs required to simultaneously optimize accuracy, robustness, and natural movement. Thus, the progress in this field depends on the gathered-evolution of engineering, neuroscience, and medical technology, with direct implications for functional rehabilitation and the quality of life of upper-limb prosthesis users.
6. Conclusions
Recent advancements in neuromuscular robotic prostheses reflect a transformative synergy between artificial intelligence, sensorimotor engineering, and neurotechnology. EMG-based control systems, brain–computer interfaces, and machine learning have significantly enhanced the precision and intuitiveness of robotic prostheses. This review has outlined the evolution of the main subsystems of these devices, highlighting the technical innovations driving performance gains in real-world applications.
Modern signal acquisition techniques combined with deep learning architectures can achieve good decoding accuracy. These methods exhibit resilience to signal drift, electrode displacement, and noise. Moreover, recent developments in hybrid control systems, fusing EMG and EEG inputs, offer enhanced robustness across dynamic conditions. Sensory feedback mechanisms, both invasive and noninvasive, have matured significantly in recent years, which simultaneously improves signal selectivity and enables bidirectional flow of information, facilitating artificial proprioception and tactile feedback.
The convergence of three pillars, intelligent control algorithms, surgically optimized neuromuscular interfaces, and osseointegrated anchoring, has shifted the clinical paradigm from passive assistance to active neurointegration. Osseointegration, beyond providing mechanical fixation, is now recognized as an electromechanical interface supporting implanted electronics and transcutaneous energy transfer, crucial for embedded sensing and stimulation without compromising skin integrity. These systems pave the way for fully implantable, maintenance-free prosthetic architectures with long-term usability.
Despite these progresses, critical challenges persist. The long-term reliability of surface EMG remains hindered by motion artifacts, muscle fatigue, and variable skin-electrode contact. Implanted neural interfaces, while offering higher fidelity, are limited by biological encapsulation, signal degradation, and the risk of chronic immune responses. Power consumption also remains a significant barrier; integrating actuators, batteries, sensors, and processors into a compact, lightweight system without compromising function is a key design constraint. Additionally, many state-of-the-art control algorithms remain confined to controlled experimental settings, lacking robustness for deployment in unstructured environments.
Another major bottleneck is the absence of standardized validation frameworks. Discrepancies in signal processing pipelines, outcome metrics, and safety protocols hinder reproducibility and regulatory translation. Real-time bidirectional control also remains underdeveloped, as latency, even on the order of milliseconds, can disrupt the synchrony between user intent and execution. Energy-efficient hardware, adaptive actuators, and neuromorphic computing platforms are promising directions to address this constraint.
Future research must target these gaps through a systems-level approach. Embedding AI models within low-power edge devices enables responsive, wearable prosthetics with minimal energy overhead. Material innovation, such as bioactive electrode coatings and flexible substrates, may improve long-term biocompatibility. Meanwhile, the democratization of prosthetic development through open-source platforms and additive manufacturing is accelerating accessibility and customization. Equally vital is the refinement of user adaptation strategies and longitudinal validation studies. Rigorous clinical trials with amputee cohorts are essential to assess functional outcomes, cognitive load, and long-term neural plasticity. Cross-disciplinary collaboration among engineers, clinicians, neuroscientists, and designers is no longer optional but foundational to the field’s advancement. Development teams must address not only the mechanical and electrical interfaces but also the physiological, psychological, and social dimensions of limb loss and restoration.
In summary, the integration of advanced biosignal acquisition, AI-driven control frameworks, and closed-loop sensory feedback is redefining the frontiers of neuromuscular prosthetics. Achieving intuitive control with real-time feedback is within reach, but will require persistent investigations in embedded systems, materials science, and neuroengineering. As the field moves from proof-of-concept to clinical translation, the emphasis must remain on human-centered design, reproducible methodologies, and sustainable deployment. Ultimately, the goal is to create prosthetic systems that do not merely restore movement but reinstate embodiment, devices that respond to the user’s intent with naturalness and precision.